Back to Journals » International Journal of Nanomedicine » Volume 14
NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: in vivo and in vitro study
Authors Abbasi-Oshaghi E , Mirzaei F, Pourjafar M
Received 26 October 2018
Accepted for publication 31 January 2019
Published 15 March 2019 Volume 2019:14 Pages 1919—1936
DOI https://doi.org/10.2147/IJN.S192382
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Ebrahim Abbasi-Oshaghi,1,2,* Fatemeh Mirzaei,2,* Mona Pourjafar3
1Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran; 2Neurophysiology Research Center, Hamadan University of Medical Sciences, Hamadan, Iran; 3Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran
*These authors contributed equally to this work
Purpose: This study evaluated the effects of titanium dioxide nanoparticles (TiO2 NPs) on liver and intestine of normal rats.
Methods: Male rats were divided into four groups as follows: 1) control rats, 2) control rats that orally received 10 mg/kg TiO2 NPs, 3) control rats that orally received 50 mg/kg TiO2 NPs, and 4) control rats that orally received 100 mg/kg TiO2 NPs. After 30 days, the NLRP3 inflammasome pathway (NLRP3, caspase-1, and IL-1β), antioxidant pathway (superoxide dismutase [SOD], glutathione peroxidase [GPx], and catalase [CAT]), inflammatory pathway (inducible nitric oxide synthase [iNOS] and tumor necrosis factor-α [TNF-α]), and the apoptosis pathway (p53, Bax, Bcl-2, and caspase-3) were determined in the intestine and liver of the rats. H&E and Masson’s trichrome (MT) staining as well as TUNEL assay were used to examine the liver and the intestine. Biochemical factors, cytotoxicity, ROS generation, and apoptosis rate were also determined in HepG2 and Caco-2 cells.
Results: TiO2 NPs in a dose-dependent manner increased cytotoxicity, oxidative stress, and apoptosis rate in Caco-2 and HepG2 cells. The administration of TiO2 NPs significantly reduced antioxidant enzyme activity and gene expressions (SOD, CAT, and GPx) as well as glutathione (GSH) levels and total antioxidant capacity (TAC) in a dose-dependent manner. TiO2 NPs also induced the apoptosis pathway and inflammatory pathway gene expressions and caspase-3 activity in the intestine and liver. TUNEL assay was in agreement with gene expressions. TiO2 NPs also led to morphological changes in the liver and intestine.
Conclusion: TiO2 NPs could have cytotoxic effects on the intestine and liver structure and function by inducing oxidative stress, inflammation, and apoptosis.
Keywords: caco-2 cells, hepg2 cells, gene expression, rat
Introduction
Nanoparticles (NPs) have special physiochemical properties due to their small size, high reactivity, and large surface area. These properties lead to their extensive use in food and non-food industries, therapeutic processes, coating materials, consumer products, and fluorescent labels.1–3
Titanium dioxide nanoparticles (TiO2 NPs) are widely used in industries such as paper and also in plastics, paints, ceramics, cosmetics, water/air purifiers, agricultural chemicals, toothpastes, and sunscreens.3,4 They are also used in the food industry as whiteners in sauces and creamers.5 Due to the high use of this compound, the question arises whether TiO2 NPs can cause harm to basic tissues such as the intestine (the site of NP absorption) and the liver (the site of NP metabolism and detoxification). To the best of the authors’ knowledge, few studies have reported the effects of TiO2 NPs on the intestine and liver as well as the mechanism of action.6–9 Previous studies have shown that TiO2 NPs induce oxidative stress and inflammation in HepG2 and Caco-2 cells.10–12 It has also been shown that TiO2 NPs increase ROS, inflammation, and apoptosis in the respiratory system.13 ROS attacks biomolecules such as DNA, proteins, lipids, and carbohydrates, which cause oxidation, methylation, deamination, apoptosis, and, consequently, lead to cell death.14 Azim et al7 showed that oral administration of TiO2 NPs (150 mg/kg/day) for 2 weeks in mice markedly elevated liver enzymes, malondialdehyde (MDA) levels, and gene expressions of NF-κB and Bax in hepatic tissue. TiO2 NPs (intraperitoneal [i.p.], 50 mg/kg for 30 days) also induced oxidative stress, DNA damage, and cell apoptosis in liver and kidney of rats.15 Furthermore, it has been reported that TiO2 NPs activate the inflammasome pathway and ultimately damage lung tissue.13 Inflammasome is a multiprotein complex that regulates inflammation by activating some cytokines such as IL-1β and IL-18.16 Activation of this pathway causes tissue damage and contributes to the development of intestinal and liver diseases.16,17 In this pathway, NLR family pyrin domain containing 3 (NLRP3) is the main component and activates caspase-1. The activation of this caspase causes pro-IL-1β to become active, which results in inflammation and tissue damage.18,19
Jia et al6 showed that i.p. administration of TiO2 NPs (50–200 mg/kg) for 2 weeks exhibited toxicity in various tissues in mice. They showed that a low dose (5 mg/kg) of TiO2 NPs did not change antioxidant enzymes and MDA levels. Another study conducted on mice showed that administration of TiO2 NPs increased NLRP3 inflammasome, IL-18 and IL-1β levels and led to airway inflammation, proposing that TiO2 NPs initiate an innate immune response in the airway.13
The intestine is an important organ of the body that interacts with the external environment and is directly related to external and toxic substances.9 In this respect, the small intestine is the major site for the absorption of metabolites and affects whole-body metabolism. The small intestine is exposed to NPs in several ways, which can probably lead to functional and structural changes.9 Engineered TiO2 NPs have become a commonly ingested material, and the influences of NPs on intestinal health function and structure are not well-recognized.4 On the other hand, the liver is a multicellular organ that plays a role in the activation and elimination of many metabolites.20 Studies have shown that the liver is the most important site for NP accumulation, which may be induced directly from intentional ingestion or indirectly through many food containers or secondary ingestion of inhaled NPs.15 Since both the intestine and the liver are directly related to harmful toxic substances, the aim of this experiment was to evaluate the effects of TiO2 NPs on oxidative stress, apoptosis, inflammation, and inflammasome pathways as well as the histopathology of these organs in rats. Furthermore, cytotoxicity, apoptosis rate, biochemical factors, and ROS production were studied in Caco-2 and HepG2 cells.
Materials and methods
Characterization of TiO2
TiO2 NP (rutile phase, 30 nm, and ≥99.9% trace metals basis) was obtained from Pishgaman Nanomaterial Company (Mashhad, Iran). The morphology and structure of TiO2 NP were measured using scanning electron microscopy (SEM, Supera 50 VP; Carl Zeiss Meditec AG, Jena, Germany), transmission electron microscopy (TEM, EM208; Philips, OR, USA), and X-ray diffraction (XRD, EQUINOX 3000; INEL, Artenay, France).
Cell culture, cytotoxicity assay, and biochemical factors
The Caco-2 and HepG2 cell lines were purchased from the National Cell Bank of Pasteur Institute (Tehran, Iran) and cultured in DMEM with 10% (v/v) FBS at 37°C with 5% CO2 using a cell incubator. Cells were grown in standard situation until 80% confluent. After that, cells were treated with particle suspensions (5–300 μg/mL) for 24 hours (in 96-well culture plates).21 The MTT method was used to determine cell viability after TiO2 NP exposure based on the previously published paper.22 Caco-2 and HepG2 cells (in six-well culture plates) were lysed in the lysis buffer in order to measure glutathione (GSH) levels, and media were used for enzyme activity (n=3).23 Cytotoxicity induced by TiO2 NPs was also measured by lactate dehydrogenase (LDH) release, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in medium using an automated analyzer (Hitachi Ltd., Tokyo, Japan).24 We determined the interference of TiO2 NP with ROS levels and LDH activity according to an earlier published paper.25 No interferences with ROS generation and LDH activity were reported at any concentration evaluated (data not shown), which show that the method was suitable for cytotoxicity measurement.
Intracellular ROS levels
ROS levels in Caco-2 and HepG2 cells were determined by 2′,7′-dichlorofluorescin diacetate (DCFH-DA) based on the method of Shen et al.26 Fluorescence intensity was measured by a spectrofluorometer (FP-6200; Jasco, Portland, OR, USA) with excitation and emission wavelengths at 485 and 530 nm, respectively (n=3).26
Cell apoptosis
The Caco-2 and HepG2 cells were treated with various concentrations of TiO2 NPs in six-well culture plates, and then single cell suspension was collected. Cells were washed in cold PBS, and the cell density was adjusted to 1×106 cells/mL in PBS. Propidium iodide (PI) stock solution was added to each cell suspension and analyzed by flow cytometry (Attune NxT, Kansas, KS, USA) according to the previously published paper.27
Animal ethics
Male Wistar rats (210 g, 8-week old) were procured from Hamadan University of Medical Sciences. They were kept in an animal house with relative humidity at 65%±5%, temperature at 22°C±2°C, and exposed to a 12-hour light/dark cycle. Standard diet and water were readily available. All experiments were approved and reviewed by the animal ethics committee of the Institutional Animal Care of Hamadan Medical University (Ethic code; IR.UMSHA.REC.1396.832, Hamadan, Iran). The experiments were performed in adherence to the guidelines for the Care and Use of Laboratory Animals of the National Institute of Health.
Animals and treatment
Animals were allowed to adapt to the animal house for 1 week prior to experiments. After that, rats were randomly divided into four groups as follows (eight rats/group): 1) control rats (treated equally with sterile saline); 2) control rats that orally received 10 mg/kg TiO2 NPs; 3) control rats that orally received 50 mg/kg TiO2 NPs; and 4) control rats that orally received 100 mg/kg TiO2 NPs, once a day for 30 consecutive days. The intragastric doses of TiO2 NPs were chosen according to the study by Wang et al28 based on the intake of dietary TiO2 particles in human. After 30 days, all rats were weighed, anesthetized by ether, and then cervical dislocation was done. The blood was collected from heart of rats and intestine and liver tissues were removed to determine biochemical factors, gene expression, and histopathological analyses.
Biochemical tests
The serum levels of ALT, AST, ALP, total bilirubin, LDH, blood urea nitrogen (BUN), and creatinine were determined using a commercially available kit by using an automated analyzer (Hitachi Ltd.).
Real-time PCR (RT-PCR)
Following NP administration, the intestine and liver were processed for gene expression analysis. Total RNA was extracted using RNXplus reagent according to the manufacturer’s instructions (CinnaGen, Tehran, Iran). RNA was reverse transcribed to cDNA using the Reverse Transcription Kit (Fermentas Life Sciences, Vilnius, Lithuania). RT-PCR was performed in a Real-Time PCR Detection System (Bio-Rad Laboratories Inc., Hercules, CA, USA). All reactions were performed in duplicate, and expressions of the various genes were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GADPH) and compared with the controls. The sequencing of primers was as follows: GAPDH (NM_017008.4) F: AAGGTCGGTGTGAACGGATTTGG, GAPDH R: TCCTGGAAGATGGTGATGGGTT; Bcl2 (NM_016993.1) F: TGTGGATGACTGAGTACCTGAACC, Bcl2 R: CAGCCAGGAGAAATCAAACAGAGG; Bax F (NM_017059.2): ACTAAAGTG CCCGAGCTGA, Bax R: ACTCCAGCCACAAAGATGGT; P53 (NM_030989.3) F: GTTCCGAGAGCTGAATGAGG, P53 R: TTTTATGGCGGGACGTAGAC; IL-1β (NM_031512.2) F: CCTTGTGCAAGTGTCTGAAG, IL-1β R: GGGCTTGGAAGCAATCCTTA; NLRP3 F (NM_001191642.1): AGAAGCTGGGGTTGGTGAATT, NLRP3 R: GTTGTCTAACTCCAGCATCTG; caspase (NM_012762.2) F: AGATGCCAACCACTGAAAGG, caspase R: GCATGATTCCCAACACAGGT; iNOS (NM_012611.3) F: AGAGACGCTTCTGAGGTTC, iNOS R: TTGATGCTTGTGACTCTTAGG; TNF-α (NM_012675.3) F: TGTTCATCCGTTCTCTACCCAC, TNF-α R: CACTACTTCAGCGTCTCGT; GPx (M_21210.1) F: GCTCACCCGCTCTTTACCTT, GPx R: AATGGACGAGAACATGCCCA; SOD (NM_017050.1) F: TCACTTCGAGCAGAAGGCAA, SOD R: CCTTTCCAGCAGCCACATTG; CAT (NM_012520.2) F: ATGGCTATGGCTCACACACC, CAT R: GAGCACGGTAGGGACAGTTC.29 The calculated formulae were as follows: ΔCt = target gene Ct – GADPH Ct, ΔΔCt = test gene ΔCt – control ΔCt, and gene expression = 2−(ΔΔCt).
Antioxidant markers
The activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) as well as GSH levels was determined in the treated animals using a commercially available kit (ZellBio, Ulm, Germany). Total oxidant status (TOS) was measured using xylenol orange, and total antioxidant capacity (TAC) was measured using the ferric ion reducing antioxidant potential (FRAP) method.22,30 The amount of MDA, a lipid peroxidation marker, was measured colorimetrically using thiobarbituric acid reactive substances (TBARS).22,30
Analysis of apoptosis rates
Apoptosis rates in the intestine and liver were determined based on caspase-3 activity and TUNEL assay. To detect the apoptosis rate, a 4 mm paraffin section was prepared, and apoptosis rates in the intestine and liver were determined using the In Situ Cell Death Detection Kit (Hoffman-La Roche Ltd., Basel, Switzerland). The fluorescence-labeled images were visualized by fluorescence microscopy, and the number of TUNEL-positive cells, indicative of DNA damage, was quantified by Image-Pro software 6.0 (Silver Spring, MD, USA). The caspase-3 activity was determined using the fluorescence assay kit according to the manufacturer’s protocols (Cayman Co., Ann Arbor, MI, USA).
IL-1β, tumor necrosis factor-α (TNF-α), and NO levels
The IL-1β and TNF-α levels in the rat intestine and liver were determined using the ELISA kit according to the manufacturer’s protocols (ZellBio, Ulm, Germany). The nitric oxide (NO) levels were determined according to the Griess reagent.31
Histopathological changes
In histopathological analysis, intestine (the first part of jejemon) and liver (same lobe) tissues were dissected from rats and fixed in 10% formalin for 48 hours and embedded in paraffin. The intestine and liver sections were stained using Masson’s trichrome (MT) and H&E. The percentage of hepatic pathological alterations was evaluated using Image-Pro Plus 6.0 (Silver Spring, MD, USA) under a light microscope (three sections/sample and six fields/slide) according to the Ishak system.20 The histopathological changes in intestine were determined according to the study by Erben et al.32
Statistical analysis
All data were expressed as mean ± SD. The statistical software SPSS (version 20.0; IBM Corporation, Armonk, NY, USA) was used to perform one-way ANOVA followed by Tukey’s post hoc test. Significant difference was accepted at P<0.05.
Results
In vitro results
Characterization of TiO2 NPs
TEM images showed that the TiO2 cluster suspension had an average diameter of 30 nm. In the characterization of NPs based on SEM and TEM images, TiO2 NPs showed a spherical shape with a size of about 30 nm. For the XRD pattern of the TiO2 NPs, strong diffraction peaks were revealed at 28°C, 36°C, and 54°C (Figure S1), which were consistent with a standard spectrum of rutile TiO2 NPs. Based on the XRD results, the proportion of the rutile phase was 99.9%.
Viability assay, biochemical factors, and ROS formation
Assessing cell viability is a vital step in toxicology that describes the cellular response to a toxin and gives evidence for survival, cell death, and metabolic activities.33 In the present experiment, cell viability was measured by evaluating the conversion of MTT to formazan. Results showed a dose-dependent reduction in cell viability for both the Caco-2 and the HepG2 cell lines after 24 hours exposure to TiO2 NPs (Figure 1A). The high concentrations of TiO2 NPs (100, 150, 200, and 300 μg/mL) also significantly increased the LDH activity in the Caco-2 and HepG2 cells after 24 hours (Figure 1B and C). TiO2 NPs caused a significant concentration-dependent increase in ALT and AST activity in HepG2 cells compared with untreated cells (Figure 1C). A significant decrease in intracellular GSH levels of Caco-2 (Figure 1B) and HepG2 cells (Figure 1C) was observed at 24 hours exposure of NPs (100, 150, 200, and 300 μg/mL).
The intracellular ROS formation was markedly elevated by TiO2 NPs in a dose-dependent manner. Caco-2 and HepG2 cells treated with TiO2 NPs (100–300 μg/mL) for 24 hours showed significant elevations in ROS levels (Figure 1B and C).
Apoptosis rates in Caco-2 and HepG2 cells
Caco-2 and HepG2 cells treated with TiO2 NPs showed apoptosis induction as proved by the appearance of a major rise in the number of cells in the sub-G1 phase compared with the control cells. However, Caco-2 (Figure 1B) and HepG2 (Figure 1C) cells treated with 150, 200, and 300 μg/mL TiO2 NPs exhibited a noteworthy increase in the apoptosis rate compared with the control cells. Apoptosis rates at the concentrations <150 mg/mL were not significant compared with control.
In vivo results
Changes in biochemical parameters
Table 1 shows the biochemical factors in the serum of all treated groups. The activity of ALT, AST, LDH, and ALP as well as the total bilirubin, BUN, and creatinine levels was significantly increased in the TiO2 NP-treated groups when compared with the control rats after 30 days.
Antioxidant gene expressions
The gene expressions of SOD, GPX, and CAT were significantly reduced in the 10, 50, and 100 mg/kg groups in a dose-dependent manner in the intestine (Figure 2A) and liver (Figure 2B).
Effect of TiO2 NPs on the antioxidant system in rat
Table 2 shows antioxidant enzyme activities, antioxidant capacity, and lipid peroxidation in rats. The MDA and TOS concentrations were markedly increased, whereas TAC levels were reduced in the rats treated with 10, 50, and 100 mg/kg TiO2 NPs compared with those in the control group. The GSH levels were reduced in the three TiO2 NP-treated groups. The activity of GPX was markedly lower in the TiO2 NPs-treated groups (10, 50, and 100 mg/kg) compared with the control rat. The activity of CAT and SOD was significantly reduced in the liver of three TiO2 NP-treated groups compared with the control group. However, the reduction of CAT and SOD in the intestine of the 10 mg/kg TiO2 NPs group was not significant compared with the control rat.
Apoptosis pathway gene expressions
The gene expressions of Bax and p53 were markedly elevated, and the Bcl-2 expression was reduced in the 10, 50, and 100 mg/kg groups (P<0.001; Figure 2A and B). However, the change in the 100 mg/kg group was more significant. The cleaved caspase-3 activity was increased in the intestine and liver of three TiO2 NP-treated groups compared with the control rats (Table 2).
Number of TUNEL-positive cells in the intestine and liver
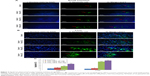
The number of TUNEL-positive cells was counted in the intestine and liver based on TUNEL and DAPI staining. In comparison with the control rat group, the mean number of TUNEL-positive cells per unit area in the intestine (Figure 3A) and the liver (Figure 3B) was significantly elevated in the TiO2 NP groups (P<0.05). Significant increase in apoptotic cells was detected at the dose of 100 mg/kg compared with the control rats. Intestine and hepatic apoptosis rates were quantified by counting the number of apoptotic cells (Figure 3C).
NLRP3 inflammasome pathway
The gene expressions of NLRP3 inflammasome, such as NLRP3, caspase-1, and IL-1β, were markedly increased in the 10, 50, and 100 mg/kg groups compared with the control rats (P<0.05, Figure 4A and B). Furthermore, IL-1β levels were markedly increased in the 10, 50, and 100 mg/kg groups in comparison with the control rat (P<0.001, Figure 4C and D).
TNF-α and iNOS levels
Expression of the TNF-α and inducible nitric oxide synthase (iNOS) genes were strongly increased in TiO2 NP-treated rats in a dose-dependent manner (P<0.001; Figure 4A and B). TiO2 NP also significantly increased TNF-α protein levels and NO levels in the intestine and liver of rats in a dose-dependent manner (P<0.001; Figure 4C and D).
Histopathological examination of intestine and liver tissues
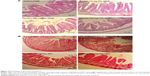
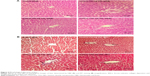
Histopathological alterations of intestine and liver tissues were examined under light microscopy with H&E and MT staining (Figures 5 and 6).
Analysis of the small intestine revealed mononuclear cell infiltration, disruption, mucosal injury, disruption of crypt structure, necrosis, submucosal edema, erosion, as well as shedding and shortening of intestinal villi along with mucosal erosion in the TiO2 NP groups, but these alterations were not present in the control group. MT staining showed intestinal fibrosis in 50 and 100 mg/kg TiO2 NP groups (Figure 5 and Table 3).
  | Table 3 Intestinal and liver histopathological changes in different treated animals |
H&E results showed liver cell degeneration, necrosis, congestion of the sinusoids, lymphocytic infiltration, and necrosis in the TiO2 NP groups in a dose-dependent manner. MT staining showed deposition of collagen in the TiO2 NP-treated groups (Figure 6; Table 3). These results indicate that following TiO2 NP administration, histopathological changes were induced in the intestine and liver after 30 days.
Discussion
It has been reported that, depending on one’s dietary habits, children receive about 1–2 mg/kg and adults receive about 0.2–0.7 mg/kg of TiO2 NPs per day.5 According to the study by Wang et al,28 oral doses in rats were chosen based on the intake of dietary TiO2 NPs in the UK, which has been expected to be about 0.1 mg/kg/day. In their study, the 100 times dose of the potential human exposure (10 mg/kg) was used as the low-dose TiO2 NPs exposure in rats.28 Meena et al15 showed that treatment of rat with TiO2 NPs at the dose of 5 mg/kg did not induce oxidative stress in liver and kidney, whereas 50 mg/kg markedly elevated ROS levels in both the organs. According to the WHO report, median lethal dose (LD50) of TiO2 for rats is >12 g/kg after oral consumption.34 In agreement with the experiments of Wang et al28 and Vasantharaja et al,35 we used TiO2 NPs at the doses of 10, 50, and 100 mg/kg.
The NP size has been defined <100 nm. Automobile and diesel exhausts are the main sources of atmospheric NPs in urban areas.13 Most NPs from vehicle exhaust are in the size range 20–60 nm for gasoline engines and 20–130 nm for diesel engines. Weir et al5 showed that chewing gums, sweets, and candies contained the highest amount of TiO2 NPs in the scale of <100 nm. NP absorption by the oral route rises with reducing particle diameter.36 Meena et al15 reported that TiO2 NPs with small distribution size (30–70 nm) can simply enter and localize inside cells. This can help in explanation of the oxidative stress observed. Furthermore, Guo et al4 reported that 30 nm TiO2 NP significantly altered nutrient transporter gene expression, ROS production, fatty acid and zinc absorption, ALP enzyme activity, and microvilli structure. In this study, TiO2 NPs showed the average diameter of 30 nm.
Based on the results from in vitro and in vivo experiments, it was found that TiO2 NP could cause oxidative stress, inflammation, apoptosis, and pathological changes in organs such as the intestine and the liver.4,38,39 In the present experiment, TiO2 NP induced toxicity, apoptosis, and increased ROS levels in HepG2 and Caco-2 cells. HepG2 cells are an appropriate in vitro model for the study of polarized human hepatocytes, as they secrete various key proteins that are vital for the study of cytotoxicity induced by NPs. Caco-2 is commonly used for drug absorption, apoptosis, and toxicity assessments.33 Hence, in this experiment, HepG2 and Caco-2 cells were selected for the assessment of target organ specificity. Hussain et al40 used the in vitro rat liver derived cell line (BRL 3A) and showed that exposure to TiO2 NPs (100–250 μg/mL) changes cellular morphology, increases LDH activity, ROS generation, and reduces GSH levels.
In this study, treatment of rats with TiO2 NP (especially at the dose of 50 and 100 mg/kg) induced oxidative damage in the intestine and liver. Changes in ROS, MDA levels (lipid peroxidation product), and antioxidant enzymes (SOD, CAT, and GPx) are sign of oxidative stress status.14,37,41 The current study showed that the antioxidant enzymes (CAT, GPx, and SOD) and non-enzyme levels (TAC and GSH) were markedly reduced and the TOS and MDA levels were increased in the intestine and liver of TiO2 NPs-treated groups. When ROS is increased, the body uses multiple antioxidant enzymes such as CAT, GPx, and SOD to clear ROS. SOD converts O2·− to H2O2 and O2, whereas GPx and CAT can eliminate H2O2 by converting it to water and O2. Therefore, CAT, GPx, and SOD keep ROS at a low concentration, effectively protecting the body from its toxic effects. However, high ROS production disrupts the balance of the oxidant/antioxidant system, causing lipid peroxidation and, consequently, liver and intestinal cell apoptosis.
The reduced CAT, GPx, and SOD activity and increased MDA and TOS concentration in the liver and the intestine indicate the oxidative stress induced by TiO2 NP. The results of Jia et al6 showed that i.p. administration of TiO2 NP to mice for 14 days led to mitochondrial damage and increased apoptotic bodies and ROS in the liver of mice. TiO2 NPs are recognized prooxidants that enter the cell by endocytosis and induce ROS generation, consequently, cause inflammation, apoptosis, and cell damage.36 On the other hand, when the mitochondria are invaded by the TiO2 NPs, the antioxidant enzymes (eg, SOD, CAT, and GPx) could be reduced.42
The current results showed that TiO2 NPs increased proapoptotic factors (BAX, caspase-3, and P53) and decreased anti-apoptotic factor (Bcl-2) in the intestine and the liver. In this experiment, the TUNEL assay also proved apoptosis in these organs. Furthermore, the activity of caspase-3 significantly increased in TiO2 NP-treated groups. The current findings are supported by other previous reports that have shown that TiO2 NPs increased liver apoptosis through the activation of apoptotic gene expression.3,39 The results of Shukla et al showed that TiO2 NPs induced oxidative DNA damage and apoptosis in HepG2 cells.3 Furthermore, Park et al showed that TiO2 NPs induced ROS production and apoptosis in cultured BEAS-2B cells.11
The caspases and Bcl-2 family of proteins (antiapoptotic and pro-apoptotic proteins) are recognized as the main regulators of the apoptotic pathway.43 Bax translocation into mitochondria could change the cytochrome C permeability, followed by stimulation of the post-mitochondrial caspase, leading to apoptotic cell death.43 Bcl-2 prevents cell apoptosis by decreasing the release of cytochrome C in order to suppress caspase-3 activation.44 Intrinsic and extrinsic pathways are two main routes of apoptosis.44 In the intrinsic pathway, various members of the Bcl-2 protein family elevate pro-apoptotic factors and mitochondrial permeability. Then, cytochrome C is released into the cytosol and that consequently leads to the activation of caspase-3.44 Previous studies have reported that NPs can induce cellular apoptosis by targeting the mitochondrial apoptotic pathway by stimulating cytochrome C release from the mitochondria, declining Bcl-2 expression, the overexpression of Bax, and by increasing caspase-3 activity.8
It has been demonstrated that caspase-3 is a major effector in the apoptosis process and that its activation marks the irreversible stage of this pathway.43 Caspase-3 is stimulated in the apoptotic cell both by intrinsic (mitochondrial) and extrinsic (death ligand) pathways.44 It has been shown that TiO2 NPs induced apoptosis in animal models by activating caspase-3.45 Shukla et al3 reported that administration of TiO2 NPs (100 mg/kg) to mice for 14 consecutive days led to pathological damage to the liver, such as increased oxidative stress, DNA damage, augmented proapoptotic factors (BAX, caspase-3 and -9, and p53), and a reduction in the anti-apoptotic factor (Bcl-2). These results indicate that TiO2 NPs can induce cell apoptosis through the caspase-dependent signaling pathway. TiO2 NPs can also lead to chromatin condensation, nuclear fragmentation, and, consequently, induce apoptosis.46 Previous studies have reported that TiO2 NPs lead to mutation in the p53 gene and other genes regulating apoptosis, thus altering their expression and, consequently, producing genotoxic effects.1 The intrinsic mitochondria apoptosis pathway has a main role in NP-induced cell death since mitochondria is one of the key target organelles for NP-induced oxidative stress. High ROS generation in the mitochondria can lead to damage to membrane phospholipids inducing mitochondrial membrane depolarization and, consequently, inducing apoptosis.47
It has been proposed that ROS contribute to inflammasome activation.17 The current data demonstrate that the administration of TiO2 NP significantly activated the NLRP3 inflammasome pathway in the intestine and liver. Inflammasomes are main contributors to inflammation, which can sense both exogenous and endogenous danger signals via intracellular nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs).19 Activation of NLRP3 inflammasome is involved in the pathogenesis of chronic inflammation-related disorders, such as chronic liver disease (CLD).19 Furthermore, another experiment reported that NLRP3 inflammasome can contribute to liver failure as well as in the development of non-alcoholic steatohepatitis (NASH) in animal models.19 It was observed that NLRP3 activation led to hepatocyte pyroptosis, severe liver inflammation, and hepatic stellate cell (HSC) activation with collagen deposition.48 Of note, the MT analysis in the current study showed that collagen deposition was significantly increased in the TiO2 NP-treated groups.
It has been documented that inflammasome is a vital regulator of gastrointestinal homeostasis and is a key factor influencing the development of intestinal inflammation through caspase-1 cleavage and the downstream production of IL-1β.16 IL-1β is a strong pro-inflammatory cytokine exerting a plethora of local and systemic effects. IL-1β stimulates the recruitment of immune cells to the inflammation site by inducing chemoattractants and adhesion molecules. IL-1β stimulates the activation and effector functions of macrophages, neutrophils, and dendritic cells.49 In the intestine, it appears that lamina propria phagocytes contain the major source of IL-1β during gastrointestinal inflammation.49 It has been documented that IL-1β signaling has a principal role in mediating intestinal inflammation.49 Abnormal activation of NLRP3 inflammasome has also been incriminated in the pathogenesis of intestinal disorders such as inflammatory bowel diseases (IBD).16 Another study showed that treatment with TiO2 NPs significantly induced the NLRP3 inflammasome pathway in lung.13 It has been reported that TiO2 NPs induce NLRP3-ASC-caspase-1 assembly and caspase-1 cleavage, leading to IL-1β release.50 TiO2 NPs can deposit inside the subcellular organelles or on the cellular surface and induce oxidative stress signaling cascades that finally lead to oxidative damage, inflammatory response, and apoptosis.47 In this study, TiO2 NPs also induced inflammatory responses in liver and intestine by increasing TNF-α and iNOS levels in a dose-dependent manner. This is in accordance with the previous experiment that showed that TiO2 NPs increased cytokine levels in rat.36 TNF-α is a potent pro-inflammatory cytokine associated with intestinal dysfunction and liver injury.51 On the other hand, iNOS in pathological situation produces high levels of NO, which is a main source of reactive nitrogen species (RNS). Peroxynitrite (ONOO−) is a main RNS, which can damage lipids, proteins, and DNA and consequently lead to liver and intestinal injury.52
We showed that exposure to TiO2 NPs led to several alterations in the cellular architecture of rat intestine and liver tissues relative to the healthy rats. The cellular degeneration induced by TiO2 NPs is a line of evidence supporting the potential of the NPs to cause biochemical changes and oxidative stress leading to cellular damage. In this experiment, high doses of TiO2 NPs markedly elevated the serum levels of ALT, AST, LDH, ALP, total bilirubin, creatinine, and BUN, which are indices for liver and kidney function. When the hepatic membrane and cells are injured or died, the amount of ALT, AST, LDH, and ALP elevates in the plasma, and this increase in amount is a sign of liver damage.35 Vasantharaja et al35 showed that administration of TiO2 NPs in rats (50 mg/kg, for 2 weeks) led to liver damage and increased hepatic marker enzymes. Previous experiments have reported the potential of NPs to sequester in tissues and thus cause cellular injury.2 Our findings indicated that administration of TiO2 NPs led to histological changes in the liver, including irregular structure of hepatocyte cells, necrosis, apoptosis features, portal inflammation, degeneration, and congestion of the sinusoids. A significant reduction in hepatocytes count per unit area was detected in TiO2 NP-treated rats. In agreement with our results, Alarifi et al39 reported that 24-hour exposure of rats to TiO2 NPs (252 mg per animal, i.p.) led to liver histopathological changes including cloudy swelling, congested dilated central veins, hydropic degeneration, fatty degeneration, and infiltration of inflammatory cells. The presence of inflammatory cells in liver tissue proposes that the TiO2 NPs can interact with enzymes and proteins in the interstitial tissue of the liver, interfering with the antioxidant defense mechanism and causing ROS production, which in turn may imitate inflammation and histopathological alterations.39 Furthermore, administration of TiO2 NPs led to histological changes in the intestine, including cell infiltration, edema, mucosal disruption, erosion, and injury, as well as shortening and shedding of intestinal villi. In agreement with the current findings, Tassinaria et al53 showed that oral administration of TiO2 NPs (2 mg/kg for 5 days) in rats can alter villous dimensions. These findings identify NLRP3 inflammasome, inflammation, oxidative stress, and apoptosis induced intestine and liver injury after exposure to TiO2 NPs.
Conclusion
The results of this study showed that the administration of TiO2 NPs in a dose-dependent manner induces hepatic and intestinal oxidative stress, inflammation, and apoptosis, which was strongly accompanied by histopathological changes in intestine and liver. Further experiments are still required to determine the mechanism of harmful effects of TiO2 NP in various cell lines and other animal species. Furthermore, comprehensive experiments are needed to determine whether low TiO2 NP doses for a long period can induce toxicity in the liver, particularly in the presence of therapeutic agents.
Acknowledgments
We thank Dr Mazaheri and Ms Bakhtiari of the Histogenotic Company for helping in carrying out TUNEL test. The authors would also like to thank Dr Amir Mirzaei form Concordia University (Montreal Canada) for his advice and support. This experiment was supported by a grant from Hamadan University of Medical Sciences (grant no. 9509025023).
Author contributions
E Abbasi-Oshaghi, F Mirzaei and M Pourjafar designed the experiment. E Abbasi-Oshaghi and F Mirzaei measured biochemical factors, gene expression and analyzed the data. M Pourjafar measured biochemical factors and analyzed the data. E Abbasi-Oshaghi and F Mirzaei participated in animal handling, and evaluated the histological changes. M Pourjafar determined cytotoxicity and in vitro tests. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Saber AT, Jacobsen NR, Mortensen A, et al. Nanotitanium dioxide toxicity in mouse lung is reduced in sanding dust from paint. Part Fibre Toxicol. 2012;9:4. | ||
Sulaiman FA, Adeyemi OS, Akanji MA, et al. Biochemical and morphological alterations caused by silver nanoparticles in Wistar rats. J Acute Med. 2015;5(4):96–102. | ||
Shukla RK, Kumar A, Vallabani NV, Pandey AK, Dhawan A. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine. 2014;9(9):1423–1434. | ||
Guo Z, Martucci NJ, Moreno-Olivas F, Tako E, Gj M, Mahler GJ. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact. 2017;5:70–82. | ||
Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46(4):2242–2250. | ||
Jia X, Wang S, Zhou L, Sun L. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res Lett. 2017;12(1):478. | ||
Azim SA, Darwish HA, Rizk MZ, Ali SA, Kadry MO. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: possible role of some antioxidants. Exp Toxicol Pathol. 2015;67(4):305–314. | ||
Duan SM, Zhang YL, Wang Y. [Effects of titanium dioxide nanoparticles and lipopolysaccharide on antioxidant function of liver tissues in mice]. Beijing Da Xue Xue Bao Yi Xue Ban. 2018;50(3):395–400. Chinese. | ||
Onishchenko GE, Erokhina MV, Abramchuk SS, et al. Effects of titanium dioxide nanoparticles on small intestinal mucosa in rats. Bull Exp Biol Med. 2012;154(2):265–270. | ||
Yang S, Xiong F, Chen K, et al. Impact of titanium dioxide and fullerenol nanoparticles on Caco-2 gut epithelial cells. J Nanosci Nanotechnol. 2018;18(4):2387–2393. | ||
Park EJ, Yi J, Chung KH, Ryu DY, Choi J, Park K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett. 2008;180:222–229. | ||
Lankoff A, Sandberg WJ, Wegierek-Ciuk A, et al. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol Lett. 2012;208(3):197–213. | ||
Kim BG, Lee PH, Lee SH, Park MK, Jang AS. Effect of TiO2 nanoparticles on inflammasome-mediated airway inflammation and responsiveness. Allergy Asthma Immunol Res. 2017;9(3):257–264. | ||
Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20(25):8082–8091. | ||
Meena R, Paulraj R. Oxidative stress mediated cytotoxicity of TiO2 nano anatase in liver and kidney of Wistar rat. Toxicol Environ Chem. 2012;94(1):146–163. | ||
Liu L, Dong Y, Ye M, et al. The pathogenic role of NLRP3 inflammasome activation in inflammatory bowel diseases of both mice and humans. J Crohns Colitis. 2017;11(6):737–750. | ||
Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12(7):387–400. | ||
Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57(3):642–654. | ||
Wu X, Dong L, Lin X, Li J. Relevance of the NLRP3 inflammasome in the pathogenesis of chronic liver disease. Front Immunol. 2017;12:1728. | ||
Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. | ||
Salla S, Sunkara R, Walker LT, Verghese M. Antioxidant and apoptotic activity of papaya peel extracts in HepG2 cells. Food Nutr Sci. 2016;07(06):485–494. | ||
Abbasi-Oshaghi E, Mirzaei F, Mirzaei A. Effects of ZnO nanoparticles on intestinal function and structure in normal/high fat diet-fed rats and Caco-2 cells. Nanomedicine. 2018;13(21):2791–2816. | ||
Kowapradit J, Opanasopit P, Ngawhirunpat T, et al. In vitro permeability enhancement in intestinal epithelial cells (Caco-2) monolayer of water soluble quaternary ammonium chitosan derivatives. AAPS PharmSciTech. 2010;11(2):497–508. | ||
Esmaeili Jahromi H, Zare Mirakabadi A, Kamalzadeh M. Evaluation of Iranian snake â Macrovipera lebetinaâ venom cytotoxicity in kidney cell line HEK-293. Asia Pac J Med Toxicol. 2016;5:49–54. | ||
Guadagnini R, Halamoda Kenzaoui B, Walker L, et al. Toxicity screenings of nanomaterials: challenges due to interference with assay processes and components of classic in vitro tests. Nanotoxicology. 2015;9(Suppl 1):13–24. | ||
Shen HM, Shi CY, Shen Y, Ong CN. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free Radic Biol Med. 1996;21(2):139–146. | ||
Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1(3):1458–1461. | ||
Wang Y, Chen Z, Ba T, et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small. 2013;9:1742–1752. | ||
Afifi M, Almaghrabi OA, Kadasa NM, Oa A, Almaghrabi OA. Ameliorative effect of zinc oxide nanoparticles on antioxidants and sperm characteristics in streptozotocin-induced diabetic rat testes. Biomed Res Int. 2015;2015:1–6. | ||
Oshaghi EA, Khodadadi I, Mirzaei F, Khazaei M, Tavilani H, Goodarzi MT. Methanolic extract of dill leaves inhibits AGEs formation and shows potential hepatoprotective effects in CCl4 induced liver toxicity in rat. J Pharm. 2017;2017(2):1–9. | ||
Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43(5):645–657. | ||
Erben U, Loddenkemper C, Doerfel K, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7(8):4557–4576. | ||
Xue Y, Zhang T, Zhang B, Gong F, Huang Y, Tang M. Cytotoxicity and apoptosis induced by silver nanoparticles in human liver HepG2 cells in different dispersion media. J Appl Toxicol. 2016;36(3):352–360. | ||
World Health Organization (WHO). FAO Nutrition Meetings Report Series No. 46A: 1969. Toxicological evaluation of some food colours, emulsifiers, stabilizers, anti-caking agents and certain other substances. WHO/FOOD ADD/70. 1970;70:36. | ||
Vasantharaja D, Ramalingam V, Aadinaath Reddy G. Oral toxic exposure of titanium dioxide nanoparticles on serum biochemical changes in adult male Wistar rats. Nanomed J. 2015;2:46–53. | ||
Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. | ||
Diaz de Barboza G, Guizzardi S, Moine L, Tolosa de Talamoni N, Stress O. Oxidative stress, antioxidants and intestinal calcium absorption. World J Gastroenterol. 2017;23(16):2841–2853. | ||
Sha B, Gao W, Wang S, Xu F, Lu T. Cytotoxicity of titanium dioxide nanoparticles differs in four liver cells from human and rat. Composites Part B: Engineering. 2011;42(8):2136–2144. | ||
Alarifi S, Ali D, Al-Doaiss AA, Ali BA, Ahmed M, Al-Khedhairy AA. Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int J Nanomedicine. 2013;8:3937–3943. | ||
Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19(7):975–983. | ||
Abbasi Oshaghi E, Goodarzi MT, Higgins V, Adeli K. Role of resveratrol in the management of insulin resistance and related conditions: mechanism of action. Crit Rev Clin Lab Sci. 2017;54(4):267–293. | ||
Gui S, Zhang Z, Zheng L, et al. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J Hazard Mater. 2011;195:365–370. | ||
Jin SJ, Yang Y, Ma L, et al. In vivo and in vitro induction of the apoptotic effects of oxysophoridine on colorectal cancer cells via the Bcl-2/Bax/caspase-3 signaling pathway. Oncol Lett. 2017;14(6):8000–8006. | ||
Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5(1):e996. | ||
Li N, Duan Y, Hong M, et al. Spleen injury and apoptotic pathway in mice caused by titanium dioxide nanoparticules. Toxicol Lett. 2010;195(2–3):161–168. | ||
Zhu Y, Eaton JW, Li C, Dioxide T. Titanium dioxide (TiO2) nanoparticles preferentially induce cell death in transformed cells in a Bak/Bax-independent fashion. PLoS One. 2012;7(11):e50607. | ||
Zhao J, Bowman L, Zhang X, et al. Titanium dioxide (TiO2) nanoparticles induce JB6 cell apoptosis through activation of the caspase-8/bid and mitochondrial pathways. J Toxicol Environ Health A. 2009;72(19):1141–1149. | ||
Wree A, Eguchi A, McGeough MD, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59(3):898–910. | ||
Lei-Leston AC, Murphy AG, Maloy KJ. Epithelial cell inflammasomes in intestinal immunity and inflammation. Front Immunol. 2017;8:1168. | ||
Ruiz PA, Morón B, Becker HM, et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome. Gut. 2017;66(7):1216–1224. | ||
Lana JP, Martins LB, Oliveira MC, et al. TNF and IL-18 cytokines may regulate liver fat storage under homeostasis conditions. Appl Physiol Nutr Metab. 2016;41(12):1295–1302. | ||
Mirzaei F, Khazaei M. Role of nitric oxide in biological systems: a systematic review. J Mazandaran Uni Med Sci. 2017;27:192–222. | ||
Tassinari R, La Rocca C, Stecca L, Tait S, De Berardis B, Ammendolia MG, editors. In vivo and in vitro toxicological effects of titanium dioxide nanoparticles on small intestine. AIP Conference Proceedings. Rome, Italy: 1667, 020016; 2015. |
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.