Back to Journals » Lung Cancer: Targets and Therapy » Volume 8
New prognostic biomarkers and therapeutic effect of bevacizumab for patients with non-small-cell lung cancer
Authors Niki M, Yokoi T, Kurata T , Nomura S
Received 5 April 2017
Accepted for publication 17 July 2017
Published 3 August 2017 Volume 2017:8 Pages 91—99
DOI https://doi.org/10.2147/LCTT.S138887
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Pan-Chyr Yang
Maiko Niki, Takashi Yokoi, Takayasu Kurata, Shosaku Nomura
First Department of Internal Medicine, Kansai Medical University, Hirakata, Osaka, Japan
Background: Several biomarkers have emerged as potential prognostic and predictive markers for non-small-cell lung cancer (NSCLC). Successful inhibition of angiogenesis with the anti-vascular endothelial growth factor antibody, bevacizumab, has improved the efficacy seen with standard cytotoxic therapy of NSCLC. However, despite such enhanced treatment strategies, the prognosis for patients with advanced NSCLC remains poor.
Patients and methods: We assessed potential biomarkers in 161 NSCLC patients and 42 control patients. Enzyme-linked immunosorbent assay methods were used to evaluate three biomarkers: platelet-derived microparticle (PDMP), high-mobility group box-1 (HMGB1), and plasminogen activator inhibitor-1 (PAI-1). We studied the effects of bevacizumab on the expression of these markers. We also analyzed the relationship of the newly designed risk factor (NDRF) to overall survival and disease-free survival. The NDRF classification of patients was determined from the levels of PDMP, HMGB1, and PAI-1. To determine the individual prognostic power of PDMP, HMGB1, and PAI-1, we evaluated associations between their levels and patient outcomes by Kaplan–Meier survival analysis in a derivation cohort.
Results: PDMP, HMGB1, and PAI-1 levels were higher in NSCLC patients compared with control patients. Notably, the difference in PDMP levels exhibited the strongest statistical significance (p<0.001). Multivariate analysis showed that HMGB1 and PAI-1 levels were significantly correlated with PDMP levels. Patients who received standard chemotherapy with bevacizumab exhibited significantly reduced levels of all three markers compared with patients who received standard chemotherapy. NDRF3 status (high levels of all three markers) was significantly correlated with a poor prognosis (p<0.05 for overall survival and disease-free survival).
Conclusion: Our results demonstrate that abnormal levels of PDMP, HMGB1, and PAI-1 are related to each other in NSCLC. Moreover, our findings suggest that the vascular complications associated with these markers may contribute to a poor prognosis for NSCLC patients.
Keywords: non-small-cell lung cancer, platelet-derived microparticle, HMGB1, PAI-1, bevacizumab
Introduction
Eighty-five percent of all lung cancers are non-small-cell lung cancer (NSCLC), and approximately two-thirds of NSCLCs are at an advanced stage at diagnosis.1 The current prognosis for patients with advanced NSCLC remains poor despite enhanced treatment strategies.2,3 Several biomarkers have emerged as potential prognostic and predictive markers for NSCLC. In particular, epidermal growth factor receptor (EGFR) has been the subject of intensive research.4 Several studies have shown that mutated EGFR can be biomarkers for NSCLC.5,6 In addition, anaplastic lymphoma kinase (ALK) mutation is another critical NSCLC biomarker.7 These biomarkers are critical for the selection of the best therapy for lung cancer patients. Inflammatory or hematologic biomarkers such as high-mobility group box-1 (HMGB1),8 mean platelet volume (MPV),9,10 neutrophil or platelet/lymphocyte rates,11,12 and microparticle (MP) levels13,14 also serve as prognostic and predictive markers for NSCLC. The utility of an inflammation-related index has also been reported.15 However, the reliability and usefulness of these markers are controversial at present.
Current topics in lung cancer therapy are focusing on tyrosine kinase inhibitors (TKIs) and programmed death receptor-1 (PD-1) blockers.16 The specific driver mutations and development of small molecular TKIs such as EGFR and ALK are reported.17 Immunotherapy in the form of checkpoint inhibitors also represents a landmark success in NSCLC treatment, and patients have experienced durable responses with these treatments with good tolerability. Nivolumab exerts antitumor activity by blocking PD-1 on T lymphocytes and is currently approved as second-line therapy for advanced NSCLC.18
Angiogenesis is particularly critical to tumor growth and metastatic dissemination, and overexpression of vascular endothelial growth factor (VEGF), an angiogenic growth factor, has been associated with a poor prognosis in patients with NSCLC.19,20 Bevacizumab is an anti-VEGF monoclonal antibody that inhibited tumor-associated angiogenesis in preclinical and clinical studies.21,22 In the Phase III Eastern Cooperative Oncology Group E4599 and AVAil in lung trials, the addition of bevacizumab to platinum-based doublet chemotherapy resulted in significant improvements in the overall response rate and median progression-free survival.23,24 Bevacizumab demonstrated good therapeutic effect in many clinical trials.23–26 We also previously reported that pemetrexed and carboplatin plus bevacizumab, followed by maintenance with pemetrexed and bevacizumab, was effective and tolerable in patients with non-squamous NSCLC.27
Many individuals with cancer are in a hypercoagulable state, and the elevated risk of thrombosis conferred by hypercoagulativity increases patient morbidity and mortality.28 Cancer patients frequently develop venous thromboembolism (VTE), and various potential predictive biomarkers have been evaluated for association with VTE in cancer progression.29–32 For example, analysis of blood cells can effectively predict the risk of VTE development.30 Additionally, D-dimer, prothrombin fragment 1+2, and soluble P-selectin levels can accurately predict VTE risk.32 Furthermore, MP levels are also an accurate marker of VTE risk.33–35 In particular, the clinical course of blood cancer such as multiple myeloma includes cytokines, chemokines, growth factors, vascular endothelial cells, and/or the hemostatic-coagulatory system.36,37 Furthermore, some anticancer drugs are related to coagulopathy.38,39 For example, bevacizumab may also be linked with the risk of thrombosis.40,41 However, some studies also reported no relationship between bevacizumab and the risk of VTE in cancer patients.42
In this study, we evaluated several thrombosis-related markers in NSCLC patients receiving standard chemotherapy with or without bevacizumab treatment. We also evaluated the utility of several thrombosis-related biomarkers for prognostic prediction for NSCLC patients.
Patients and methods
Patients
NSCLC patients and healthy volunteers were recruited from Kansai Medical Hirakata Hospital and Kansai Medical University (Osaka, Japan) from September 2011 to October 2015. NSCLC patients were grouped according to the guidelines for tumor-node-metastasis stage I–IV, based on primary tumor size, lymph node involvement, and distance between the metastasis and primary tumor.43 This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the institutional review board of Kansai Medical University (Hirakata, Japan). Written informed consent was obtained from all participants. The primary objective of this study was the analysis of thrombosis-related biomarkers in NSCLC patients with or without bevacizumab treatment. The secondary objective was the evaluation of prognostic prediction for NSCLC patients using a newly designed risk factor (NDRF) obtained from the results of the primary objective.
Treatments
The present study is an evaluation of thrombosis-related biomarkers in NSCLC patients treated with standard chemotherapy with or without bevacizumab and is thus not a clinical trial to evaluate any usefulness regarding therapeutics. The standard chemotherapy treatment included combination of the following drugs: carboplatin, pemetrexed, docetaxel, paclitaxel, gemcitabine, vinorelbine, cisplatin, etoposide, irinotecan, TS-1, and TKIs.
Measurement of biomarkers
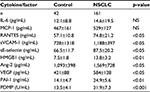
All biomarkers were measured at two points: before therapy and 1 month after therapy. In addition, we measured biomarkers if the patient’s condition took a sudden turn for the worse. Patient blood samples were collected in plain or sodium citrate containing tubes and left at room temperature for a minimum of 1 hour. Serum and citrated plasma were isolated by 20-minute centrifugation at 1,000×g at 4°C. Serum was divided into aliquots and frozen at −30°C until use. Human interleukin (IL)-6, monocyte-chemotactic protein (MCP)-1, regulated on activation normally T-cell expressed and secreted (RANTES), soluble vascular cell adhesion molecule (sVCAM)-1, soluble E (sE)-selectin, angiopoietin (Ang)-2, VEGF, and plasminogen activator inhibitor-1 (PAI-1) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Thermo Fisher Scientific (Waltham, MA, USA). HMGB1 was measured using the HMGB1 ELISA Kit II (Shino-test Corp, Kanagawa, Japan). Serum levels of cytokines and soluble factors were measured according to the manufacturer’s instructions. Recombinant products and standard solutions provided with commercial kits served as positive controls. Normal ranges were as follows: IL-6: 0.2–4.5 pg/mL, MCP-1: 170–570 pg/mL, RANTES: 23.9–58.5 ng/mL, sVCAM-1: 395–714 ng/mL, sE-selectin: 23.0–79.2 ng/mL, HMGB1: 1.2–4.8 ng/mL, Ang-2: 500–2,000 pg/mL, VEGF: 40–500 pg/mL, and PAI-1: 1.1–10.5 ng/mL.
Assessment of platelet-derived microparticle (PDMP)
Blood samples were collected with a 21-gauge needle from a peripheral vein into vacutainers containing EDTA-ACD (NIPRO Co Ltd, Japan) to minimize platelet activation. The samples were handled as described in the manufacturer’s protocol. Briefly, the samples were gently mixed by inverting the tube once or twice, stored at room temperature for 2–3 hours, and centrifuged at 8,000×g for 5 minutes at room temperature. Storage of samples at room temperature for 2–3 hours did not affect the PDMP level. Immediately after centrifugation, 200 µL of the upper layer of supernatants from 2-mL samples were collected to avoid contamination of platelets44,45 and the samples were stored at −40°C until analysis. PDMP levels were measured in duplicate using an ELISA kit (JIMRO Co Ltd, Japan) and monoclonal antibodies against glycoprotein CD42b and CD42a.44–48 The range of normal PDMP values was 3–8 U/mL.
Statistical analysis
Results are shown as the mean ± standard errors. Statistically significant differences between groups were identified using the chi-square or Student’s t-tests. Correlations between PDMP level and continuous variables were assessed using multivariable linear regression analyses. Receiver operating characteristics curve analysis was used to estimate an optimal cutoff value for biomarkers. Overall survival (OS) was defined as the time from initial diagnosis to the time of death from any cause or the date the patient was last known to be alive. Disease-free survival (DFS) was measured from the date of diagnosis until the date of disease recurrence or death, or until the date the patient was last known to be disease-free. Univariate analyses of OS were performed using the Kaplan–Meier product-limit method with the log-rank test and the Cox proportional hazards model. The 95% confidence interval (CI) for the survival rate was calculated using Greenwood’s method. The Brookmeyer and Crowley method was used to calculate the 95% CI of the median survival time. p-values <0.05 were considered statistically significant. All analyses were performed using the StatFlex program (version 6) (Artec Inc, Osaka, Japan).
Results
Clinical characteristics of the study subjects
A total of 161 NSCLC and 42 control (bronchial asthma or chronic obstructive pulmonary disease) patients were recruited for this study. The mean age of the patients was 68 years, with a range of 21–84 years. Among the 161 NSCLC patients, a total of 106 reported a history of smoking while the remaining 55 had never smoked. Table 1 details the histological classification of tumors from NSCLC patients. The performance status (PS) was 0–2 in 121 patients and 3 or 4 in 40 patients. Sixteen patients had stage IIIb disease, and 135 patients had stage IV disease. One hundred and thirty seven patients had received at least one regimen of systemic chemotherapy, and 22 patients had received best supportive care alone.
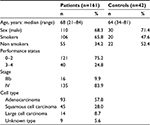  | Table 1 Clinical characteristics of the study subjects |
Levels of various biomarkers in NSCLC
We examined a panel of potential biomarkers for NSCLC in the NSCLC patient group and the healthy controls. The levels of IL-6 and MCP-1 were not significantly different between the two groups (Table 2). However, the levels of RANTES, sVCAM-1, sE-selectin, HMGB1, Ang-2, VEGF, PAI-1, and PDMP were higher in NSCLC patients compared with control patients. Notably, PDMP levels exhibited the strongest statistical significance between the two groups (p<0.001).
Variable analysis of various biomarkers
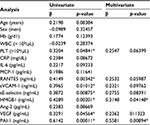
We next used univariate and multivariate regression analyses to investigate the associations between 15 variables and PDMP concentration in NSCLC patients (Table 3). Univariate analyses revealed that platelet, RANTES, sVCAM-1, sE-selectin, HMGB1, VEGF, and PAI-1 were significantly associated with PDMP. HMGB1 and PAI-1 levels also significantly correlated with PDMP levels using the multivariate analysis.
Effects of bevacizumab on biomarkers
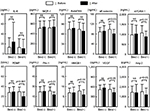
We next evaluated the concentrations of IL-6, MCP-1, RANTES, sE-selectin, sVCAM-1, PDMP, PAI-1, HMGB1, Ang-2, and VEGF in NSCLC patients treated with standard chemotherapy with or without bevacizumab (Figure 1). In the patients who received standard treatment without bevacizumab (n=70), the concentrations of all markers showed no significant changes. However, in the patients who received standard therapy with bevacizumab (n=91), the concentrations of sE-selectin, sVCAM-1, PDMP, PAI-1, HMGB1, Ang-2, and VEGF were significantly altered after treatment (n=91). IL-6, MCP-1, and RANTES levels showed no statistically significant difference.
Survival analysis using PDMP, HMGB1, and PAI-1
We focused our attention on three biomarkers, PDMP, HMGB1, and PAI-1, because of the following reasons: 1) PDMP levels exhibited the strongest statistically significant difference between NSCLC patients and controls (p<0.001); 2) HMGB1 and PAI-1 levels in NSCLC patients were significantly correlated with PDMP levels by the multivariate analysis; and 3) the concentrations of PDMP, PAI-1, and HMGB1 were also significantly altered after treatment with bevacizumab.
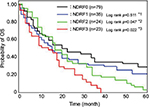
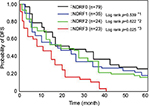
Therefore, we determined NDRF classification based on the levels of PDMP, HMGB1, and PAI-1, and analyzed the contribution of NDRF to OS or DFS. The number of NSCLC patients with high levels of PDMP, HMGB1, or PAI-1 was 63, 34, or 41, respectively (Table 4). NDRFs were classified as 0–3 according to the following definitions – NDRF0: patients with low levels of PDMP, HMGB1, and PAI-1; NDRF1: patients with one factor (HMGB1 or PAI-1) elevated; NDRF2: patients with two factors (PDMP, HMGB1, or PAI-1) elevated; and NDRF3: patients with all three factors elevated (Table 4).
We next evaluated the association between the levels of PDMP, HMGB1, and PAI-1 and patient outcome using Kaplan–Meier survival analysis in the derivation cohort to determine the individual prognostic power of each biomarker. NDRF3 was significantly correlated with a poor prognosis (p<0.05 for both OS and DFS; Figures 2 and 3). NDRF2 was also associated with an unfavorable OS (p<0.05; Figure 2), but was not associated with DFS (Figure 3). Neither NDRF1 nor NDFR0 was correlated with either OS or DFS (Figures 2 and 3).
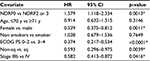
Multivariate analysis clearly revealed that NDRF2 or NDRF3 was an independent unfavorable prognostic factor for OS (hazard ratio: 1.579, 95% CI: 1.118–2.334, p=0.0013). In contrast, being female (p=0.0011), having a PS of 0–2 (p<0.0001), having non-squamous cell carcinoma (p=0.0039), and having stage IIIb disease (p=0.0416) were independent favorable prognostic factors (Table 5). Being younger than 70 years (p=0.3697) was, however, not a significant factor. Furthermore, no significant difference in OS was observed between patients with and without a history of smoking (p=0.7649).
Discussion
Multiple studies have attempted to identify prognostic factors or molecular biomarkers to predict the likelihood of lung cancer metastasis or recurrence. At present, useful prognostic factors include disease staging, PS, histology, sex, and age.49–51 Additionally, recent efforts have focused on identifying potential endothelial, hematological, or inflammatory biomarkers for NSCLC.9,13–15,52 We found that levels of RANTES, sVCAM-1, sE-selectin, HMGB1, Ang-2, VEGF, PAI-1, and PDMP were higher in NSCLC patients than in control individuals. Some of these biomarkers have been previously associated with NSCLC prognosis.8,14,53–55
In the present study, we studied the effect of bevacizumab on the expression of various markers. Bevacizumab improved PDMP, HMGB1, and PAI-1 levels as well as sE-selectin, sVCAM-1, Ang-2, and VEGF. Angiogenesis is important for tumor growth and metastasis, with the pro-angiogenic protein, VEGF, being a major regulator of angiogenesis in normal and malignant tissues.56–58 The overexpression of VEGF has been correlated with a poor prognosis in patients with NSCLC.20 Some reports have shown that bevacizumab may be linked with a risk of thrombosis.40,41 However, another study also reported no relationship between bevacizumab and risk of VTE in cancer patients.42 Our results support the latter because bevacizumab improved thrombosis-related biomarkers such as PDMP, HMGB1, and PAI-1. Together, this suggests that PDMP may be an important biomarker in NSCLC.
PDMP is a platelet-related biomarker with procoagulant activity and contributes to thrombosis formation and atherosclerosis.44–46 Several associations between platelet-based parameters and NSCLC progression have been previously reported.9,10,50 Inagaki et al9 reported that the MPV:platelet count ratio was closely associated with survival in patients with advanced NSCLC. Kumagai et al10 reported that a low MPV predicted an unfavorable prognosis following curative resection of NSCLC. Furthermore, Zhang and Ran51 conducted a meta-analysis and demonstrated that an elevated platelet count confers a poor prognosis for patients with lung cancer. Therefore, we investigated whether PDMP or PDMP-associated biomarkers were associated with NSCLC prognosis. We investigated 15 variables and their associations with PDMP in NSCLC patients by multivariable analysis. Our results showed that levels of HMGB1 and PAI-1 were significantly correlated with PDMP levels.
HMGB1 has been previously reported as a potential prognostic factor for NSCLC.8,53–55 Naumnik et al8 identified increased levels of HMGB1 in patients with advanced NSCLC undergoing chemotherapy. However, they concluded that HMGB1 concentration did not influence survival time following NSCLC treatment because there was no significant difference in HMGB1 levels before and after chemotherapy. In contrast, Wang et al53 reported that HMGB1 was highly expressed in NSCLC and may be valuable as a prognostic and predictive marker for NSCLC. Therefore, the relevance of HMGB1 in NSCLC prognosis is controversial.
Su et al55 reported that high PAI-1 expression in NSCLC correlated with a poor prognosis. However, the authors also observed that PAI-1 correlation is dependent on PAI-2. Therefore, the individual relevance of these markers on NSCLC prognosis remains unclear. Nevertheless, PAI-1 and/or HMGB1 levels may be important prognostic factors for lung cancer.54,55,59
We found that NSCLC patients with combined high levels of PDMP, HMGB1, and PAI-1 had a poor prognosis. A previous report has described a significant elevation of PDMP in lung cancer.60 However, we did not identify any individual effects of PDMP, HMGB1, or PAI-1 on NSCLC prognosis.
The level of endothelial cell-derived microparticles (EDMP) has also been identified as a prognostic biomarker for NSCLC.13,14,60 Fleitas et al13 reported that circulating levels of EDMP and circulating endothelial cells correlate with prognosis, and could be useful prognostic markers for patients with advanced NSCLC. Consistent with these findings, Wang et al14 suggested that circulating EDMPs may be a useful biomarker predictive of 1-year mortality in end-stage NSCLC patients. Furthermore, Tseng et al60 reported that of all MPs investigated, only an increased level of EDMP was significantly associated with lung cancer. Unfortunately, we could not measure EDMP in the present study. Therefore, whether high levels of PDMP, HMGB1, and PAI-1 in NSCLC patients are associated with the level of EDMP remains unknown.
We propose that HMGB1 plays an important role in the relationships between HMGB1, PDMP, and PAI-1. HMGB1 is a nuclear protein that binds nucleosomes and promotes DNA bending.61 However, HMGB1 released from intracellular stores into the blood stream also plays a crucial role in the cellular response to tissue damage.62 HMGB1 expression is detectable in multiple immune and inflammatory diseases,63 and HMGB1 protein is sequestered by thrombomodulin in vivo.64 Moreover, HMGB1 stimulates toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE). Therefore, HMGB1 can activate platelets through TLR4. Activation of TLR or RAGE on endothelial cells or platelets stimulates release of PAI-1 and PDMP into the circulation and promotes vasculopathy. Therefore, the presence of HMGB1, PDMP, and PAI-1 could contribute to vascular complications such as thrombosis formation. As many individuals with cancer are in a hypercoagulable state, the elevated risk of thrombosis conferred by hypercoagulativity increases patient morbidity and mortality.20
Our findings have several potential implications. First, we have shown that a combined increase in the levels of HMGB1, PDMP, and PAI-1 is related to NSCLC prognosis. Second, we have described how vascular complications may result from increased levels of these biomarkers to contribute to poor prognosis for NSCLC. Third, we demonstrated that bevacizumab improved the levels of HMGB1, PDMP, and PAI-1 after treatment. Nevertheless, our study has some limitations. We were unable to determine whether any relationship between EDMP and the three biomarkers exists. Additionally, we did not investigate how different therapeutic strategies other than bevacizumab, for example, TKIs and PD blockers, affect the utility of the identified prognostic markers. Furthermore, the mechanism of the decrease of the thrombosis-related biomarkers after bevacizumab treatment has not been clarified. Finally, we could not recognize the relationship between certain clinical proof of vascular complications and the elevation of PDMP, HMGB1, and PAI-1. Further confirmation of our observations in prospective studies is necessary.
Acknowledgments
This study was supported in part by a grant from the Japan Foundation of Neuropsychiatry and Hematology Research, a Research Grant for Advanced Medical Care from the Ministry of Health and Welfare of Japan, and a grant (13670760 to SN) from the Ministry of Education, Science and Culture of Japan.
Disclosure
The authors report no conflict of interests in this work.
References
Herbst RS, Heymach J, Lippman S. Lung cancer. N Engl J Med. 2008;359:1367–1380. | ||
Johnson DH, Schiller JH Jr, Bunn PA. Recent clinical advances in lung cancer management. J Clin Oncol. 2014;32:973–982. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. | ||
Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. | ||
Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34. | ||
Wang S, Tsui ST, Liu C, Song Y, Liu D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J Hematol Oncol. 2016;9:59. | ||
Iragavarapu C, Mustafa M, Akinleye A, et al. Novel ALK inhibitors in clinical use and development. J Hematol Oncol. 2015;8:17. | ||
Naumnik W, Nilklińska W, Ossolińska M, Chyczewska E. Serum levels of HMGB1, survivin, and VEGF in patients with advanced non-small cell lung cancer during chemotherapy. Folia Histochem Cytobiol. 2009;47:703–709. | ||
Inagaki N, Kibata K, Tamaki T, Shimizu T, Nomura S. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer. 2014;83:97–101. | ||
Kumagai S, Tokuno J, Ueda Y, et al. Prognostic significance of preoperative mean platelet volume in resected non-small-cell lung cancer. Mol Clin Oncol. 2015;3:197–201. | ||
Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013;14:5237–5242. | ||
Kemal Y, Yucel I, Ekiz K, et al. Elevated serum neutrophil to lymphocyte and platelet to lymphocyte ratios could be useful in lung cancer diagnosis. Asian Pac J Cancer Prev. 2014;15:2651–2654. | ||
Fleitas T, Martínez-Sales V, Vila V, et al. Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS One. 2012;7:e47365. | ||
Wang CC, Tseng CC, Hsiao CC, et al. Circulating endothelial-derived activated microparticle: a useful biomarker for predicting one-year mortality in patients with advanced non-small cell lung cancer. BioMed Res Int. 2014;2014:173401. | ||
Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. | ||
Dholaria B, Hammond W, Shreders A, Lou Y. Emerging therapeutic agents for lung cancer. J Hematol Oncol. 2016;9:138. | ||
Smith AD, Roda D, Yap TA. Strategies for modern biomarker and drug development in oncology. J Hematol Oncol. 2014;7:70. | ||
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. | ||
O’Byrne KJ, Koukourakis MI, Giatromanolaki A, et al. Vascular endothelial growth factor, platelet-derived endothelial cell growth factor and angiogenesis in non-small-cell lung cancer. Br J Cancer. 2000;82:1427–1432. | ||
Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer. 2006;51:143–158. | ||
Kim K, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. | ||
Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. | ||
Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. | ||
Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. | ||
Daz-Ares L, deMarinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–255. | ||
Estevinho F. Bevacizumab for the treatment of nonsquamous non-small-cell lung cancer in Portugal: a retrospective, multicenter study. Cancer Manag Res. 2012;4:91–97. | ||
Yokoi T, Torii Y, Katashiba Y, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab, followed by maintenance pemetrexed and bevacizumab in Japanese patients with non-squamous non-small cell lung cancer. Oncol Lett. 2014;8:2453–2457. | ||
Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839–4847. | ||
Blom JW, Vanderschool JP, Oostindiër MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–535. | ||
Simanek R, Vormittag R, Ay C, et al. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost. 2010;8:114–120. | ||
Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122:2331–2336. | ||
Ferroni P, Martini F, Portarena I, et al. Novel high-sensitive D-dimer determination predicts chemotherapy-associated venous thromboembolism in intermediate risk lung cancer patients. Clin Lung Cancer. 2012;13:482–487. | ||
Manly DA, Wang J, Glover SL, et al. Increased microparticle tissue factor activity in cancer patients with venous thromboembolism. Thromb Res. 2010;125:511–512. | ||
Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–1880. | ||
Nomura S, Niki M, Nishizawa T, Tamaki T, Shimizu M. Microparticles as biomarkers of blood coagulation in cancer. Biomark Cancer. 2015;7:51–56. | ||
Oshima K, Kanda Y, Nannya Y, et al. Clinical and pathologic findings in 52 consecutively autopsied cases with multiple myeloma. Am J Hematol. 2001;67:1–5. | ||
Ducros E, Mirshahi SS, Faussat AM, et al. Soluble endothelial protein C receptor (sEPCR) is likely a biomarker of cancer-associated hypercoagulability in human hematologic malignancies. Cancer Med. 2012;1:261–267. | ||
Chong BH, Lee SH. Management of thromboembolism in hematologic malignancies. Semin Thromb Hemost. 2007;33:435–448. | ||
Franchini M, Di Minno MN, Coppola A. Disseminated intravascular coagulation in hematologic malignancies. Semin Thromb Hemost. 2010;36:388–403. | ||
Nalluri SI. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–2285. | ||
Meyer T, Robles-Carrillo L, Robson T, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost. 2009;7:171–181. | ||
Hurwitz HI, Sallz LB, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol. 2011;29:1757–1764. | ||
Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. | ||
Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123:8–23. | ||
Nomura S, Shimizu M. Clinical significance of procoagulant microparticles. J Intensive Care. 2015;3:2. | ||
Nomura S. Microparticle and atherothrombotic diseases. J Atheroscler Thromb. 2016;23:1–9. | ||
Osumi K, Ozeki Y, Saito S, et al. Development and assessment of enzyme immunoassay for platelet-derived microparticles. Thromb Haemost. 2001;85:326–330. | ||
Nomura S, Uehata S, Saito S, Osumi K, Ozeki Y, Kimura Y. Enzyme immunoassay detection of platelet-derived microparticles and RANTES in acute coronary syndrome. Thromb Haemost. 2003;89:506–512. | ||
O’Byrne KJ, Gatzemeier U, Bondarenko I, et al. Molecular biomarkers in non-small-cell lung cancer: a retrospective analysis of data from the phase 3 FLEX study. Lancet Oncol. 2011;12:795–805. | ||
Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol. 2011;3:127–138. | ||
Zhang X, Ran Y. Prognostic role of elevated platelet count in patients with lung cancer: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8:5379–5387. | ||
Zhang T, Jiang Y, Qu X, Shen H, Liu Q, Du J. Evaluation of preoperative hematologic markers as prognostic factors and establishment of novel risk stratification in resected pN0 non-small-cell lung cancer. PLoS One. 2014;9:e111494. | ||
Wang JL, Wu DW, Cheng ZZ, Han WZ, Xu SW, Sun NN. Expression of high mobility group box-B1 (HMGB-1) and matrix metalloproteinase-9 (MMP-9) in non-small cell lung cancer (NSCLC). Asian Pac J Cancer Prev. 2014;15:4865–4869. | ||
Wang H, Li Y, Yu W, Ma L, Ji X, Xia W. Expression of the receptor for advanced glycation end-products and frequency of polymorphism in lung cancer. Oncol Lett. 2015;10:51–60. | ||
Su CY, Liu YP, Yang CJ, et al. Plasminogen activator inhibitor-2 plays a leading prognostic role among protease families in non-small cell lung cancer. PLoS One. 2015;10:e0133411. | ||
Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. | ||
Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. | ||
Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. | ||
Bayramoglu A, Gunes HV, Metintas M, et al. Plasminogen activator inhibitor-1 and susceptibility to lung cancer: a population genetics perspective. Genet Test Mol Biomarkers. 2014;18:587–590. | ||
Tseng CC, Wang CC, Chang HC, et al. Levels of circulating microparticles in lung cancer patients and possible prognostic value. Dis Markers. 2013;35:301–310. | ||
Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. | ||
Matsuoka N, Itoh T, Watarai H, et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120:735–743. | ||
Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. | ||
Abeyama K, Stern DM, Ito Y, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267–1274. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.