Back to Journals » Journal of Inflammation Research » Volume 7
NFκB activation in cutaneous lesions of leprosy is associated with development of multibacillary infection
Authors Wambier C, Ramalho L, Frade MA, Foss N
Received 17 February 2014
Accepted for publication 30 April 2014
Published 25 August 2014 Volume 2014:7 Pages 133—138
DOI https://doi.org/10.2147/JIR.S62562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Carlos G Wambier,1 Leandra Naira Z Ramalho,2 Marco Andrey C Frade,1 Norma T Foss1
1Division of Dermatology, Department of Internal Medicine, 2Department of Pathology, Ribeirão Preto School of Medicine, University of São Paulo, Ribeirão Preto, São Paulo, Brazil
Background: Nuclear factor kappa B (NFκB) transcription factors play a central role in controlling the expression of genes involved in inflammatory reactions, proliferation, and survival of human cells. However, the in situ evaluation of NFκB activity in leprosy has not been completed previously. The aim of this study was to determine whether NFκB activity correlates with susceptibility or resistance to Mycobacterium leprae infection in biopsies from skin lesions of 38 patients with the clinical and laboratory diagnosis of leprosy.
Methods: The NFκB activation profile was evaluated in biopsies from skin lesions of 38 patients with the clinical and laboratory diagnosis of leprosy. NFκB activation was evaluated and quantified by Southwestern histochemistry, and its activation index (range, 0–4) was calculated according to the percentage of nuclear positivity by the histochemistry. Activation index >1 was considered representative of activation of NFκB.
Results: Fifteen patients (39.5%) demonstrated activated NFκB. Multibacillary leprosy was associated with activated NFκB (54.5%, P=0.028). Borderline leprosy was most strongly associated with NFκB activation (80%), with an odds ratio of 32.7 (P=0.016). These clinical forms are characterized by increased susceptibility to M. leprae and by immunological instability. Activation of NFκB was absent in the granulomas in tuberculoid leprosy, which represents an effective inflammatory reaction pattern against M. leprae.
Conclusion: These results indicate that NFκB activation could favor susceptibility and immunological instability to M. leprae infection, potentially by the stimulation of phagocytosis and the regulation of apoptotic mechanisms of infected cells, leading to the proliferation of this intracellular bacillus. Further studies are needed to evaluate if inhibition of NFκB activation in multibacillary leprosy could favor resistance and an effective granulomatous immune response.
Keywords: transcription factors, nuclear factor kappa B, immunomodulation, Mycobacterium leprae, leprosy resistance, leprosy susceptibility
Introduction
Leprosy is a chronic mycobacteriosis caused by Mycobacterium leprae that may produce a limited or disseminated infection in skin and peripheral nerves, leading to a spectrum of clinical manifestations based upon the level of cell mediated immunity (CMI) against the bacilli. At one end of the spectrum is tuberculoid leprosy (TT), characterized by restricted growth of the pathogen, and high CMI. At the opposite end of the spectrum is lepromatous leprosy (LL), characterized by widespread dissemination of bacilli, strikingly absent CMI, and a predominant humoral immune response against M. leprae. Between these two polar forms, there is the borderline group, which can be divided into three subgroups according to the CMI response: borderline tuberculoid (BT), borderline (BB), and borderline lepromatous (BL).1,2
The immunological aspects of leprosy have been extensively investigated, but the factors that facilitate or impair CMI in the polar manifestations of the disease are still not completely understood. It is known that the family of transcription factors named nuclear factor kappa B (NFκB) control the global inflammatory response by playing a central role in modulation of inflammatory reactivity. In resting cells, NFκB subunits are inactivated in the cytosol.3 Upon cytokine signaling, tumor necrosis factor alpha (TNFα), or environmental stress, NFκB activation is triggered to modulate inflammatory reactivity.4 NFκB also acts as an important survival factor for human cells, by preventing apoptosis induced by TNFα.5 This anti-apoptotic effect is mediated through the transcriptional up-regulation of NFκB-dependent anti-apoptotic proteins, such as TNFα receptor-associated factor 1 and factor 2 (TRAF-1 and TRAF-2) and the cellular inhibitor of apoptosis protein 1.5,6 Although M. leprae may induce apoptosis of Schwann cells via Toll-like receptor 2,7 the contact of viable M. leprae in Schwann cells has also been shown to cause survival of these cells, instead of apoptosis,8 which could be associated with NFκB-dependent anti-apoptotic mechanisms.
Peripheral blood mononuclear cells (PBMC) from multibacillary leprosy (MB) patients and healthy blood donors exposed to inactivated M. leprae induced nuclear translocation of NFκB (p65/p50 and p50/p50 dimers) in both groups. Such activation was considered essential to the production of TNFα by in vitro culture of PBMC from both patients and healthy controls.9 In vitro cultures of adherent PBMC from healthy donors showed lower levels of activation of NFκB (p65) when exposed to M. leprae than when exposed to bacillus Calmette-Guérin, indicating a possible NFκB signaling deficit in response to M. leprae.10
In situ studies of NFκB activation could elucidate its role in the pathophysiology of the cutaneous manifestations of leprosy. It could be hypothesized that the cutaneous manifestations at the opposite ends of the spectrum of leprosy could be related to the activity of NFκB. For example, during the development of TT lesions, NFκB signaling induced by TNFα could be one of the factors that favor the effectiveness of the inflammatory reaction and complete bacilli destruction by CMI. On the other hand, during the development of LL lesions, the anti-apoptotic activity of NFκB could allow intracellular parasitic activity and multiplication of the bacilli. The aim of this study was to determine whether NFκB activity correlates with susceptibility or resistance to M. leprae infection in biopsies from skin lesions of 38 patients with the clinical and laboratory diagnosis of leprosy.
Material and methods
This study used previously archived formalin-fixed and paraffin-embedded skin biopsy specimens, and data from the medical records of leprosy patients followed in the Leprosy Outpatient Clinic of the National Reference Centre for Sanitary Dermatology with Emphasis on Leprosy of the Hospital of Clinics at the Ribeirão Preto School of Medicine at the University of São Paulo. During routine follow-up at the outpatient clinic, all patients are classified according to the Ridley–Jopling spectrum (TT, BT, BB, BL, and LL) and by the World Health Organization (WHO) operational classification (MB, more than five skin lesions; and paucibacillary leprosy (PB), up to five skin lesions).
The ethics committee at the Hospital of Clinics, Ribeirão Preto Medical School, University of São Paulo, approved this study under consent number 2763/2011. Patients were not required to give written consent as data was analyzed anonymously; medical record information, all samples (including biopsies), and test results were previously gathered and were not obtained specifically for this study.
The patients included in the study had a clinical diagnosis of leprosy and had a skin biopsy performed at time of diagnosis, with adequate leprosy classification by Ridley–Jopling spectrum (TT, BT, BB, BL, and LL). Epidemiological and clinical data were obtained from the patients’ medical records, including classification by Ridley–Jopling criteria, WHO operational classification, comorbidities, and the use of alcohol, tobacco, and medications. The selection criteria were based on all patients whose first biopsy by the Leprosy Outpatient Clinic was performed between 2006 and 2010.
Patients were excluded from the study if medical records indicated: the use of non-steroidal anti-inflammatory drugs or corticosteroids 14 days before the date of the biopsy; leprosy reactions in the disease course; inflammatory diseases; drug eruptions in the past 3 months; higher doses than the standard multidrug therapy for treatment of leprosy or previous complete treatment; severe comorbidity or life threatening event in the past 6 months; pregnant or breast-feeding patients; and chronic disease that might interfere with inflammatory mechanisms such as diabetes mellitus, lymphedema, chronic renal disease, chronic heart failure, smoking, alcoholism, and malnutrition. A total of 24 initially selected patients were excluded from the study based on one or more of these exclusion criteria, (nine BB, six BL, five LL, two BT, and two TT). Clinical forms outside the typical leprosy spectrum such as indeterminate leprosy and pure neural leprosy were also not included in this study.
Skin biopsies were performed with a disposable skin biopsy punch (diameter 4 mm) after local anesthesia (2% lidocaine plus epinephrine tartrate 1:200,000 IU). All specimens were formalin-fixed and paraffin-embedded. Histopathological examination with standard hematoxylin-eosin staining, and the Fite–Faraco staining technique was used for the detection of M. leprae bacilli.
Biopsies underwent Southwestern histochemistry for in situ detection and distribution of the DNA-binding activity of NFκB. The non-radioactive in situ detection of activated NFκB in paraffin-embedded skin tissue preparations was performed as previously described,11 using digoxigenin labeling and detection kits (Roche Applied Science, Indianapolis, IN, USA). Synthetic sense DNAs (Imprint Genetics Corporation, Hialeah, FL, USA), which contained NFκB sequences, were used as the probe. After annealing with the complementary sequence, the DNA probe 5′-AGTTGAGGGGACTTTCCCAGGC-3′ was labeled with digoxigenin. The sections were then incubated with the labeled probes for 12 hours at 37°C. They were then incubated with an anti-digoxigenin antibody conjugated with alkaline phosphatase and detected using a nitro-blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate solution. 5′-AGTTGAGGCTCCTTTCCCAGGC-3′ (mutant form of the probe) was used as a negative control. Only cells exhibiting homogenous distinct purple nuclear staining were considered positive. The number of NFκB-positive cell nuclei was estimated in percentage in ten randomly chosen high-power fields (400×) in each sample, from which mean values were calculated. NFκB activation index (range, 0–4) was set to quantify the final percentage of positivity, ie, “0” when 0% of nuclei were positive, “1” when 1%–10% of nuclei were positive, “2” when 11%–25% of nuclei were positive, “3” when 26%–50% of nuclei were positive, and “4” when >50% of nuclei were positive, as shown in Figure 1. The criteria for activated NFκB was the presence of an activation index >1 (ie, activation indices of 2, 3, and 4). Hematoxylin-eosin staining is not performed with this technique; therefore, violet staining indicates only nuclei with activated NFκB. The pathologist who scored NFκB indexes was blinded regarding the previous classification of leprosy of the subjects.
Among the 38 selected patients, the mean age was 47.4±21.2 years, and 22 (57.9%) were male. Twenty-two patients (57.9%) were classified as MB: five had BB (13.1%), eight had BL (21.1%), and nine had LL (23.7%). Sixteen patients (42.1%) were classified as PB: seven had TT (18.4%), and nine had BT (23.7%).
Fisher’s exact test was used to evaluate the significance of the association between categorical variables. Logistic regression was used to determine the strength of association (odds ratio [OR]) between NFκB activation and the clinical classification of leprosy, adjusted for age and sex. P-values <0.05 were considered statistically significant. Statistical analysis was performed using software STATA™ 12 (StataCorp LP College Station, TX, USA).
Results
The median NFκB activation index was 1 (range, 0–4). NFκB was activated (activation index >1) in 15 skin lesions (39.5%), with an activation index of 4, 3 and 2 in one, five, and nine patients, respectively. In the remaining 23 lesions (60.5%) NFκB was not activated, with an activation index of 1 and 0 in 22 and seven patients, respectively. The presence of NFκB activation in the skin lesions varied strikingly according to the clinical form of leprosy. TT lesions had 0% with NFκB activation, BT had 33% with NFκB activation, BB had 80% with NFκB activation, BL had 50% with NFκB activation, and LL had 44% with NFκB activation.
NFκB activation indices were higher in MB patients (median =2; range, 0–4), than in PB patients (median =1; range, 0–2), with a higher frequency of activated NFκB in MB patients (54.5%) than in PB patients (18.8%; P=0.028), as shown in Table 1. All three PB patients with activated NFκB had the clinical form of BT. TT was associated with absence of activation of NFκB (P=0.019), with an activation index of 1 in six patients and 0 in one patient. BB leprosy had the highest frequency of activated NFκB (80%), with an OR of 32.7 (P=0.016; Table 2), and an activation index of 4 in one patient (the highest activation index of this study), 3 in one patient, 2 in two patients, and 1 in one patient (Table 3).
  | Table 1 Data from 38 leprosy patients evaluated for activation of NFκB |
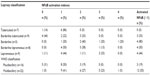  | Table 3 Distribution of NFκB activation indexes by leprosy classification and WHO classification |
Discussion
Based on the data of this study, patients with the most effective immune reactivity against M. leprae bacilli (TT) had the least activated NFκB, with 0% activated NFκB. BT had a low frequency of NFκB activation at 33.3%. These findings may depict the low inflammatory profile in the chronic and stable granulomatous reaction, which is associated with previous destruction of bacilli and efficient deprivation of mycobacterial antigens. On the other hand, the clinical forms with immunological instability had a higher frequency of activated NFκB: BB (80%), and BL (50%). It is noteworthy that within MB, the forms that usually present with upgrading or downgrading leprosy reactions (BB and BL) had more cells with NFκB activation than LL (44.4%). Likewise, within PB the form which is more unstable and prone to leprosy reactions presented with increased NFκB activation BT (33.3%), whereas TT, which is the most stable form presented with null activation (0%).
MB demonstrated a higher frequency of activated NFκB than PB (P=0.028), suggesting the possible role of bacillary antigens in modulatory processes, which may be activated through Toll-like receptors, cytokine modulation, and/or intracellular mycobacterial products. NFκB activation also regulates neural physiology by modulating synaptic plasticity and by regulating the growth of dendrites,12 suggesting that MB patients may have a higher activity of reparatory or adaptive mechanisms to the damage caused by the inflammatory response to, or intracellular activity of M. leprae bacilli.
TNFα may also trigger up-regulation of NFκB-dependent anti-apoptotic proteins.5 Activated NFκB may inhibit apoptosis pathways and stimulate cell proliferation, allowing sufficient cell survival to host parasitic activity of these slowly duplicating mycobacteria. NFκB activation and its associated transcription modulation might be one of various bacillary adaptive mechanisms to allow chronic infection, which may in turn result in less effective host immunity. For example, if the inflammatory status mediated by NFκB in MB leprosy is not efficient enough to destroy M. leprae, this could even stimulate phagocytosis and consequent intracellular infection, similar to what has been reported with Trypanosoma cruzi,13 where microorganism invasion and survival is favored by NFκB activation induced by TNFα. Apoptosis has been shown to be more frequent in PB than in MB, suggesting a possible mechanism for containing bacillary multiplication.14,15
Although age >60 years was associated with NFκB activation (66.7%, P=0.025; Table 1), it was not an independent risk factor by logistic regression analysis (OR =1.2, P=0.905; Table 2). Seventy-five percent of these older patients presented with MB leprosy, and also the majority of LL patients (55.6%) were aged >60 years.
This study reviewed patients treated at a large leprosy referral center between the years 2006–2010 and followed strict exclusion criteria, so was therefore limited by a small sample size. However, we feel that these initial findings are significant, and important in gaining a better understanding of the varied immunologic responses in leprosy patients, and we hope that this research will stimulate similar studies with a larger sample size.
Conclusion
Our study suggests that activation of NFκB is associated with increased susceptibility to M. leprae infection, as well as immunological instability. The results show increased NFκB activation in skin lesions of MB and BB patients, in contrast to scarce activation in skin lesions of PB, with no activation in TT. Future studies are needed to analyze the role of NFκB in acute leprosy inflammatory reactions, its activation after multidrug therapy, and the possibility of NFκB inhibition to favor resistance and an effective granulomatous immune response against the bacilli.
Acknowledgments
The authors thank Mark A Cappel, MD, Department of Dermatology, Mayo Clinic, Jacksonville, Florida, for his help with the English language revision. The authors are also grateful to Ms Auristella de Melo Martins for her excellent laboratory assistance. The authors also wish to thank FAEPA (Fundacao de Apoio ao Ensino, Pesquisa e Assistencia, Hospital of Clinics, Ribeirão Preto School of Medicine, University of São Paulo) for funding and support.
Disclosure
The authors report no conflicts of interest related to this work.
References
Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34(3):255–273. | |
Arnoldi J, Gerdes J, Flad HD. Immunohistologic assessment of cytokine production of infiltrating cells in various forms of leprosy. Am J Pathol. 1990;137(4):749–753. | |
Kempe S, Kestler H, Lasar A, Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005;33(16):5308–5319. | |
Denk A, Goebeler M, Schmid S, et al. Activation of NF-kappa B via the Ikappa B kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J Biol Chem. 2001;276(30):28451–28458. | |
Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281(5383):1680–1683. | |
Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10(5):927–939. | |
Oliveira RB, Ochoa MT, Sieling PA, et al. Expression of Toll-like receptor 2 on human Schwann cells: a mechanism of nerve damage in leprosy. Infect Immun. 2003;71(3):1427–1433. | |
Rambukkana A, Zanazzi G, Tapinos N, Salzer JL. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science. 2002;296(5569):927–931. | |
Hernandez Mde O, Fulco Tde O, Pinheiro RO, et al. Thalidomide modulates Mycobacterium leprae-induced NF-κB pathway and lower cytokine response. Eur J Pharmacol. 2011;670(1):272–279. | |
Sinsimer D, Fallows D, Peixoto B, Krahenbuhl J, Kaplan G, Manca C. Mycobacterium leprae actively modulates the cytokine response in naive human monocytes. Infect Immun. 2010;78(1):293–300. | |
Hernández-Presa MA, Gómez-Guerrero C, Egido J. In situ non- radioactive detection of nuclear factors in paraffin sections by Southwestern histochemistry. Kidney Int. 1999;55(1):209–214. | |
Gutierrez H, Hale VA, Dolcet X, Davies A. NF-kappaB signalling regulates the growth of neural processes in the developing PNS and CNS. Development. 2005;132(7):1713–1726. | |
Pinto AM, Sales PC, Camargos ER, Silva AM. Tumour necrosis factor (TNF)-mediated NF-κB activation facilitates cellular invasion of non-professional phagocytic epithelial cell lines by Trypanosoma cruzi. Cell Microbiol. 2011;13(10):1518–1529. | |
Walsh DS, Lane JE, Abalos RM, Myint KS. TUNEL and limited immunophenotypic analyses of apoptosis in paucibacillary and multibacillary leprosy lesions. FEMS Immunol Med Microbiol. 2004;41(3):265–269. | |
Brito de Souza VN, Nogueira ME, Belone Ade F, Soares CT. Analysis of apoptosis and Bcl-2 expression in polar forms of leprosy. FEMS Immunol Med Microbiol. 2010;60(3):270–274. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


