Back to Journals » OncoTargets and Therapy » Volume 13
Next-Generation Sequencing of Synchronous Multiple Primary Lung Cancers in a Patient with Squamous Cell Carcinoma and Small Cell Lung Cancer
Authors Wu X , Huang W , Geng T, Wei Y
Received 3 August 2020
Accepted for publication 19 October 2020
Published 12 November 2020 Volume 2020:13 Pages 11621—11626
DOI https://doi.org/10.2147/OTT.S274329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Xinggang Wu,1,2,* Wenhua Huang,3,* Tao Geng,2 Yutao Wei2,4
1Medicine Department, Shihezi University, Shihezi, Xinjiang 832000, People’s Republic of China; 2Cardiothoracic Surgery, The First Affiliated Hospital of Shihezi University School of Medicine, Shihezi, Xinjiang 832000, People’s Republic of China; 3Department of Cardiovascular Surgery, Affiliated Central People’s Hospital of Zhanjiang of Guangdong Medical University, Zhanjiang 524045, People’s Republic of China; 4Thoracic Surgery, Jining First People’s Hospital, Jining, Shandong 272000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yutao Wei
Thoracic Surgery, Jining First People’s Hospital, Cardiothoracic Surgery, The First Affiliated Hospital of Shihezi University School of Medicine, Beier Road, Shihezi 832000, People’s Republic of China
Tel +86-993-2858456
Email [email protected]
Abstract: The incidence of synchronous multiple primary malignancies is low. The presence of different lung tumor types in one patient is rare. Here, we report a rare case of synchronous lung squamous cell cancer and small cell lung cancer in a 60-year-old man. Because of the presence of two different tumor types, the proper treatment must be determined. To identify treatment targets, the genetic features of primary tumor tissues from the lungs were analyzed by next-generation sequencing (NGS). The objective was to analyze the origin and evolution of multiple primary lung cancers. NGS can find the genetic mutation sites of patients to guide treatment and promote the advancement of precision medicine. The effects of standard treatments were evaluated by response evaluation criteria in solid tumors. The results suggest that early treatment of synchronous multiple primary malignancies is a favorable outcome.
Keywords: sMPLC, NGS, chemotherapy, immunotherapy
Introduction
Multiple primary lung cancer (MPLC) refers to the simultaneous or sequential occurrence of two or more primary malignancies in the lungs of the same patient.1 According to the time of tumor discovery, MPLCs can be divided into < 6 months of simultaneous MPLC (sMPLC) and≥ 6 months of metachronous MPLC (mMPLC). sMPLC is a rare entity, with an incidence rate that ranges from 1% to 7%,2 and the majority are of the same histologic type. The coexistence of small and non-small cell carcinoma has been reported in a small fraction of cases.3–9 Here, We report a rare case of synchronous MPLC of different histological types [squamous cell carcinoma (SCC) and small-cell lung carcinoma (SCLC)].
Case Report
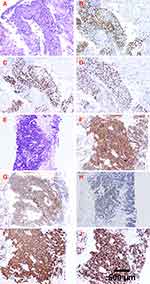
A 60-year-old man was admitted to the First Affiliated Hospital of Shihezi University School of Medicine on 25 March 2020 with a 2-month history of cough, sputum, and wheezing. He was a road worker with a 30-year history of smoking 40 cigarettes/day; he quit smoking 9 years ago. Laboratory examination revealed elevated tumor markers, including CEA (15.94 ng/mL), CA15-3 (27.37 U/mL), CA19-9 (31.19 U/mL), CA72-4 (17.07 U/mL), and cytokeratin 19 fragment (35.15 ng/mL). Autoimmune markers, including anti-SM antibodies, anti-nucleosome antibodies (AnuA), and anti-histone antibodies (AHA), were positive. Chest computed tomography (CT) revealed two solid lesions located at the lower lobes of the right lung (48 × 36 mm) and left lung (78 × 45 mm) (Figure 1), suggesting the possibility of a malignant tumor. Magnetic resonance imaging (MRI) examination of the head indicated possible brain involvement. Tumor stage was determined stage IV (T4N3M1b). The patient reported no family history of lung cancer. To determine the histological type, needle biopsies of both lesions were performed under CT guidance. Pathology examination of needle biopsy specimens and immunohistochemistry results showed that the two lesions were histologically different. The left lower lobe was SCC and was positive for P40, P63, Ki-67 (80%), and negative for CD56, Syn, cgA, TTF-1. The right lung lower lobe tumor was diagnosed as SCLC; tumor cells were positive for CD56, Syn, cgA, TTF-1, and Ki-67 (90%) and negative for P40 and P63 (Figure 2). Immunohistochemistry suggested that the tumor origins differed between the two sites, and the molecular profiles were heterogeneous. The final clinical diagnosis of this patient was sMPLC.
MRI and CT detected a head mass diagnosed as a metastatic lesion and lymph node metastasis, which were contraindications for surgery. Because this patient presented with two different tumor types in the lungs, determining a treatment strategy was challenging. SCC and SCLC have huge differences in biological behavior, treatment, and prognosis. To identify treatment targets, we used NGS technology to analyze the genetic features of the primary tumor tissues from the lungs. During the waiting period, the patient received systemic chemotherapy. According to the National Comprehensive Cancer Network guidelines for small cell lung cancer, the patient received systemic chemotherapy with carboplatin and etoposide because SCLC is more malignant. After 40 days, the results of NGS were as follows (Table 1): somatic mutations: 19 (Tables 2 and 3); germline mutations: 0; tumor cell microsatellite instability detection: microsatellite stability; tumor mutation load: high tumor mutational burden (TMB-H). The NGS results suggested that the patient should receive immunotherapy with PD-1/PD-L1 inhibitors.
 |
Table 2 Point Mutations, Small Fragments of Indel Detection Results Indel Detection Results |
 |
Table 3 Copy Coefficient Mutation Detection Results |
Follow-Up
The response of the patient to chemotherapy was evaluated in accordance with the response evaluation criteria in solid tumors. Chest CT images obtained after one cycle of chemotherapy, showed a dramatic shrinkage of the right lung lower lobe tumor from 48 × 36 mm to 30 × 15 mm. The left lung lower lobe tumor also showed from 78 × 45 mm to 29.1 × 18.7 mm (Figure 3). Full-body examination and CT scan results showed no changes in the volume of the lesions in the brain. The patient refused immunotherapy and continued with the previous chemotherapy regimen. The patient remains under follow-up.
Discussion
In this report, we describe a rare case of synchronous MPLC with two histologically distinct pulmonary tumors, namely, SCC and SCLC in the same patient. Martini and Melamed first proposed the diagnostic criteria for MPLC.1 However, determining whether the additional lesion represents a second primary lung cancer or an additional tumor nodule that corresponds to the dominant cancer is difficult.10 If the histological examination shows differences between two tumors, determining whether these pulmonary cancer foci are separate primary tumors is relatively easy.11 Multiple pulmonary resections are indicated for patients with synchronous or metachronous lung cancer with multiple pulmonary sites of involvement.12,13 Chen et al Indicated that sublobar resection was acceptable for patients with MPLC detected at an early stage, because it has a similar prognosis than standard resection and better pulmonary function preservation.14 However, the presence of distant metastasis is a contraindication, for surgery, and chemotherapy. Is used to slow the progression of the disease. Small and non-small cell lung cancer are very different biological entities, and the treatment options differ significantly between the two tumor types. The treatment of synchronous multiple primary small cell and non-small cell lung cancers in patients with distant metastasis remains challenging. Comprehensive genetic profiling is needed to provide complete molecular information, which can be achieved using a clinical NGS test targeting 1021 cancer-relevant genes. The tumor mutational burden (TMB) has emerged as a new biomarker for predicting the response to programmed cell death ligand-1 (PD-L1) treatment.15–17 The TMB results of NGS in the present patient were TMB-H (Table 1), which suggests that PD-1/PD-L1 inhibitors can be used for immunotherapy. In addition, the presence of ERCC3, RAD54L, and TP53 mutations (Table 2) indicated that the patient would benefit from PD-1/PD-L1 inhibitor therapy. Therefore, immunotherapy with PD-1/PD-L1 inhibitors was recommended in the present patient. However, after consultation with the patient and his family, this treatment plan was not adopted, chemotherapy was adopted, and good results were achieved. The results of NGS showed the presence of other mutations (Table 2), which could provide new directions for future research. Although determining an effective treatment strategy is one of the many challenges associated with sMPLC, the data generated by NGS can provide information on treatment targets, thereby advancing the era of precision medicine and improving the prognosis of patients. The objective of this case report was to analyze the origin and evolution of MPLC. The results suggest that NGS is important for identifying therapeutic targets, as well as for the discovery of new potential driver genes.
Ethics
This patient provided written informed consent for the publication of the case details and images. And ethical approval for this study was obtained from the meeting of ethics committee of The First Affiliated Hospital of Shihezi University School of Medicine.
Acknowledgments
This research was supported by the Natural Science Foundation of China (grant number 81460059), the doctor/scientific Grant of Xinjiang Production and Construction Crops (grant number 2014BB019), the Regional Innovation Guidance Program of Corps (2017BA043), and the Achievement Transformation and Technology Promotion Project at Shihezi University (CGZH201703). Xinggang Wu and Wenhua Huang are co-first authors for this study.
Disclosure
Dr Wenhua Huang is now affiliated with Department of Thoracic and Cardiovascular Surgery, Ganzhou Municipal Hospital, Ganzhou, Jiangxi 341000, China. The author reports no conflicts of interest in this work.
References
1. Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70(4):606–612. doi:10.1016/S0022-5223(19)40289-4
2. Ferguson MK, DeMeester TR, DesLauriers J, et al. Diagnosis and management of synchronous lung cancers. J Thorac Cardiovasc Surg. 1985;89(3):378–385. doi:10.1016/S0022-5223(19)38787-2
3. Hiraki A, Ueoka H, Yoshino T, et al. Synchronous primary lung cancer presenting with small cell carcinoma and non-small cell carcinoma: diagnosis and treatment. Oncol Rep. 1999;6:75–80.
4. Gogakos Apostolos S, Dimitrios P, Thomas R, et al. Double primary non-small cell lung cancer with synchronous small cell lung cancer N2 nodes: a case report. Ann Transl Med. 2015;3::157.
5. Sitki CM, Rudy L, Whitney W, et al. Synchronous bilateral lung cancer with discordant histology. Oncology (Williston Park, N Y). 2020;34:55–60.
6. Elisabetta F, Tiziana D, Giovanni F, et al. Three different synchronous primary lung tumours: a case report with extensive genetic analysis and review of the literature. Lung Cancer. 2008;59:395–402.
7. Laith N, Samia A, Abughanimeh Omar K. Isolated renal metastasis from primary lung squamous cell carcinoma with synchronous small cell lung cancer. Cureus. 2019;11:e4891.
8. Jiang L, Zheng X, Wu S, et al. A rare case of synchronous multiple primary lung cancer: squamous cell cancer and small cell lung cancer. Onco Targets Ther. 2019;12:8801–8806. doi:10.2147/OTT.S213259
9. Hirokazu T, Mika H, Soichiro I, et al. Multiple lung cancers including squamous cell carcinoma with strong PD-L1 expression and adenocarcinoma with EGFR exon 19 deletion: a case report. Respir Med Case Rep. 2020;29::100976.
10. Kozower Benjamin D, Larner James M, Detterbeck Frank C, et al. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5):e369S–e399S. doi:10.1378/chest.12-2362
11. Cheng H, Lei B-F, Peng P-J, et al. Histologic lung cancer subtype differentiates synchronous multiple primary lung adenocarcinomas from intrapulmonary metastases. J Surg Res. 2017;211:215–222. doi:10.1016/j.jss.2016.11.050
12. Kang X, Zhang C, Zhou H, et al. Multiple pulmonary resections for synchronous and metachronous lung cancer at two Chinese Centers. Ann Thorac Surg. 2020;109(3):856–863. doi:10.1016/j.athoracsur.2019.09.088
13. Zhirong Z, Shugeng G, Yousheng M, et al. Surgical outcomes of synchronous multiple primary non-small cell lung cancers. Sci Rep. 2016;6(1):23252. doi:10.1038/srep23252
14. Chen T-F, Xie C-Y, Rao B-Y, et al. Surgical treatment to multiple primary lung cancer patients: a systematic review and meta-analysis. BMC Surg. 2019;19(1):185. doi:10.1186/s12893-019-0643-0
15. Steve L, Stein Julie E, Rimm David L, et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5(8):1195–1204.
16. Ozaki Y, Muto S, Takagi H, et al. Tumor mutation burden and immunological, genomic, and clinicopathological factors as biomarkers for checkpoint inhibitor treatment of patients with non-small-cell lung cancer. Cancer Immunol Immunother. 2020;69(1):127–134. doi:10.1007/s00262-019-02446-1
17. Kim HS, Cha H, Kim J, et al. Genomic scoring to determine clinical benefit of immunotherapy by targeted sequencing. Eur J Cancer. 2019;120:65–74. doi:10.1016/j.ejca.2019.08.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.