Back to Journals » OncoTargets and Therapy » Volume 12
NLRP3 inflammasome activation by estrogen promotes the progression of human endometrial cancer
Authors Liu SG, Wu XX, Hua T, Xin XY, Feng DL, Chi SQ, Wang XX, Wang HB
Received 4 June 2019
Accepted for publication 30 July 2019
Published 27 August 2019 Volume 2019:12 Pages 6927—6936
DOI https://doi.org/10.2147/OTT.S218240
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Shuang-Ge Liu,1,* Xiao-Xiong Wu,2,* Teng Hua,1,* Xiao-Yan Xin,1 Di-Lu Feng,1 Shu-Qi Chi,1 Xiao-Xiao Wang,1 Hong-Bo Wang1
1Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, People’s Republic of China; 2Department of Emergency, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, People’s Republic of China
Correspondence: Hong-Bo Wang
Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, No. 1277 Jiefang Avenue, Wuhan 430022, People’s Republic of China
Tel +86 1 592 742 7529
Fax +86 278 572 6362
Email [email protected]
*These authors contributed equally to this work
Background: Activation of NLPR3 inflammasome is associated with the development and progression of some types of malignant tumors, but its role in endometrial cancer is unclear. This study aimed to investigate the expression and function of NLRP3 inflammasome in endometrial cancer.
Materials and methods: The expression levels of NLRP3, its inflammasome components and estrogen receptor β in endometrial cancer and paired non-tumor tissues were detected. The effects of NLPR3 silencing or overexpression on the proliferation, migration, and invasion of Ishikawa and HEC-1A cells were determined. The impact of NLPR3 silencing on the growth of implanted tumors was determined in vivo. The effects of estrogen on NLPR3 inflammasome activation and Ishikawa cell proliferation were determined.
Results: The upregulation of NLRP3, ASC, caspase-1, and IL-1β was associated with the progression of endometrial cancer and poor survival. NLPR3 silencing inhibited the proliferation, migration, and invasion of endometrial cancer cells while NLPR3 overexpression had opposite effects. NLPR3 silencing reduced IL-1β and caspase-1 expression and the growth of implanted endometrial tumors, accompanied by decreased pro-IL-1β maturation. Estrogen enhanced NLPR3, ERβ, pro-IL-1β, IL-1β expression, and endometrial cancer cell proliferation, which were mitigated by treatment with ERβ inhibitor but not ERα inhibitor.
Conclusion: Our results suggest that estrogen acts through ERβ to enhance the activation of NLPR3 inflammasome and promote the progression of endometrial cancer. NLPR3 inflammasome may be a new therapeutic target for endometrial cancer.
Keywords: NLRP3, endometrial cancer, estrogen, inflammasome, ERβ
Introduction
Endometrial cancer is the most common gynecological malignancy in the developed countries and its incidence is increasing.1 Although the pathogenesis of endometrial cancer remains unclear, several risk factors such as late menopause onset, post-menopausal estrogen therapy, obesity, hypertension, and diabetes are associated with the development and progression of endometrial cancer. It is notable that obesity, hypertension, and diabetes are associated with chronic inflammation, which may promote the development and progression of cancer.2,3 However, how inflammation modulates the development and progression of endometrial cancer has not been clarified.
The inflammasome is a large complex in the innate immune response to pathogen-associated molecular patterns or danger-associated molecular patterns.4 The nucleotide-binding domain, leucine-rich family (NLR), pyrin-containing 3 (NLRP3) inflammasome can respond to many stimuli.5 NLPR3 interacts with the adaptor protein of apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) through the pyrin domain and recruits pro-caspase-1, which activates and cleavages pro-IL-1β and pro-IL-18 to promote their maturation.6,7 Recent studies have suggested that NLRP3 inflammasome has a different role in the development of many types of cancers, such as colorectal cancer, melanoma, breast cancer, gastric cancer, and liver cancer.8,9 While the activation of NLRP3 inflammasome can inhibit colitis-related colorectal cancer, aberrant activation of NLPR3 inflammasome can promote the development of gastric carcinoma, breast cancer, and lung metastasis.10 However, the role of NLPR3 inflammasome in human endometrial cancer remains elusive.
In this study, we examined the expression levels of NLPR3 and other inflammasome components in human-paired endometrial cancer and adjacent non-tumor tissues. Furthermore, we examined how altered NLPR3 expression affected the proliferation, invasion, migration, and tumor growth of human endometrial cancer cells. Since estrogen is a risk factor of endometrial cancer, we further investigated whether exogenous estrogen can change NLPR3 expression and NLPR3 inflammasome activation in endometrial cancer cells. Our findings indicated that NLPR3 inflammasome activation was associated with the progression of endometrial cancer, and estrogen enhanced NLPR3 inflammasome activation and the proliferation of endometrial cancer cells through ERβ.
Materials and methods
Patients
A total of 31 patients with endometrial cancer were recruited at the Department of Gynecology at the Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) from January 2014 to December 2016. Their tissue samples were obtained while they underwent surgery. Individual patients were excluded if they received any hormone therapy, radiotherapy, chemotherapy, or immunotherapy before operation, and had any endocrine disease, immunological disease, or other malignancies. Their endometrial cancer and adjacent non-tumor tissue samples were subjected to liquid nitrogen-frozen and paraffin-embedded for immunohistochemistry and qRT-PCR in the Tumor Tissue Sample Library and the Department of Pathology. All experiments were performed following Medical Ethics and Human Clinical Trial Committee of Union Hospital and all patients provided written informed consent. The demographic and clinical characteristics of patients are shown in Table 1.
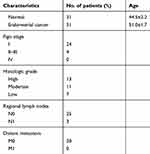 |
Table 1 Clinicopathological characteristics of 31 patients with endometrial cancer |
Immunohistochemistry
NLRP3 and ERβ expression in human tissue sections were examined by immunohistochemistry.11 Briefly, the paraffin-embedded endometrial cancer and their adjacent non-tumor tissue sections (4 μM) were deparaffinized, rehydrated, treated with 3.0% H2O2 and subjected to antigen retrieval in 10 mM sodium citrate. After blocking with 5% fetal bovine serum(FBS) in TBST for 30 mins, the sections were incubated with rabbit polyclonal anti-NLRP3 (1:300, Proteintech, USA), anti-ERβ (1:1,000, Proteintech, USA) at 4°C overnight. After being washed, the bound antibodies were detected with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG and visualized with diaminobenzidine, followed by counterstained with hematoxylin. The staining was evaluated by a semi-quantitative scoring system as described previously.12 In detail, the staining intensity was graded as: 0, no staining; 1, light yellow; 2, brown; and 3, dark brown. The proportion of cells stained was assessed as: 1, <30% staining; 2, 30–70% staining; and 3, >70% staining. The combined score (the value of staining intensity plus the proportion of stained cells) was assessed as follows: 0, negative staining (−); 1–2, weak or focal (+), 3, moderate staining (++), and ≥4 strong staining (+++). For correlation analysis, the expression of NLRP3 and ERβ was judged as negative for (−) and positive for (+), (++), and (+++), and the expression of NLRP3 and ERβ was judged as low for (+), and high for (++) and (+++).
In addition, the levels of Ki67, VEGF, NLRP3, caspase-1, and IL-1β in the xenograft tumors were determined by immunohistochemistry using antibodies for Ki67 (1:1,000, Santa Cruz, USA), VEGF (1:500, Proteintech, USA), NLRP3 (1:300, Proteintech, USA), caspase-1 (1:800, Proteintech, USA) and IL-1β (1:500, ABclonal, USA).
Cell culture
Human endometrial carcinoma Ishikawa and HEC-1A cells were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China), and cultured in RPMI-1640 and DMEM/F12 medium supplemented with 10% FBS (Gibco, Auckland, NZ) at 37ºC in a humidified atmosphere with 5% CO2, respectively. In addition, Ishikawa cells were treated in triplicate with vehicle (control) or ICI182870 (0.1–2.5 µM) or PHTPP (0.5 or 5 µM, Sigma-Aldrich, USA) for 24 h.
Cell transfection
Ishikawa and HEC-1A cells were cultured in 24-well plates overnight and transduced with control lentivirus (mock) or shNLRP3-1, shNLRP3-2, or NLRP3 lentivirus (Genechem, China) at a MOI of 20. The cells were treated with puromycin for 5 days to generate Ishikawa/mock, Ishikawa/shNLRP3, Ishikawa/NLRP3, HEC-1A/mock, HEC-1A/shNLRP3, and HEC-1A/NLRP3 cells.
Quantitative real-time PCR
Total RNA was extracted from endometrial tissue or cells by TRIzol (Invitrogen. USA) and each sample of RNA (2 µg) was reversely transcribed into cDNA using the PrimeScript RT kit (TaKaRa, China) according to the manufacturer’s instruction. qRT-PCR was performed using the TaKaRa SYBR Green PCR kit in the LightCycler CFX96 (Bio-Rad, USA). The primers were as follows: NLRP3 GCTTGCCGACGATGCCTTC and CTGTCATTGTCCTGGTGTCTTCC; ASC CGTTGAGTGGCTGCTGGATG and CAGGCTGGTGTGAAACTGAAGAG; Caspase-1 ACACCGCCCAGAGCACAAG and TTTCTTCCCACAAATGCCTTCCC; IL-1β TGGCTTATTACAGTGGCAATGAGG and AGTGGTGGTCGGAGATTCGTAG; IL-18 GAGGCGGGCAGATCACCAG and CAATCTCGGCTCACCCACAACC. The parameters were: 95°C for 5 mins and then 40 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 15 s. The data were normalized to the GAPDH and analyzed by the 2–ΔΔCt method.
Western blotting
Endometrial tissues were homogenized and cancer cells were lysed in RIPA buffer. After centrifuged, the concentrations of total proteins were determined by BCA. Individual samples (50 μg/lane) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10–12% gels and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% dry milk in TBST and incubated with rabbit polyclonal anti-NLRP3, anti-ERβ, anti-Caspase-1, anti-IL-1β, anti-IL-18 (ABclonal, USA), anti-GAPDH (Santa Cruz, USA) or rabbit monoclonal anti-ERα (Cell Signaling Technology, USA) at 4°C overnight. After being washed, the bound antibodies were detected with HRP-conjugated secondary antibodies and visualized using the enhanced chemiluminescent reagent (Bio-Rad, USA). The relative levels of target to GAPDH expression were determined by densitometric analysis using the ImageJ software.
Cell viability assay
Cell viability was analyzed using a CCK-8 kit (Google Biological, Wuhan, China), according to the manufacture’s instruction. The absorbance at 450 nm in individual wells was measured using the EL800 Universal Microplate Reader (BioTek Instruments, Winooski, VT, USA).
Transwell migration and invasion assays
Cell migration and invasion were determined by transwell assay. Briefly, cells (1×106 cells/well) were cultured in the upper chambers of 24-well transwell plates (8-μm pores) in serum-free medium. The bottom chambers were added with 20% FBS and the cells were cultured for 24 hrs. The cells at the surface of the upper chamber membrane were removed using a cotton ball. The cells on the bottom membrane of the upper chambers were fixed in 4% paraformaldehyde and stained with 0.5% crystal violet, and the migrated cells were counted in a blinded manner. For invasion assay, the upper chambers were precoated with Matrigel (BD Biosciences, San Jose, CA, USA).
Colony formation assay
The clonogenicity of cells was determined by colony formation assay.13 Briefly, individual groups of cells (500 cells/well) were cultured in triplicate in six-well plate for 10 days. The formed clones with >50 cells were stained with 0.5% crystal violet and counted under a microscope in a blinded manner.
Wound healing
Cells were cultured in six-well plates and when the cells reached at 85% of the confluency, the monolayer of cells was wounded using a sterile 100-μL pipette tip. The cells were cultured in 2% FBS medium for 24 hrs and then imaged.
Xenograft model of tumors in mice
The animal experiment protocols were approved by Animal Care and Use Committee of Union Hospital, and all animal operations were performed following NIH Guide for the Care and Use of Laboratory Animals. Female BALB/c nude mice at 4 weeks of age were purchased from Huafukang Biotechnology (Beijing, China) and housed in a specific pathogen-free facility in Experimental Animal Centre of Tongji Medical College with free access to food and water. The mice were randomized and implanted subcutaneously with Ishikawa or Ishikawa/shRNA cells (2×106/mouse, 4 mice per group). The growth of implanted tumors was monitored every 5 days using a caliper up to 40 days after inoculation. The mice were sacrificed and their tumor volumes and weights were measured. The tumor volume was determined by the formula: V (mm3)=Length (mm)×Width2 (mm2)×0.5236.
Statistical analysis
Data are present as the mean ± standard error of mean. Comparison of categorical variables was analyzed by χ2 test. Comparison of continuous variables was analyzed by two-tailed Student’s t test or one-way ANOVA. Comparison of non-normally distributed data was analyzed by nonparametric test of two independent samples. A two-tailed P-value of <0.05 was considered statistically significant. All statistical analyses were conducted using SPSS statistical software (SPSS Inc., Chicago, IL, USA), version 17.0.
Results
Increased NLRP3 expression in endometrial cancer tissues
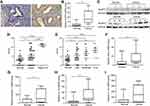
To determine the potential role of NLRP3 inflammasome in the development and progression of endometrial cancer, the expression of NLRP3 was examined by immunohistochemistry in 31 cancer and their adjacent non-tumor tissues. NLRP3 was predominantly expressed in the cytoplasm of tissue cells, and NLRP3 staining was stronger in cancer tissues than in non-tumor tissues (Figure 1A). Quantitative PCR and Western blot revealed that NLRP3 mRNA and protein levels were significantly higher in cancer tissues than in non-tumor tissues (Figure 1B and C). Statistical analysis indicated that NLRP3 expression was positively associated with cancer stage, but negatively associated with differentiation grade (Figure 1D and E). Moreover, mRNA levels of ASC, caspase-1, and IL-1β, but not IL-18 were significantly higher in cancer tissues than that in non-tumor tissues (Figure 1F–I). Taken together, these data indicate that upregulated NLRP3 inflammasome is associated with the development and progression of endometrial cancer.
Altered NLRP3 expression modulates biological behaviors of endometrial cancer cells
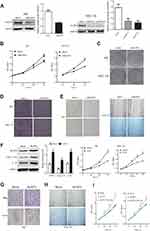
Next, Ishikawa and HEC-1A cells were transduced with lentivirus to knockdown or overexpress NLRP3. Knockdown of NLRP3 significantly reduced the proliferation, clonogenicity, invasion, and migration in both Ishikawa and HEC-1A cells (Figure 2A–E). In contrast, NLRP3 overexpression enhanced the proliferation, migration, and invasion in both Ishikawa and HEC-1A cells (Figure 2F–H). Furthermore, NLRP3 overexpression increased caspase-1 activation and the release of IL-1β in endometrial cancer cells (Figure 2F). The proliferation ability was decreased by YVAD-cmk, an inhibitor of caspase-1 (Figure 2I). Collectively, these results indicate that upregulated NLRP3 expression promotes the progression of endometrial cancer.
Knockdown of NLRP3 inhibits the growth of implanted endometrial tumors in vivo
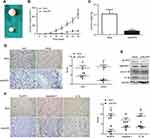
To further determine oncogenic role of NLRP3 in vivo, BALB/c nude mice were injected subcutaneously with Ishikawa/mock or Ishikawa/shNLRP3. At the end of the experiment, the tumors were dissected (Figure 3A). NLRP3 silencing significantly attenuated the growth of implanted endometrial tumors (Figure 3B). Ishikawa/shNLRP3 tumor weight decreased significantly compared to Ishikawa/mock tumor (Figure 3C). Immunohistochemistry and Western blot analysis showed that NLRP3 silencing reduced Ki67, VEGF, NLRP3, caspase-1, and IL-1β levels in tumor tissues (Figure 3D–F). These results confirm the oncogenic role of NLRP3 in endometrial tumor.
Estrogen enhances ishikawa cell proliferation by upregulating NLRP3 through ERβ
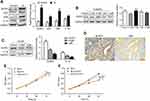
Excess estrogen stimulation is associated with the development of endometrial carcinoma, but it is unclear whether and how estrogen regulates NLRP3 mediated tumor growth. Thus, we treated Ishikawa cells with estradiol and found that estradiol not only upregulated ERα and ERβ expression, but also increased NLRP3 and IL-1β expression in Ishikawa cells (Figure 4A). The enhanced NLRP3 expression by estradiol was demolished by treatment with ERβ inhibitor PHTPP but not by ERα inhibitor ICI182870 (Figure 4B and C). Immunohistochemistry detected high levels of ERβ in human endometrial tumors (Figure 4D, Table 2), and ERβ expression was positively associated with NLRP3 expression (Table 3). In addition, estradiol promoted the proliferation of Ishikawa cells, but NLRP3 silencing dramatically reversed the effects (Figure 4E). Finally, PHTTP treatment inhibited the proliferation of Ishikawa cells, but NLRP3 overexpression dramatically reversed the effects (Figure 4F). Collectively, these results suggest that estrogen enhances the proliferation of endometrial cancer cells by upregulating NLRP3 expression in an ERβ-dependent manner.
 |
Table 2 Expression of NLRP3 and ERβ in endometrial cancer tissues |
 |
Table 3 NLRP3 expression is positively associated with ERβ expression |
Discussion
Inflammation is associated with the development and progression of endometrial cancers.14 Actually, non-steroidal anti-inflammatory drugs may inhibit the growth of endometrial cancer.15,16 In this study, we investigated the potential role of NLRP3 inflammasome activation in endometrial cancer. We found that upregulated NLPR3 inflammasome activation, such as NLRP3, ASC, caspase-1, and IL-1β, occurred in endometrial cancer tissues and associated with the progression of endometrial cancer. Our data extend previous observations that NLRP3 inflammasome activation promotes breast cancer metastasis, cervical cancer, lung cancer, and prostate cancer.17–20 However, our data are in disagreement with the report that NLPR3 inflammasome activation decreased liver cancer progression.21 Therefore, the role of NLRP3 inflammasome activation may depend on the tumor origination and stage. The promoting effect of NLPR3 inflammasome activation suggests that NLPR3 inflammasome may be a new valuable therapeutic target for the design of therapies for endometrial cancer.
It is well known that the activation of NLPR3 inflammasome can promote the maturation of IL-1β and epithelial-mesenchymal transition.22 In this study, we found that knockdown of NLPR3 expression inhibited the proliferation, migration, and invasion of endometrial cancer cells while NLPR3 overexpression had opposite effects. Furthermore, NLPR3 silencing reduced caspase-1 activation and IL-1β maturation in endometrial cancer cells. The increased levels of IL-1β may enhance IL-8, IL-6, IL-24, and TNF-α to promote inflammatory cascade and the proliferation and migration of endometrial cancer cells.23–25 Therefore, our findings may provide new insights into the pathogenesis of endometrial cancer.
Chronic estrogen stimulation is the main risk factor of endometrial cancer.26 Interestingly, while downregulated ERα expression is associated inversely with the progression of endometrial cancer, upregulated ERβ expression is detected in the low-differentiated endometrial cancer. In this study, we found that the expression of ERβ increased in endometrial cancer, and was positively correlated with the expression of NLRP3, similar to that in endometriosis.27 Our data are not consistent with previous observations that decreased levels of NLRP3 and ERβ expression were associated with the progression and metastasis of liver cancer, and that estrogen inhibited the expression of ASC, NLRP1b, and NLRP3 in the spinal cord tissues in a rat model of spinal cord injury.28 Importantly, we found that estrogen enhanced NLPR3 inflammasome activation and promoted the proliferation of endometrial cancer cells via ERβ. Therefore, the different role of estrogen/ERβ/NLPR3 inflammasome activation in different pathological conditions may be attributed to estrogen dependence and ERβ expression.
Our study has some limitations. First, we had a small sample size and short follow-up so we could not conclude the relationship between NLRP3 inflammasome activation and the survival of endometrial cancer patients. Second, the mechanistic interaction between estrogen receptor and NLRP3 should be further investigated.
To the best of our knowledge, this is the first study to demonstrate that upregulated NLPR3 inflammasome activation was associated with the progression of endometrial cancer, and NLPR3 inflammasome activation promoted malignant behaviors of endometrial cancer via ERβ. NLPR3 inflammasome may be a new therapeutic target for endometrial cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
2. Wallace AE, Gibson DA, Saunders PT, Jabbour HN. Inflammatory events in endometrial adenocarcinoma. J Endocrinol. 2010;206(2):141–157. doi:10.1677/JOE-10-0072
3. Liu M, Zhang X. An integrated analysis of mRNA-miRNA transcriptome data revealed hub regulatory networks in three genitourinary cancers. Biocell. 2017;41(1):19–26.
4. Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265(1):6–21. doi:10.1111/imr.12296
5. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi:10.1038/nri3452
6. von Moltke J, Ayres JS, Kofoed EM, Chavarria-Smith J, Vance RE. Recognition of bacteria by inflammasomes. Annu Rev Immunol. 2013;31:73–106. doi:10.1146/annurev-immunol-032712-095944
7. Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi:10.1038/nature10759
8. Mearini E, Poli G, Cochetti G, Boni A, Egidi MG, Brancorsini S. Expression of urinary miRNAs targeting NLRs inflammasomes in bladder cancer. Onco Targets Ther. 2017;10:2665–2673. doi:10.2147/OTT.S132680
9. Weichand B, Popp R, Dziumbla S, et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1ß. J Exp Med. 2017;214(9):2695–2713. doi:10.1084/jem.20160392
10. Karki R, Man SM, Kanneganti TD. Inflammasomes and cancer. Cancer Immunol Res. 2017;5(2):94–99. doi:10.1158/2326-6066.CIR-16-0269
11. Zhang J, Wang L, Qiu M, et al. The protein levels of MCM7 and p63 in evaluating lesion severity of cervical disease. Int J Gynecol Cancer. 2013;23(2):318–324. doi:10.1097/IGC.0b013e31827f6f06
12. Liu S, Hua T, Xin X, Shi R, Chi S, Wang H. Altered expression of hormone receptor, integrin ß3 and pinopode in the endometrium of luteal phase defect women. Gynecol Endocrinol. 2017;33(4):315–319. doi:10.1080/09513590.2016.1259405
13. Wei Q, Guo P, Mu K, et al. Estrogen suppresses hepatocellular carcinoma cells through ERß -mediated upregulation of the NLRP3 inflammasome. Lab Invest. 2015;95(7):804–816. doi:10.1038/labinvest.2015.63
14. Ueno T, Yabushita H, Iwasaki K, Wakatsuki A. Role of adiponectin and leptin in non-diabetic, non-obese patients with endometrial cancer. Eur J Gynaecol Oncol. 2018;39(2):199–204.
15. Takiuchi T, Blake EA, Matsuo K, Sood AK, Brasky TM. Aspirin use and endometrial cancer risk and survival. Gynecol Oncol. 2018;148(1):222–232. doi:10.1016/j.ygyno.2017.10.026
16. Verdoodt F, Friis S, Dehlendorff C, Albieri V, Kjaer SK. Non-steroidal anti-inflammatory drug use and risk of endometrial cancer: a systematic review and meta-analysis of observational studies. Gynecol Oncol. 2016;140(2):352–358. doi:10.1016/j.ygyno.2015.12.009
17. Zhang B, Zhang Y, Zhang X, Lv Y. Suspension state promotes extravasation of breast tumor cells by increasing integrin β1 expression. Biocell. 2018;42(1):17–24. doi:10.32604/biocell.2018.06115
18. He A, Shao J, Zhang Y, Lu H, Wu Z, Xu Y. CD200Fc reduces LPS-induced IL-1ß activation in human cervical cancer cells by modulating TLR4-NF-κB and NLRP3 inflammasome pathway. Oncotarget. 2017;8(20):33214–33224.
19. Wang Y, Kong H, Zeng X, et al. Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells. Oncol Rep. 2016;35(4):2053–2064. doi:10.3892/or.2016.4569
20. Dupaul-Chicoine J, Arabzadeh A, Dagenais M, et al. The Nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity. 2015;43(4):751–763. doi:10.1016/j.immuni.2015.08.013
21. Wei Q, Mu K, Li T, et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94(1):52–62. doi:10.1038/labinvest.2013.126
22. Wang H, Wang Y, Du Q, et al. Inflammasome-independent NLRP3 is required for epithelial-mesenchymal transition in colon cancer cells. Exp Cell Res. 2016;342(2):184–192. doi:10.1016/j.yexcr.2016.02.010
23. Berry KK, Varney ML, Dave BJ, Bucana CD, Fidler IJ, Singh RK. Expression of interleukin-8 in human metastatic endometrial carcinoma cells and its regulation by inflammatory cytokines. Int J Gynecol Cancer. 2001;11(1):54–60. doi:10.1046/j.1525-1438.2001.011001054.x
24. Oh K, Lee OY, Park Y, Seo MW, Lee DS. IL-1ß induces IL-6 production and increases invasiveness and estrogen-independent growth in a TG2-dependent manner in human breast cancer cells. BMC Cancer. 2016;16(1):724. doi:10.1186/s12885-016-2746-7
25. Someya A, Ikegami T, Sakamoto K, Nagaoka I, Esteban FJ. Glucosamine downregulates the IL-1ß-induced expression of proinflammatory cytokine genes in human synovial MH7A cells by O-GlcNAc modification-dependent and -independent mechanisms. PLoS One. 2016;11(10):e0165158. doi:10.1371/journal.pone.0165158
26. Haring J, Skrzypczak M, Stegerer A, et al. Estrogen receptor ß transcript variants associate with oncogene expression in endometrial cancer. Int J Mol Med. 2012;29(6):1127–1136.
27. Han SJ, Jung SY, Wu SP, et al. Estrogen receptor ß modlulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. doi:10.1016/j.cell.2015.10.034
28. Zendedel A, Mönnink F, Hassanzadeh G, et al. Estrogen attenuates local inflammasome expression and activation after spinal cord injury. Mol Neurobiol. 2018;55(2):1364–1375. doi:10.1007/s12035-017-0400-2
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.