Back to Journals » OncoTargets and Therapy » Volume 13
Nivolumab in Heavily Pretreated Metastatic Gastric Cancer Patients: Real-Life Data from a Western Population
Authors Petrillo A , Tirino G, Zito Marino F, Pompella L , Sabetta R, Panarese I, Pappalardo A, Caterino M , Ventriglia A, Laterza MM, Morgillo F, Orditura M, Ciardiello F, Franco R , De Vita F
Received 4 September 2019
Accepted for publication 10 January 2020
Published 29 January 2020 Volume 2020:13 Pages 867—876
DOI https://doi.org/10.2147/OTT.S229813
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Angelica Petrillo,1 Giuseppe Tirino,1 Federica Zito Marino,2 Luca Pompella,1 Rosalaura Sabetta,2 Iacopo Panarese,2 Annalisa Pappalardo,1 Marianna Caterino,1 Anna Ventriglia,1 Maria Maddalena Laterza,1 Floriana Morgillo,1 Michele Orditura,1 Fortunato Ciardiello,1 Renato Franco,2 Ferdinando De Vita1
1Division of Medical Oncology, Department of Precision Medicine, School of Medicine, University of Study of Campania “Luigi Vanvitelli”, Naples 80131, Italy; 2Pathology Unit, University of Study of Campania “Luigi Vanvitelli”, Naples 80138, Italy
Correspondence: Angelica Petrillo Tel +39-0815666729
Email [email protected]
Ferdinando De Vita Tel +39-0815666713
Email [email protected]
Purpose: ATTRACTION-2 trial assessed the role of Nivolumab as a new standard treatment for Asian patients with pretreated metastatic gastric cancer (mGC). The aim of this analysis was to evaluate the safety and efficacy of Nivolumab in a real-life Western population, considering the lack of evidence to date.
Patients and Methods: Patients progressed after ≥ 2 chemotherapy regimens and able to receive Nivolumab (3 mg/kg q14) were eligible for the analysis.
Results: 16 patients received Nivolumab as third (81.3%) or fourth line (18.7%) from September 2017 to July 2019. The safety was in line with the literature and only one patient discontinued treatment due to persistent hematological toxicity. Overall response rate and disease control rate were 18.7% and 31.2%, respectively. Median duration of response was 5 months. With a median follow-up of 21 months, median OS was 6 months (7, 21 and 22 months in the responders) and median PFS 3 months. PD-L1 and microsatellite status were retrospectively collected in 12 patients. All the major responders were MSI, although no statistically significant difference in OS or PFS was observed according to molecular analysis.
Conclusion: Nivolumab is feasible and effective in Western patients with mGC. Further investigation is urgently needed also in non-Asians.
Keywords: immune-checkpoint inhibitors, nivolumab, immunotherapy, third line, PD-L1, MSI
Introduction
Gastric cancer (GC) represents the fifth most common tumor and the third-leading cause of cancer-related death worldwide,1 showing similar trends in Europe.2
Although GC is a potential curative disease at an early stage, in Western countries this tumor is always detected at an advanced or metastatic stage due to a lack of specific symptoms. Palliative chemotherapy is the current treatment for metastatic GC (mGC);3 however, despite the advances in the research, the prognosis remains poor with a median overall survival (OS) of 1 year and a 5-year survival rate of 5.2%.4
Recently, the Phase III trial ATTRACTION-2 showed that nivolumab – a fully humanized anti-programmed death-1 (PD-1) antibody – improves survival in Asian patients with mGC after at least two lines of chemotherapy, regardless of programmed death-1 ligand (PD-L1) expression.5 The trial showed a median OS benefit of 1.1 months for the treatment arm (5.26 months (95% confidence interval (CI): 4.60–6.37) versus 4.14 months (95% CI: 3.42–4.86) in the nivolumab and placebo group, respectively), with durable responses and good safety profile. Based on these results, Nivolumab was approved in Japan for the treatment of patients with chemotherapy-refractory gastric and gastroesophageal junction tumors regardless of PD-L1 status. However, today is known that Asian and non-Asian GC exhibit distinct tumor immunity signatures also related to T-cell functions, which may influence geographical differences in clinical outcome.6 Therefore, it is not clear if the results obtained from an entirely Asian population could be transferred to non-Asian one and still today there is a lack of evidence about the efficacy of Nivolumab in non-Asian patients.
In this context, recently the Phase I/II Checkmate-032 assessed the safety and efficacy of nivolumab as a single agent and in combination with ipilimumab in Western patients with chemotherapy-refractory esophagogastric cancers.7 The trial showed an Overall response rate (ORR) of 12%, 24% and 8% in the three study arms (nivolumab 3 mg/Kg, nivolumab 1mg/kg plus ipilimumab 3mg/kg and nivolumab 3 mg/kg plus ipilimumab 1 mg/kg, respectively), regardless of PD-L1 status. Additionally, 12-month OS rates were 39%, 35%, and 24%, respectively, showing promising efficacy and safety results in this setting.
Based on this background, the aim of our analysis was to evaluate the safety and efficacy of Nivolumab in a single institution cohort of patients from a real-life western population.
Materials and Methods
Patients
We retrospectively collected clinical data for patients treated with Nivolumab at the Division of Medical Oncology of the University of Study of Campania “Luigi Vanvitelli”. Patients aged 18 years or older with histologically confirmed adenocarcinoma of the stomach or gastroesophageal junction, able to receive Nivolumab due to the evidence of metastatic disease progressed after at least two lines of standard chemotherapy (according to the inclusion and exclusion criteria of the ATTRACTION-2 trial5) were considered eligible for our analysis. Patients previously treated with immune checkpoint inhibitors in clinical trials were excluded.
Nivolumab was required for each patient after signing of written informed consent as off-label use and approved by our institutional committee basing on the results showed in the oral presentation of ATTRACTION-2 trial at ASCO GI 20178 and ESMO congress 20179 and the following paper.5 Additionally, the institutional board of the University of Study of Campania “Luigi Vanvitelli” approved the collection of data and the study was done in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent also before data collection.
The following clinical and pathological variables were recorded for each patient: gender, age, Eastern Cooperative Oncology Group performance status (ECOG PS) before starting treatment (on a 5-point scale, with 0 indicating no symptoms and higher numbers indicating greater disability up to 5 for dead10), tumor site (proximal or distal), pathological disease’s stage at diagnosis according to tumor-nodes-metastasis (TNM) system,11 tumor grading, histological tumor type (adenocarcinoma with or without signet ring cells), Lauren’s classification (intestinal or diffuse), human epidermal growth factor receptor 2 (Her-2) expression (positive or negative), site of metastasis (with or without peritoneal involvement). Additionally, a complete blood count test was routinely obtained from each patient within 2 weeks before starting Nivolumab. Then we recorded the neutrophils/lymphocytes ratio (NLR), defined as the ratio of the absolute neutrophils and platelets count to the absolute lymphocytes count.
Treatment
Patients received Nivolumab 3 mg/Kg as intravenous infusion every 14 days according to ATTRACTION-2 trial schedule.5,8,9 A radiological assessment with total body computed tomography (CT) scan was performed every 2 months in all patients in order to evaluate the response, according to the criteria of Response Evaluation Criteria in Solid tumors (RECIST) 1.1.12 Magnetic resonance imaging, scintigraphic bone scan, brain CT scan or 18-fluorodeoxyglucose positron emission tomography were performed as needed in addition to CT evaluation in controversial cases. Patients received Nivolumab until progression of disease (PD), death for any reason, unacceptable toxicity, worsening of conditions, patient’s or physician’s decision. Treatment toxicity was evaluated according to Common Terminology Criteria for Adverse Events (CTCAE) v 5.0.13
Molecular Characteristics
Molecular analysis was performed at our institution (Pathology unit) on formalin-fixed paraffin-embedded (FFPE) tissue from archival tissue sample of primary or metastatic tumors. The analysis was not mandatory before starting treatment, because the test was not a part of our routine clinical practice at that time. Therefore, the analysis was done only in case of sample availability and the patients were not selected for biomarkers’ expression.
The following analyses were performed: immunohistochemical (IHC) evaluation of mismatch repair (MMR) proteins status and PD-L1 expression (according to tumor proportional score), in addition to Her-2 status evaluation. According to international recommendation, Her-2 status was assessed before starting first-line treatment and defined by IHC as follows: score 0/1+, negative; score 2+ equivocal (ISH assessment required); score 3+, positive.14
From January 2019, determination of Epstein-Barr early RNA in situ hybridization (EBER) for Epstein-Barr virus (EBV) was added for the new samples analyzed.
Evaluation of MMR Proteins Status and EBV in situ Hybridization
MMR proteins expression was analyzed by IHC assay, using monoclonal antibodies directed against MutL homolog 1 (MLH1: VENTANA MLH1, M1), PMS homologue 2 (PSM2: VENTANA PMS2, EPR3947), mutS homologue 2 (MSH2: VENTANA MSH2, G219-1129) and mutS homologue 6 (MSH6: VENTANA MSH6, 44). The staining was regarded positive when the tumor nuclei stained positively with the same intensity of the internal positive control including infiltrating lymphocytes, stromal cells and adjacent non-neoplastic epithelium. All cases with loss (absence) of nuclear immunostaining or reduced protein expression, when compared with internal positive control, were considered negative. An abnormal MMR expression was represented by a patchy and/or weak expression consisting of a nuclear loss associated with a gain in cytoplasmic staining or a heterogeneous expression within adjacent tumor areas.15
Microsatellite instability status (MSI) molecular test will be performed by comparison of the allelic profiles of the mononucleotide repeat markers BAT-25, BAT-26, NR-21, NR-24, and NR-27 in tumor and corresponding normal tissue. EBER using VENTANA EBER Probe was performed to assess EBV status.
Evaluation of PD-L1 Expression
PD-L1 expression was performed using VENTANA PD-L1 SP263 primary antibody. IHC PD-L1 expression was evaluated both on tumor and on immune cells. Staining for PD-L1 in FFPE tissue sections was considered positive in the cases with histological evidence of cytoplasmic and/or membranous staining (≥1%) and divided into two groups: low positivity (positivity in 1–49% of the neoplastic cells) and high positivity (more than 50% of neoplastic cells). To reduce inter-observer variation, all the cases were reviewed by two pathologists (RF and IP) and in cases of disagreement, the final interpretation was determined by consensus and using the multi-head microscope.
Statistical Analysis
SPSS software was used for statistical analysis (version 23.00; SPSS, Chicago, IL). A level of 0.05 was chosen to assess the statistical significance. Survival distribution was estimated by the Kaplan–Meier method with 95% CI. We considered OS and progression-free survival (PFS) as the time from the start of treatment with Nivolumab to the date of death from any cause and PD, respectively. The median follow-up time was calculated from the Kaplan–Meier curve with reversed censoring. Median value was used for determining the cut-off for NLR and patients were divided into two groups according to that (NLR low vs NLR high). The differences in survival were evaluated by the Log rank test and described by the Kaplan–Meier method.
Results
Patients’ Characteristics
Sixteen patients received Nivolumab as off-label treatment for mGC progressed after at least two lines of standard chemotherapy at our institution between September 2017 and July 2019. The last follow-up time was July 10th, 2019.
Patients’ characteristics are summarized in Table 1. The median age was 66.5 years old (range 36–84 years old) and the majority of patients were male (56.2%). All patients were Caucasian. They mostly presented an ECOG PS of 1 (43.8%) and had a primary tumor located in the stomach (56.2%). It is important to note that three patients (18.6%) had an ECOG PS pf 2 before starting treatment, since their clinical condition worsened during the waiting for the approval of Nivolumab as off-label process, but they were considered able to receive the treatment. The majority of patients had a metastatic disease at diagnosis (75%) and did not show peritoneal involvement during their history (56.2%). Tumors showed the following characteristics: poorly differentiated adenocarcinoma (50%) without signet ring cells (81.3%), intestinal according to Lauren’s classification (43.8%).
 |
Table 1 Patients’ Baseline Characteristics |
The median NLR value before starting Nivolumab was 3.73 (range: 1.69–11.9) and patients were divided into two groups according to that cut-off value (NLR low and high for NLR < 3.73 or ≥3.73, respectively). All patients received platinum, pyrimidine analogues and taxanes as previous chemotherapeutic agents in their history; 43.8% of patients received trastuzumab (100% of Her-2 positive tumors) and 87.5% received ramucirumab as part of first- and second-line treatments, respectively.
Treatment and Toxicities
Patients received a median of three infusions of Nivolumab (range 1–39) that represents one treatment cycle according to ATTRACTION-2 trial,5 as third (81.3%) or fourth line of treatment (18.7%) for metastatic disease.
The safety profile was in line with the literature and the most common adverse events were grade 2 arthralgia and grade 1 skin rash, each of them in one patient (6.2%). No patients had immune-related adverse events of special interest or serious adverse events. Only one patient (6.2%) showed unacceptable toxicity, requiring the discontinuation for prolonged grade 2 anemia and grade 3 thrombocytopenia after the first administration, whereas eleven patients (68.5%) discontinued Nivolumab due to PD, worsening of conditions or death. The treatment is still ongoing in 4 patients (25%) at the time of data cut-off.
Tumor’s Molecular Characteristics
Tumor’s molecular characteristics are summarized in Table 2. Her-2 status determination was performed in each patient at the time of the first diagnosis of metastatic diseases as part of our clinical practice, according to international guidelines.16 The majority of tumors showed Her-2 negative status (50%).
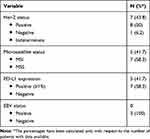 |
Table 2 Tumor’s Molecular Characteristics |
Data for PD-L1 expression and microsatellite status were obtained on archival specimens required for this analysis from 12 patients (75%), showing the following results: Microsatellite stability status (MSS)/MSI: 7/5; PD-L1 positive (≥1%)/negative: 5/7. Of note, in two patients (12.5%) there was the evidence of both MSI status and PD-L1 positive expression. EBV status was required on archival specimen only in three patients (18.7%), showing negative results in all cases.
Objective Tumor Response and Survival
The best response for each patient and the correlation with the molecular characteristics of tumors are summarized in Table 3. At the time of data cut-off, response evaluation was available for 7 patients (43.8%), due to the fact that 8 patients (50%) stopped treatment before assessing the first response and that in one patient (6.2%) the response has not been evaluated yet.
 |
Table 3 Best Response for Each Patient and Correlation with the Molecular Characteristics of Tumors |
Best overall responses were complete response (CR) in 2 patients, partial response (PR) in one patient and stable disease (SD) in 2 patients, resulting in an overall response rate (ORR) of 18.7% and a disease control rate (DCR) of 31.2%. Of note, the only patient who had a PR showed a tumor reduction more than 50%. PD was the best response in two cases (Figure 1).
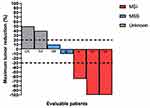 |
Figure 1 Waterfall plot of response to Nivolumab in patients with advanced gastric cancer according to MSI status. |
If we consider the response according to microsatellites status, the majority of patients with MSI showed a major radiological response (3/5 patients, ORR in MSI patients: 60%), whereas the response was not assessed (NA) in two patients due to the worsening of condition in one case and the early phase of treatment in the other one.
Globally, median duration of response was 5 months (range: 2–17 months). All patients with a major response (PR, CR) had durable responses and were still in remission. Additionally, the response was better and durable in the responders with MSI status (Figure 2) and the only two patients that received the treatment for more than 1 year are still alive and in remission.
 |
Figure 2 Swimmer plot of response to Nivolumab in patients with advanced gastric cancer. Single patient’s data are reported in each line (see Table 3 for patients ID). |
With a median follow-up of 21 months (95% CI: 7.8–34.2), 10 patients died (62.5%) and 6 (37.5%) are still alive at the time of the analysis. Median OS was 6 months (95% CI: 0.0–12.2) in the entire population and median PFS was 3 months (95% CI: 1.9–4.0). Of note, OS was 7, 21 and 22 months in patients who showed a major response. There was no statistically significant difference in median OS between patients according to microsatellites status (2 months versus not reach (NR) in MSS and MSI group, respectively; p: 0.13) (Figure 3A), PD-L1 expression (2 months in both groups, p: 0.53) (Figure 3B) or NLR (6 versus 3 months in NLR low and NLR high group, respectively; p: 0.64). Same results were reported for median PFS according to microsatellite status (2 months versus NR in MSS and MSI group, respectively; p: 0.07) (Figure 3C), PD-L1 expression (2 months in both groups, p: 0.5) (Figure 3D) and NLR (3 versus 2 months in NLR low and NLR high group, respectively; p: 0.66).
Discussion
In our retrospective analysis, we evaluated the efficacy and safety of Nivolumab in a real-life population of 16 mGC non-Asian patients progressed after at least two standard chemotherapy lines for metastatic disease. Our study showed a median OS of 6 months (95% CI: 0.0–12.2) and median PFS of 3 months (95% CI: 1.9–4.0), in line with the data showed in the nivolumab arm of the ATTRACTION-2 trial5 (median OS: 5.26 months (95% CI: 4.6–6.37); median PFS: 1.61 months (95% CI: 1.54–2.3)). In our analysis, the ORR and DCR were 18.7 and 31.2%, respectively; also in this case, these findings were quite similar to the data showed in nivolumab arm of the ATTRACTION-2 trial,5 in which ORR and DCR were 11.2 and 40.3%, respectively. Additionally, we recorded two cases of complete responses (not reported in the Asiatic trial) with durable responses after more than one year of treatment (21 and 22 months). However, even if the ATTRACTION-2 was the first randomized trial to demonstrate a clear benefit by using Nivolumab in heavily pretreated mGC patients, we should consider that the trial involved all Asian patients (from South Korea, Japan and Taiwan). Nevertheless, data from more than 1000 GC showed that Asian and non-Asian GC exhibit distinct tumor immunity signatures, also related to T-cell functions.6 Tumors from non-Asian patients, in fact, were associated with enrichment of tumor-infiltrating T cells and T-cell gene expression signatures, suggesting that non-Asian patients might have stronger immune signatures than Asian ones. Based on these assumptions, it is clear that the results obtained from an Asian population could represent only the first step for further evaluations on non-Asian patients.
The first trial that assessed the safety and efficacy of Nivolumab in Western patients was the Phase I/II Checkmate-032.7 The trial evaluated three different schedules of treatment: Nivolumab 3 mg/Kg every 2 weeks, Nivolumab 1 mg/Kg plus Ipilimumab (anti-CTLA-4 antibody) 3 mg/Kg every 3 weeks and Nivolumab 3 mg/Kg plus Ipilimumab 1 mg/Kg. The study showed an ORR of 12% (nivolumab monotherapy), 24% and 8%, respectively, with a median duration of response of 7.1 (nivolumab monotherapy), 7.9 and NR, respectively. Additionally, DCR were 32% (nivolumab single agent), 41% and 37%, respectively; median PFS was 1.4 (nivolumab single agent), 1.4 and 1.6 months and median OS were 6.2 (nivolumab single agent), 6.9 and 4.8 months, respectively, with evidence of durable responses. Of note, the Checkmate-032 trial showed one CR per investigator assessment (not confirmed at blinded central review) in the Nivolumab monotherapy arm. Therefore, taking together all these data, our results are in line also with the data shown in the Nivolumab single agent arm of the Checkmate-032 trial on a fully western population. However, the follow Phase III trial is expected in order to confirm the data of Phase I/II Checkmate-032 study.7
Unlike the ATTRACTION-25 and Checkmate-032 trials,7 our patients received a previous treatment with: taxanes in 100% (versus 86% in ATTRACTION-2 and 64% in Checkmate-032), irinotecan in 18.7% (versus 75% in ATTRACTION-2, not reported in Checkmate-032), trastuzumab in 43.7% (not reported ATTRACTION-2, 24% in Checkmate-032), and ramucirumab in 87.5% of cases (versus 11% in ATTRACTION-2, not reported in Checkmate-032). However, we did not perform any survival analysis according to previous treatment due to the small sample included in our study.
The safety profile of treatment reported in our analysis was in line with the literature. In fact, the treatment was globally well tolerated and the most common adverse events were grade 2 arthralgia and grade 1 skin rash, each of them in one patient (6.2%). Only one patient (6.2%) showed unacceptable toxicity, requiring the discontinuation for prolonged grade 2 anemia and grade 3 thrombocytopenia after the first administration. However, in that case, we were not sure that the toxicities were related to Nivolumab, because the patient received only one administration of Nivolumab after a long second-line period and the type of toxicity is not typical for immunotherapy. Finally, no patients had immune-related adverse events of special interest or serious adverse events.
To investigate the role of potential biomarkers, we evaluate the MSI status and PD-L1 expression in the archival samples from 12 patients. As already indicated, we performed the analysis only on the available samples after the beginning of treatment and the patients were not selected for these biomarkers, according to the ATTRACTION-25 and Checkmate-032 trials.5 Additionally, we assessed the EBV status only on three samples because this determination was not part of our clinical practice at the time of our analysis and we required it case by case after the publication of the evidences in literature. In fact, recently Kim et al17 showed dramatic response to pembrolizumab in patients with MSI status or EBV positivity (that are mutually exclusive) with ORR of 85.7% and 100% in MSI high and EBV positive tumor, respectively. The authors concluded that these biomarkers should be routinely tested in the everyday clinical practice in order to select the patients who may benefit from immunotherapy. However, these relevant data should be considered with caution, because the analysis was made on a very small sample (MSI: 7/61 patients (11.5%); EBV positive: 6/61 patients (9.8%)). In our study, we reported MSI status and PD-L1 positive expression each in 5 patients (31.2%) and EBV negative in all patients. There was no statistically significant difference in median OS and median PFS between patients according to microsatellites status or PD-L1 expression. However, if we consider the response according to microsatellites status, the majority of patients with MSI showed a major radiological response (3/5 patients, ORR in MSI patients: 60%), whereas the response was not assessed in two patients due to the worsening of condition in one case and the early phase of treatment in the other one. Additionally, the response was better and durable in the responders with MSI status and the only two patients that received the treatment for more than 1 year are still alive and in remission.
In this context, we analyzed also the impact of new promising biomarkers as predictor of response to immunotherapy18 such as NLR. In fact, moving from the evidences in the literature that elevated pretreatment NLR is an independent prognostic factor also in mGC,19 the role of NLR was recently investigated also in mGC patients treated with Nivolumab. Both the experiences presented as abstract at ASCO GI congress, 2019 (a subset analysis of the ATTRACTION-2 trial and a retrospective study) showed that baseline low NLR was related with better outcomes.20,21 Although in our analysis we reported a median OS of 6 versus 3 months in NLR low (<3.73) and NLR high (≥3.73) group, respectively, and a median PFS of 3 versus 2 months in NLR low and NLR high group, respectively; however, these differences are not statistically significant (p: 0.64 for OS and p: 0.66 for PFS), may be due to the small sample analyzed. Additionally, we must consider that the baseline NLR – that is the value before starting the last line of treatment – could not be representative of the immunological state of the host, because the previous chemotherapy lines could have changed NLR value through the induction of some mechanisms, such as neutropenia. Basing on these assumptions, we think that the role of NLR as prognostic and predictive factor requires further analysis in this field.
Finally, we believe that our analysis could represent an important experience in the real-life clinical practice, because the profile of patients outside of clinical trials may differ from the population randomized in the Phase III studies. In this context, to the best of our knowledge, our study is the first real-life experience in a full western population.
Nevertheless, our study had some limitations: first, it was a single institution experience with a very small sample of patients. However, it is important to underline that we decided to use Nivolumab after the presentation of the first data of the ATTRACTION-2 trial8,9 in order to offer a new therapeutic chance to the patients due to their poor outcome. Based on this fact, we required Nivolumab through a process for off-label treatment after approval by our ethical committee for every single patient, limiting the access to a larger number of patients. Additionally, due to the time required to obtain the drug, we included in our analysis also three patients with ECOG PS 2, but that were suitable to receive the treatment, as already indicated.
Another limitation of our analysis was that we did not analyze the correlation between clinic-pathological characteristics and survival in the univariate and multivariate analysis, due to the small number of patients. Additionally, we did not analyze PD-L1 according to the more recent combined positive score (CPS) required to select patients to receive pembrolizumab,17 for example, but we considered the method used in the ATTRACTION-25 and Checkmate-032 trials.7 At least, we consider the RECIST 1.1 criteria12 to assess the response, according to the landmarks trial,5,7 even if the RECIST modified for immunotherapy (iRECIST)22 should be used in case of treatment with immune checkpoint inhibitors.
Conclusions
Our results showed that Nivolumab is feasible and effective in real-life unselected western patients affected by mGC, awaiting for further Phase III trial in the Western mGC after the publication of Checkmate-032 trial’s results. Additionally, the determination of microsatellite status, PD-L1 expression and EBV status could be useful in the clinical practice to select patients that could benefit from immunotherapy. However, these biomarkers require additional validation before the routine use of them.
Acknowledgments
We thank all patients and their families for the participation in this study.
Author Contributions
Conceptualization: A.Pe, F.D.V; methodology, formal analysis, resources and data curation: A.Pe; molecular assessment: F.Z.M., I.P, R.S, R.F; investigation and interpretation of data: all authors; writing – original draft preparation, A.Pe., G.T., L.P, F.Z.M.; supervision, F.C, R.F, F.D.V. All authors contributed to revising the article, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
A.Pe: honoraria from Lilly; G.T: travel accommodation from Italfarma, Servier; M.O.: Honoraria from Italfarmaco, EISAI, epionpharma, Roche; F.C: Advisory Boards: Roche, Amgen, Merck, Pfizer, Sanofi, Bayer, Servier, BMS, Celgene, Lilly; Institutional Research Grants: Bayer, Roche, Merck, Amgen, AstraZeneca, Takeda; F.D.V: Advisory Boards: Roche, Amgen, Celgene, Lilly. The authors declare that all these conflicts of interest are not connected with the issue of this paper. The other authors have no conflicts of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a Cancer J Clin. 2018;68:394–424.
2. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi:10.1016/j.ejca.2012.12.027
3. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and metanalysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi:10.1200/JCO.2005.05.0245
4. SEER Cancer Stat Facts: Stomach Cancer. National Cancer Institute. MA, USA. Available from: http://seer.cancer.gov/statfacts/html/stomach.html.,
5. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi:10.1016/S0140-6736(17)31827-5
6. Lin SJ, Gagnon-Bartsch JA, Tan IB, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. 2015;64(11):1721–1731. doi:10.1136/gutjnl-2014-308252
7. Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836–2844. doi:10.1200/JCO.2017.76.6212
8. Kang Y-K, Satoh T, Riu MH, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): a double-blinded, randomized, phase III trial. J Clin Oncol. 2017;35(4_suppl). doi:10.1200/JCO.2017.35.4_suppl.2.
9. Boku N, Kang Y, Riu MH, et al. A Phase III study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer: updated results and subset analysis by PD-L1 expression (ATTRACTION-02). Ann Oncol. 2017;28(suppl_5):v209–v268. doi:10.1093/annonc/mdx369
10. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–655. doi:10.1097/00000421-198212000-00014
11. Sobin LH, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumors.
12. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi:10.1016/j.ejca.2008.10.026
13. Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: november 27, 2017.U.S. Department of Health and Human services. NIH. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
14. Lordick F, Al-Batran SE, Dietel M, et al. HER2 testing in gastric cancer: results of a German expert meeting. J Cancer Res Clin Oncol. 2017;143(5):835–841. doi:10.1007/s00432-017-2374-x
15. Bae YS, Kim H, Noh SH, Kim H. Usefulness of immunohistochemistry for microsatellite instability screening in gastric cancer. Gut Liver. 2015;9(5):629–635. doi:10.5009/gnl15133
16. Smyth EC, Verheij M, Allum W, Cunningham D. Cervantes A and Arnold D. ESMO clinical practice guidelines: gastric cancer. Ann Oncol. 2016;27(Suppl. 5):v38–v49. doi:10.1093/annonc/mdw350
17. Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458. doi:10.1038/s41591-018-0101-z
18. Panarese I, De Vita F, Ronchi A, et al. Predictive biomarkers along gastric cancer pathogenetic pathways. Expert Rev Anticancer Ther. 2017;17(5):417–425. doi:10.1080/14737140.2017.1301207
19. Petrillo A, Laterza MM, Tirino G, et al. Systemic-inflammation-based score can predict prognosis in metastatic gastric cancer patients before first-line chemotherapy. Future Oncol. 2018;14(24):2493–2505. doi:10.2217/fon-2018-0167
20. Ogata T, Satake H, Ogata M, et al. The combination of the changes and the value of neutrophil-to-lymphocyte ratio is useful for prediction of response for advanced gastric cancer treated with nivolumab: a multicenter retrospective study. Int J Clin. 2019;37(4_suppl):150. doi:10.1200/JCO.2019.37.4_suppl.150
21. Kim JH, Ryu MH, Park YS, et al. Predictive biomarkers for the efficacy of nivolumab as ≥ third-line therapy in patients with advanced gastric cancer (AGC): from a subset analysis of ATTRACTION-2 phase III trial. Int J Clin. 2019;37(4_suppl):152. doi:10.1200/JCO.2019.37.4_suppl.152
22. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi:10.1016/S1470-2045(17)30074-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

