Back to Journals » Cancer Management and Research » Volume 12
NLCIPS: Non-Small Cell Lung Cancer Immunotherapy Prognosis Score
Authors Song P , Yang D, Cui X, Wang H, Si X, Zhang X, Zhang L
Received 12 April 2020
Accepted for publication 25 June 2020
Published 17 July 2020 Volume 2020:12 Pages 5975—5985
DOI https://doi.org/10.2147/CMAR.S257967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Peng Song,1,* Dongliang Yang,1,2,* Xiaoxia Cui,1 Hanping Wang,1 Xiaoyan Si,1 Xiaotong Zhang,1 Li Zhang1
1Department of Respiratory Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, People’s Republic of China; 2Department of General Education Courses, Cangzhou Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Zhang
Department of Respiratory Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science & Peking Union Medical College., Xinkai Road, Dong Cheng, Beijing, People’s Republic of China
Tel +86 18811630866
Email [email protected]
Introduction: Currently in China, many immune checkpoint inhibitors (ICIs) have been approved for the treatment of non-small cell lung cancer (NSCLC). Some patients can not benefit from ICIs, and approximately 50% of patients have immunotherapy-related toxicity. Therefore, it is necessary to monitor carefully the selection of immunotherapy population using biomarkers to maximize the benefit of patients with NSCLC.
Methods: A prospective analysis was performed on patients with advanced NSCLC who were treated with ICIS at our hospital from March 2018 to June 2019, up to the follow-up deadline of December 31, 2019. The primary end points were overall survival (OS) and progression-free survival (PFS), and the secondary end points were objective response rate and disease control rate. A lasso regression was used for the univariate analysis, and Cox regression analysis was used for the multivariate analysis. An efficacy prediction line chart was developed.
Results: A total of 63 patients were included in the study. The median PFS was 7.0 months (95% CI, 5.0– 11.0) and did not reach the median OS. According to the lasso regression, significant univariate factors were smoking index, PD-ligand 1 expression, and neutrophil to lymphocyte ratio (NLR). According to the multivariate analysis, the Cox proportional hazards model showed that smoking index and NLR are independent predictors of PFS in immunotherapy. A model comprised of independent predictors was developed based on a multivariate logical analysis of the main cohort—non-small cell lung cancer immunotherapy prognosis score. This model is shown as a nomogram with a C-index of 0.801 (95% CI, 0.744, 0.858), which has high prediction accuracy.
Conclusion: This predictive model, including NLR and smoking index, can achieve a 1-year PFS in immunotherapy of patients. PD-1 inhibitors have been demonstrated to be effective and safe in the clinical treatment of patients with NSCLC.
Keywords: immunotherapy, prognosis score, non-small cell lung cancer, smoking index
Introduction
Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancers.1 Immunotherapy of tumors can be traced back to the application of Colli toxin in 1863, and so far has experienced active immunotherapy (ie Bacillus Calmette-Guérin (BCG)), adoptive cell transfer (ie transfer factor (TF), tumour-infiltrating lymphocytes (TIL), dendritic cell-cytokine induced killer (DC-CIK), and antigen-specific cancer vaccines (melanoma-associated antigen 3 (MAGE-A3) and L-BLP25), etc.2,3 Until recently, Immune checkpoint inhibitors (ICIs) act on cell surface checkpoint proteins to detect and destroy cancer cells through the autoimmune system, and can effectively be used to treat many types of malignant tumors. However, such treatment could lead to immune-related adverse events (irAEs). ICIs include monoclonal antibodies (mAbs) against programmed cell death receptor-1 (PD-1), PD-1 ligand (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), which have been approved for the treatment of advanced malignant tumors. PD-1 and CTLA-4 belong to the CD28 superfamily. PD-1 transmits a negative signal to T cells after binding to one of its two ligands (PD-L1 or PD-L2).4 When PD-1 binds to its ligand, it inhibits the kinase involved in T cell activation, which allows tumor cells to escape immune detection and attack. ICIs have been approved by the US Food and Drug Administration (FDA) for the treatment of advanced malignancies such as melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), urothelial carcinoma, head and neck squamous cell carcinoma, and Hodgkin’s lymphoma.5
To date, PD-L1 expression on tumor cells is considered to be the most effective biomarker predicting the efficacy of ICI in NSCLC and other malignancies.6,7 However, the sensitivity and specificity of PD-L1 expression in predicting the effect of immunotherapy are lower.8,9 Recently, a high tumor mutation burden (TMB) was found to be a positive predictive biomarker for immunotherapy response to multiple tumor types, including lung cancer. However, evaluating TMB using next-generation sequencing is expensive and requires sufficient pathological material, and advanced NSCLC is often scarce. Besides, TMB can change dynamically during disease progression and treatment.10−12 Some scholars have proposed that the inflammatory process is a possible mechanism for immunotherapy resistance in patients with cancer, promotes the growth and spread of cancer, and activates oncogenic signals.12 Therefore, many conventional blood parameters have been studied as potential inflammatory biomarkers for the efficacy of immunotherapy in patients with cancer, such as elevated circulating albumin concentrations, absolute neutrophil counts, absolute platelet counts, and lactate dehydrogenase (LDH) levels.13–15 To date, other biomarkers, such as nonsynonymous mutations and neoantigens, CD8 + T cell counts in tumor regions, defective genes for mismatch repair or high microsatellite instability, and gut microbiota have also been considered as predictable ICIs that respond well to treatment; however, there is currently no “gold standard” for predicting the efficacy of immunotherapy.
In addition to a single indicator for predicting the efficacy of immunotherapy, there are currently studies on the combination of multiple indicators to construct a prediction model. Mezquita et al16 have generated a lung immune prognosis index (LIPI) based on the data from 161 patients receiving ICIs, and they found that LIPI was associated with survival using independent ICI treatment. Dickran et al17 combined randomized trials of 3987 patients with NSCLC and reached similar conclusions. These studies are performed mainly in Western populations. Due to the differences between Eastern and Western populations, it is necessary to explore effective biomarkers for immunotherapy in Asian populations. In our study, we collected clinical and pathological data from 63 Chinese patients with NSCLC who were treated with anti-PD-1 monoclonal antibodies (including pembrolizumab, nivolumab, and sintilimab). We performed PD-L1 tests on pathological tissues to establish lung cancer immunotherapy efficacy prediction score in an accurate selection of immunotherapy benefit population.
Patients and Methods
The Ethics Committee of Peking Union Medical College Hospital approved this study, which was performed in accordance with the ethical principles of the Declaration of Helsinki. All of the patients provided their written informed consent.
Study Population, Treatment, and Response Evaluation
We prospectively analyzed the samples from 63 patients with advanced NSCLC who visited Peking Union Medical College Hospital from March 2018 to June 2019 and who were prescribed PD-1 or PD-L1 inhibitors. According to the response evaluation criteria in solid tumor 1.1 standard, the patients’ responses were divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The number of patients with an objective response rate (ORR) of CR + PR accounted for the total number of patients, and the disease control rate (DCR) indicated the number of patients with CR + PR + SD who accounted for the total number of patients. Efficacy is determined every 6 to 8 weeks after the start of the immunotherapy. In special cases, the time interval can be adjusted to suit the patients’ needs. The enrollment deadline for patients was June 30, 2019, and the follow-up deadline was December 31, 2019.
Statistical Analysis
We used a descriptive analysis to summarize the clinicopathological features. We measured continuous variables using median and quartile ranges, and we described the frequency distribution of categorical variables. We used the Kaplan–Meier method to draw survival curves and compared them using the Log rank test. A Cox regression analysis was used for the multivariate analysis. Based on the results of the multivariate analysis, a line chart was developed (using the R version 2.14.1 of the rms26 software package). The performance of the nomogram was measured by the C-index. A P<0.05 was considered to be statistically significant. All of the dynamic variables in this study (such as body mass index, the absolute value of CD4 + T cells, the absolute value of CD8 + T cells, routine blood measurements, and lactate dehydrogenase) were based on the patients’ medical records before immunotherapy.
Results
Patients’ Characteristics, Response, and Survival Outcome
Table 1 summarizes the demographic, clinical, and pathological characteristics of 63 NSCLC patients. The median age of the population was 61 years (39–81 years). There were 53 males (84.13%) and 10 females (15.87%). 58 people (92.07%) had an ECOG score ≤1. The proportion of adenocarcinoma (42.86%) is less than that of squamous cell carcinoma (57.14%). The percentage of heavy smokers (smoker index > 400) was 46.03%. Most patients have stage IV disease (84.12%) during immunotherapy. PD-L1 expression was <1% in 19 cases (35.19%), 1% −49% in 28 cases (51.85%), and ≥50% in 7 cases (12.96%). A total of 30 people had autoantibodies (either ANA antibody or anti-neutrophil antibody were positive), 12 cases were positive and 18 cases were negative. There were 29 people (46.03%) with NLR> 4, and 34 people (53.97%) with ≤ 4. There were 31 cases (49.21%) of PLR>220, and 32 cases (50.79%) of ≤220. Lactate dehydrogenase increased in 16 cases (25.4%) and normal in 47 cases (74.6%). Patients received 32 cases of first-line immunotherapy, 22 cases of second-line, and 9 cases of third-line and above. Drugs used included pembrolizumab in 42 cases, nivolumab in 4 cases, and sintilizumab in 17 cases. The interval from the diagnosis of the disease to the start of immunotherapy is 1 to 38 months, and the medication cycle is 1 to 35 cycles.
 |
Table 1 Demographic, Clinical and Pathological Characteristics of 63 Non-Small Cell Lung Cancer Patients |
The follow-up deadline was December 31, 2019. Sixty-three patients were included in the statistical analysis. The best effect was a partial response (PR) in 27 (42.8%) cases, SD in 14 (22.2%) cases, and PD in 22 (34.9%) cases; no CRs were observed. The ORR was 42.8%, and the disease control rate (DCR) was 65.1%. There were 50 (79.4%) cases of disease progression. Those who did not show disease progression (n=13, 20.6%) were regarded as censored data. Fourteen (22.2%) patients died during the study period. The median PFS was 7.0 months (95% CI, 5.0–11.0 months) (Figure 1), and the median OS was not reached.
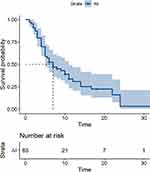 |
Figure 1 Progression-free survival curves for 63 patients. |
Non-Small Cell Lung Cancer Immunotherapy Prognosis Score
Because the study did not achieve the median OS, the prognostic factors were mainly analyzed by PFS. Because some variables have missing data values, to avoid the statistical test efficiency reduction and bias caused by missing values, we initially filled in the missing values of the original data. The multiple imputation by chained equations (MICE) package in R software is used to implement multiple interpolation. Table 2 provides a preliminary description of the data after filling.
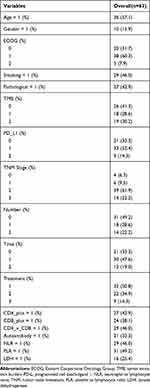 |
Table 2 Statistical Description Chart of Data After Multiple Interpolation |
Lasso Regression
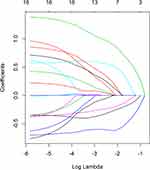
Taking PFS and disease progression as dependent variables, we used the variables in the Table 1 as independent variables to perform lasso regression, and the R software package such as glmnet. It is used to remove unimportant variables from the original data and focus on selecting the most useful predictive features. Figure 2 shows the lasso coefficient curve for 18 independent variables. The adjustment parameter (lambda) in the LASSO model is chosen to pass the 10-fold cross-validation through the minimum standard. When the penalty parameter lambda = lambda.1se = 0.3096236, the model error is relatively small. Figure 3 shows a graph of the distribution of coefficients for a log (lambda) sequence. Vertical lines were drawn at the values selected using 10-fold cross-validation. The best lambda resulted in three non-zero coefficients, namely, the smoking index, PD-L1 expression, and neutrophil to lymphocyte ratio (NLR). Blank indicates that the coefficients are compressed to 0 (Table 3).
 |
Table 3 18 Independent Variable Coefficient |
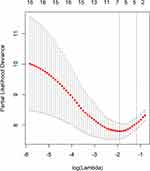 |
Figure 3 Logarithmic (lambda) sequence coefficient distribution. |
Cox Multivariate Risk Regression Analysis
Significant variables were incorporated into the Cox proportional hazards model for multivariate analysis to eliminate the effects of confounding factors on each variable. The factors included in the Cox model were three non-zero coefficients, namely, the smoking index, PD-L1 expression, and NLR. Multivariate analysis showed that smoking index (hazards ratio [HR], 0.37; 95% CI, 0.19–0.73) and NLR (HR, 3.14; 95% CI, 1.45–6.83) were independent factors affecting the prognosis of PFS in immunotherapy. Comparing heavy smokers with light smokers, it can be seen that P<0.05, risk function HR is 0.37, suggesting that the risk of disease progression of heavy smoking NSCLC immunotherapy is 0.37 times that of the light smoking group (95% CI, 0.19–0.73). Heavy smoking is a protective factor for PFS. Comparing NLR >4 group and NLR <4 group, it can be seen that P<0.05, risk function HR is 3.14, suggesting that NLR >4 group immunotherapy disease progression risk is 3.14 times that of NLR <4 group (95% CI, 1.45–6.83). NLR <4 is a protective factor for PFS. However, there was no statistical correlation between PD-L1 expression and the prognosis of immunotherapy of NSCLC (P>0.05) (Figure 4).
 |
Figure 4 Multivariate analysis results (**P <0.05). Abbreviations: PD-L1, programmed cell death-ligand 1; NLR, neutrophil to lymphocyte ratio; AIC, Akaike information criterion. |
To provide a quantitative tool to predict the likelihood of lung cancer immunotherapy progression, we developed a model that includes independent predictors (NLR and smoking index) based on multivariate logical analysis of the main cohort —NLCIPS—and displayed as a nomogram (Figure 5). The PFS prediction consistency index (C-index) is 0.801 (95% CI, 0.744–0.858), which has a high prediction accuracy.
Calculating each person’s risk score based on NLCIPS involves recording the risk scores greater than the median values as high, and the scores below the median values as low, and making Kaplan–Meier curves with different groups of high and low. The results are shown in Figure 6. Note the worse prognosis in the high-risk group, with significant statistical differences (P<0.0001).
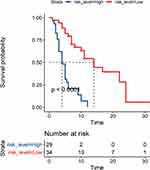 |
Figure 6 K-M method progression-free survival curve for non-small cell lung cancer patients with different risk scores. |
After drawing the nomogram, the predictive ability of the model was evaluated using the graphic calibration method. The calibration curve describes the terminology of the consistency between the model’s predicted risk and the actual risk of progress. In theory, the standard curve (black dotted line) is a straight line that passes through the origin of the coordinate axis and has a slope of 1. If the prediction calibration curve is closer to the standard curve, the better the predictive ability of the nomogram. The 1-year calibration plot (Figure 7) shows a good fit.
Discussion
Although survival benefits can be obtained with ICIs, PD-1 blockade is only effective in 20% to 30% of patients with NSCLC, with a 1-year survival rate of 42% and a decline to 18% at 3 years,18 well below the survival rate of other malignancies, such as melanoma and Hodgkin’s lymphoma. Predictive biomarkers for PD-1 treatment are urgently needed in NSCLC.19 Identifying predictive biomarkers in patients most likely to respond to immunotherapy is a key point in ongoing clinical trials. Tumor PD-L1 expression is the only biomarker approved and studied the most in patients with NSCLC, but it is limited by many biological and technical issues due to its heterogeneous and temporally and spatially varying expression in tumors.20 Recently, other potential biomarkers have also been studied, such as TMB, immune scores, differentiation cluster 8 (CD8) positive tumor-infiltrating lymphocytes, immune gene markers, and intestinal flora, but so far, no biomarker has consistently demonstrated the ability to effectively screen cancer patients.
This study summarized the clinicopathological characteristics of 63 patients with NSCLC, and selected NLR and smoking index as independent predictors of PFS. In lung cancer, systemic inflammatory status is closely related to poor prognosis.21 In advanced disease, adverse predictive reactions to NLR in patients treated with platinum-based chemotherapy and targeted therapy have been observed.22,23 However, the effect of inflammatory states on immunotherapy is unclear. Many conventional blood parameters have been studied as potential inflammatory biomarkers, such as elevated neutrophils, platelets, LDH, hypoalbuminemia, and NLR. Among them, NLR has been used as a representative indicator of systemic inflammation, and its elevation is related to the poor prognosis of various cancers, which is theoretically feasible. Lymphocytes are essential in tumor defense and are associated with a good prognosis.24 In the tumor microenvironment, neutrophils can be manipulated, including early in the differentiation process, thereby developing different phenotypes and functional polarization and inducing antitumor or tumor-promoting effects.25 In the pro-inflammatory state, it will rapidly increase the production of neutrophils and release immature or poorly differentiated neutrophils. The recruitment of these immature neutrophils into the tumor stroma can inhibit apoptosis, promote metastasis and angiogenesis, and cause tumorigenicity.26
Recently, NLR has been most extensively studied in solid tumors, and high NLR is associated with a shorter survival rate in patients with multiple tumor types.27,28 In the advanced melanoma cohort, of which 720 patients were treated with ipilimumab28 and 512 patients were treated with pembrolizumab30, the two largest retrospective analyses reported the independent prognostic value of NLR. Both studies showed that the pro-inflammatory status of ICI-treated melanoma patients is associated with poor prognosis. Jin et al29 retrospectively analyzed the data of 54 patients with NSCLC treated with anti-PD-1 antibodies (NSCLC). Of 54 patients, 18 (33.3%) had an objective response to treatment. The multivariate analysis indicated that the higher NLR after treatment was an independent prognostic factor for shorter PFS and OS. Zer et al30 found that baseline NLR ≤4 in 88 patients treated with PD-1 axis inhibitors was associated with better DCR, time to treatment, time to progression, and OS. Lower NLR and Neutrophil absolute value during treatment were associated with the response to treatment relative to the duration of treatment. Our results suggested that NLR can be used as a predictor of immunotherapy response and can help make clinical decisions in specific situations. Besides, the calculation of NLR is very simple, easy to obtain, and can be reproduced in nearly all institutions without any additional expenditure, making it very popular.
Our study found that smoking index ≥400 is a favorable prognostic factor for lung cancer immunotherapy. Garon et al31 published the results of the longest follow-up study of keynote-001, showing the long-term efficacy of immunotherapy; however, at the same time, this study demonstrated that smokers seemed to be more likely to benefit from immunotherapy among 16 patients who have lived for 5 years, 14 (87.5%) had a history of smoking or were smoking. Borghaei et al32 found that patients with a history of smoking had longer PFS. Rizvi et al33 showed that higher nonsynonymous mutations and load in tumors from smokers improve the efficacy of immunotherapy. However, Wang et al34 found that certain carcinogens in tobacco promote the immune escape of tumor cells, which can induce the increase of PD-L1 expression, and then allow tumor cells to escape T-cell surveillance. Coincidentally, the level of PD-L1 in tumor tissues determines the efficacy of immunotherapy to a certain extent. The higher the expression, the better the efficacy. Therefore, the public cannot simply think that smoking is helpful for the immunotherapy of lung cancer. Smoking is still recognized as the greatest high-risk factor for lung cancer. If you do not smoke, the incidence of lung cancer will drop significantly.
This study developed a new prognostic scoring system including NLR and smoking index—namely, NLCIPS, which can assess the 1-year PFS rate of immunotherapy patients with NSCLC. The results showed that the prognosis of NLCIPS high group and NLCIPS low group was significantly different. Evaluating different biomarkers in a single prognosis score rather than focusing on a single biomarker can better identify patients most likely to benefit from ICIs.35 Numerous studies have used clinical characteristics and blood biomarkers to study many immune-based prognostic scores, such as lung immune prognosis index (LIPI),36 advanced lung cancer inflammation index (ALI),37 and immunotherapy gender-ECOG-NLR-Delta NLR (iSEND),38 systemic inflammation index (SII),39 and biochemical-clinical index score (EPSILoN).40 Mezquita et al36 proposed the lung immune prognosis index (LIPI) as a method to identify a subgroup of patients with NSCLC and different disease control rates and survival outcomes after immunotherapy. In several populations treated with PD-1 or PD-L1 inhibitors for NSCLC, the predictive ability of LIPI was validated and a significant prognostic association with survival outcomes was observed. Shiroyama et al37 proposed the concept of ALI, and the ALI score was calculated as BMI × ALB/NLR. An ALI score of <18 was more likely to be associated with poor PFS and early progress. Park et al38 proposed a multivariate risk prediction model iSEND, which includes clinical and analytical variables (gender, ECOG performance status, NLR and NLR difference before and after treatment). ISEND was good and OS was better than the poor iSEND group. De Giorgi and others proposed the concept of SII in renal cell carcinoma immunotherapy.41 The specific calculation was platelet count × neutrophil count/lymphocyte count. Liu et al39 found that SII ≤603.5 was independently associated with longer PFS and OS in 44 patients with advanced lung cancer. These results suggested that NLCIPS may be a new predictive score for immunotherapy, and that larger prospective data are needed to confirm these outcomes. It is worth noting that this study is done in the Chinese population. Therefore, the immunotherapy prognostic score of this study does not have applicability for people outside mainland China.
However, while PD-1/PD-L1 inhibitors and CTLA-4 inhibitors bring long-term and sustained clinical benefits to patients with advanced tumors, they may also cause life-threatening systemic adverse effects (immune-related adverse effects, irAEs). Tartarone et al found in a meta-analysis involving more than 4000 cancer patients that the incidence of adverse reactions of immune checkpoint inhibitors is close to 50%.42 Sun et al found that the overall incidence of irAEs was 22% for all grades and 4% for high-grade irAEs.43 Mild irAEs (grade 1–2) are effective for steroid therapy. Patients with grade 3–4 irAEs should be hospitalized or stay in intensive care unit (ICU) to receive systemic steroid treatment; patients whose symptoms have not been relieved after 3d-5d of steroid treatment can be further treated under the guidance of a specialist. Considering the serious side effects of immune checkpoint inhibitors, the immunotherapy prognostic score is very important for selecting NSCLC patients who may have clinical benefit from ICIs treatment.
Conclusion
Smoking index and NLR are independent predictors of PFS in NSCLC immunotherapy. Non-small cell lung cancer immunotherapy prognosis scores including NLR and smoking index can predict 1-year progression-free survival of patients.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Disclosure
The authors declared that they have no conflicts of interest to this work, and do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Zhu JW, Li R, Tiselius E, et al. Immunotherapy (Excluding Checkpoint Inhibitors) for Stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev. 2017;12(12):CD011300.
3. Mohsenzadegan M, Peng RW, Roudi R. Dendritic cell/cytokine-induced killer cell-based immunotherapy in lung cancer: what we know and future landscape. J Cell Physiol. 2020;235(1):74–86. doi:10.1002/jcp.28977
4. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a Phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi:10.1016/S0140-6736(16)32517-X
5. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi:10.1126/science.aar4060
6. Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non–small-cell lung cancer: a review. JAMA Oncol. 2016;2:1217. doi:10.1001/jamaoncol.2016.0639
7. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa1200690
8. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
9. Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi:10.1158/1078-0432.CCR-13-3271
10. McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi:10.1126/science.aaf1490
11. Cyriac G, Gandhi L. Emerging biomarkers for immune checkpoint inhibition in lung cancer. Semin Cancer Biol. 2018;52:269–277. doi:10.1016/j.semcancer.2018.05.006
12. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
13. Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54(7):961–970. doi:10.3109/0284186X.2015.1043026
14. Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23(1):31–39. doi:10.1016/j.suronc.2013.12.001
15. Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204–1212. doi:10.1158/1055-9965.EPI-14-0146
16. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi:10.1126/science.aao3290
17. Kazandjian D, Gong Y, et al. Prognostic value of the lung immune prognostic index for patients treated for metastatic non–small cELL lung cancer. JAMA Oncol. 2019;5(10):1481–1485. doi:10.1001/jamaoncol.2019.1747
18. Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–2608. doi:10.1158/1535-7163.MCT-17-0386
19. Davar D, Socinski MA, Dacic S, et al. Near complete response after single dose of nivolumab in patient with advanced heavily pre-treated KRAS mutant pulmonary adenocarcinoma. Exp Hematol Oncol. 2015;4:34. doi:10.1186/s40164-015-0029-7
20. Prelaj A, Tay R, Ferrara R, et al. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer. 2019;106:144–159. doi:10.1016/j.ejca.2018.11.002
21. Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer.J. Thorac Oncol. 2015;10(2):280–285. doi:10.1097/JTO.0000000000000399
22. Han Y, Wang J, Hong L, et al. Plateletlymphocyte ratio is an independent prognostic factor in patients with ALK-positive non-small-cell lung cancer. Future Oncol. 2017;13(1):51–61. doi:10.2217/fon-2016-0317
23. Song Y-J, Wang L-X, Hong Y-Q, et al. Lymphocyte to monocyte ratio is associated with response to first-line platinum-based chemotherapy and prognosis of early-stage non-small cell lung cancer patients. Tumour Biol. 2016;37(4):5285–5293. doi:10.1007/s13277-015-4397-8
24. Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5. doi:10.1016/j.jss.2011.03.068
25. Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8(3):125–158.
26. Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol. 2004;5:182–190. doi:10.1016/S1470-2045(04)01414-7
27. Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732–738. doi:10.1093/annonc/mdw016
28. Suzuki R, Takagi T, Hikichi T, et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol Lett. 2016;11(5):3441–3445. doi:10.3892/ol.2016.4381
29. Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459–470. doi:10.1007/s00262-017-2092-x
30. Zer A, Sung MR, Walia P, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19(5):426–434. doi:10.1016/j.cllc.2018.04.008
31. Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the Phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527. doi:10.1200/JCO.19.00934
32. Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two Randomized, open-label, Phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–3933. doi:10.1200/JCO.2017.74.3062
33. Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi:10.1126/science.aaa1348
34. Wang GZ, Zhang L, Zhao XC, et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat Commun. 2019;10(1):1125. doi:10.1038/s41467-019-08887-7
35. Tay R, Prelaj A, Califano R. Immune checkpoint blockade for advanced non-small cell lung cancer: challenging clinical scenarios. J Thorac Dis. 2018;10:1494–1502. doi:10.21037/jtd.2018.01.80
36. Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi:10.1001/jamaoncol.2017.4771
37. Shiroyama T, Suzuki H, Tamiya M, et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non–small cell lung cancer. Cancer Med. 2018;7:13–20. doi:10.1002/cam4.1234
38. Park W, Mezquita L, Okabe N, et al. Association of the prognostic model iSEND with PD-1/L1 monotherapy outcome in non-small-cell lung cancer. Br J Cancer. 2020;122(3):340–347. doi:10.1038/s41416-019-0643-y
39. Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33:e22964. doi:10.1002/jcla.22964
40. Prelaj A, Ferrara R, Rebuzzi SE, et al. EPSILoN: a prognostic score for immunotherapy in advanced non-small-cell lung cancer: a validation cohort. Cancers (Basel). 2019;11(12):E1954. doi:10.3390/cancers11121954
41. De Giorgi U, Procopio G, Giannarelli D, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019;25(13):3839–3846. doi:10.1158/1078-0432.CCR-18-3661
42. Tartarone A, Roviello G, Lerose R, et al. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol. 2019;15(20):2423–2433. doi:10.2217/fon-2018-0868
43. Sun XY, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a prisma systematic review and meta-analysis. BMC Cancer. 2019;19(1):558. doi:10.1186/s12885-019-5701-6
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.