Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
New-onset of postoperative atrial fibrillation is likely to recur in the absence of other triggers
Authors Park-Hansen J, Greve AM , Clausen J , Holme SJ, Carranza CL, Irmukhamedov A , Sabah L, Lin Q, Madsen AS , Domínguez H
Received 9 February 2018
Accepted for publication 23 April 2018
Published 7 September 2018 Volume 2018:14 Pages 1641—1647
DOI https://doi.org/10.2147/TCRM.S165155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Jesper Park-Hansen,1 Anders M Greve,2,3 Johan Clausen,1 Susanne J Holme,4 Christian L Carranza,4 Akhmadjon Irmukhamedov,5 Lubna Sabah,1 Qing Lin,1 Anne Sofie Madsen,1 Helena Domínguez1,2
1Department of Cardiology, Bispebjerg/Frederiksberg University Hospital, Copenhagen, Denmark; 2Department of Biomedicine, Faculty of Health and Medical Sciences, 3Department of Cardiology, Herlev/Gentofte University Hospital, Copenhagen, Denmark; 4Department of Thoracic Surgery, Rigshospitalet, Copenhagen, Denmark; 5Department of Thoracic Surgery, Odense University Hospital, Odense, Denmark
Background: Incident atrial fibrillation (AF) is reported in 10%–65% of patients without previous AF diagnosis after open heart surgery. The risk of late AF recurrence after a postoperative AF onset is unclear, and it is controversial whether AF limited to the postoperative period should elicit oral anticoagulation (OAC) therapy. The primary objective of this study was to evaluate the long-term recurrence of AF in patients developing new-onset peri-procedural AF.
Patients and methods: Patients (n=189) with available baseline and follow-up data included in Left Atrial Appendage Closure with Surgery trial were coded for known AF at baseline and for postoperative first-time AF diagnosis. AF occurrence was classified as follows: peri-procedural ≤7 days postoperatively, early >7 days but ≤3 months and late >3 months. Patients with no AF recurrence registered during follow-up were invited to undergo Holter monitoring.
Results: A total of 163 (86.2%) patients had no history of AF. Among these, 80 (49.1%) developed new-onset peri-procedural AF. After a mean follow-up of 3.7±1.6 years, late AF occurred in 35 of the 80 (43.8%) patients who developed peri-procedural AF and in 6 additional patients (7.2%) who remained in sinus rhythm until discharge (hazard ratio [HR] 9.3, 95% CI 3.8–22.4, p<0.001). Patients with peri-procedural AF and early AF had 12.24 times higher risk of late AF (95% CI 4.76–31.45, p<0.001) as compared to the group with no postoperative AF.
Conclusion: New-onset of AF after open heart surgery has a high rate of recurrence and should not be regarded as a self-limiting phenomenon secondary to surgery.
Keywords: atrial fibrillation, postoperative atrial fibrillation, heart surgery
Introduction
Oral anticoagulation (OAC) is well established to prevent cardiac thromboembolism in patients with atrial fibrillation (AF) not secondary to reversible conditions according to their risk profile.1 AF is very common following open heart surgery with a prevalence of 10%–65%, highest after a combination of coronary artery bypass grafting (CABG) and valve surgery,2 and is associated with prolonged hospital stay, hemodynamic instability, an increased risk of stroke and mortality.2–5
However, it is uncertain whether patients with AF detected in the postoperative setting should be treated with OAC. The American Association for Thoracic Surgery (AATS) acknowledges in the 2014 guidelines for the prevention and management of postoperative AF and flutters for thoracic surgical procedures that the ideal duration of anticoagulation after postoperative AF is unknown.6 The newest guidelines from the European Society of Cardiology (ESC) 2016 state that “good quality data are needed to determine whether long-term anticoagulation can prevent strokes in patients with post-operative AF at high stroke risk.”7 The newest guidelines from the American Heart Association 2014 state that “it is reasonable to administer antithrombotic medication in patients who develop postoperative AF, as advised for non-surgical patients” (level of evidence: B).8
We hypothesized that postoperative new-onset AF recurs after new-onset postoperative AF and therefore represented the onset of AF rather than a transient phenomenon.
Patients and methods
Study design
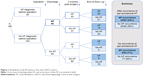
The study cohort consisted of all patients included in the Left Atrial Appendage Closure with Surgery (LAACS) study without prior AF diagnosis. In brief, LAACS study was a prospective, open-label study on patients scheduled for open heart surgery. In LAACS, we randomized patients to surgical closure of left atrial appendage closure in addition to the planned surgery procedure or control (LAACS study, ClinicalTrials.gov unique identifier ID NCT02378116). The study was conducted according to the Declaration of Helsinki. Patients were included after providing written informed consent according to the protocol approved by the Ethics Committee of the Capital Region, Denmark (registration number H-3-2010-017). The protocol included planned open heart surgery (coronary artery bypass, valve surgery and combination of both and surgery of the aorta) not related to endocarditis.
At the time of inclusion, we registered prior AF, other medical history and medication, including OAC. All patients included in the LAACS trial were followed through their electronic hospital medical records for at least 1 year after surgery, until the end of the study. This study was based on the total cohort of patients without known AF, who underwent open heart surgery and who were included in the LAACS trial.
Perioperative AF (POAF) detection and management
All patients were routinely monitored with telemetry for the first 3 days after surgery and longer if there had been cases of arrhythmia. We classified the occurrence of AF within the first 7 days following surgery as POAF. Patients were managed with rhythm or rate control and OAC at the discretion of the treating physician. The occurrence of AF in 3 months following surgery was considered “early recurrence.”
End point definitions
The primary end point was AF recurrence beyond the first 3 months after the surgery (late recurrence) among patients with POAF. If there were no episodes of AF in the patient’s clinical records, they received continuous heart rhythm monitoring (Holter) for at least 7 days with any device available at the outpatient clinic.
All-cause mortality was recorded as the secondary end point.
Statistical analyses
All analyses were performed using SAS statistical software package version 9.4 (SAS Institute Inc., Cary, NC, USA). Continuous variables are presented as mean ± SD and categorical as number and percentages. After inspection for normality, differences between the LAACS and control groups were evaluated by Student’s t-test (continuous variables) and by Chi-square and Fisher’s exact tests for categorical variables where appropriate. Logistic and Cox regressions were used to estimate risk ratios for peri-procedural and late AF/mortality, respectively. Multivariable models were developed by including predictor variables with a priori presumed influence on the risk of AF occurrence. Due to the randomized design, LAACS was a forced covariate in all multivariable models. The probability of late AF over time among patients with and without POAF was evaluated by Fine and Gray9 competing risk regression using death as a competing event. For all hypothesis testing, a two-tailed p-value <0.05 was required for statistical significance.
Results
Of the 189 patients included in the main LAACS study, 163 patients had no history of AF and were included in this subanalysis. The mean follow-up was 3.7±1.6 years.
A total of 36 patients were monitored by the study group, and the recurrence of AF was found in 21 patients. The numbers were added to the findings of AF in clinical setting, and the cumulative findings of AF recurrence are mentioned in the “Results” section.
POAF
A total of 80 patients (49%) had new-onset POAF, while 83 (51%) did not have AF. Table 1 depicts the baseline characteristics according to POAF.
In multivariable analyses, older age (p=0.03), calcium blocker (p=0.01) and surgery type compared to CABG alone (p=0.04) remained associated with increased risk of POAF. LAACS was significantly associated with a lower risk of postoperative AF (odds ratio [OR] 0.40, 95% CI 0.19–0.82, p=0.01; Table 2).
Early and late AF after surgery
During the first 3 months following surgery, 43 of the 80 patients with POAF (54%) had early recurrence of AF, while it occurred in three out of 83 (4%) of those without POAF (Figure 1).
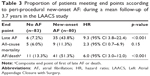
Importantly, of the 80 patients with POAF, 35 (43.8%) had late recurrence of AF as compared to six (7.2%) patients with no postoperative AF. Mortality was not significantly different (nine out of 80 patients with POAF and five out of 83 without AF, p=0.15). Univariate survival analyses demonstrated that patients with POAF had 9.3 times higher risk of late AF (p≤0.001; Table 3).
As depicted in Table 4, adjusted analyses showed that POAF (p<0.001), renin–angiotensin system (RAS) blockers (p=0.01), valve surgery alone or combined with CABG compared to CABG alone (p=0.01) was associated with late recurrence of AF. In addition, POAF followed by early AF had a 12-fold higher risk of late AF (hazard ratio [HR] 12.24, 95% CI 4.76–31.45, p<0.001; Table 4).
Figures 2 and 3 show the cumulative probability of late AF according to POAF and early AF, respectively. Patients with both POAF and early recurrence had highest probability of late recurrence (Table S1).
  | Figure 2 Cumulative probability of late AF (after 90 days) according to peri-procedural new-onset AF. |
  | Figure 3 Cumulative probability of late AF (after 90 days) according to peri-procedural new-onset AF with or without early AF recurrence (within 90 days). |
Discussion
The main finding in our study is that POAF after open heart surgery, in patients without previous history of AF, recurs in 44% of the cases. This argues that peri-procedural AF is a marker of AF well beyond the stress of the surgery. Overall, this supports the need to consider a more aggressive approach both regarding follow-up of these patients and in the prescription of OAC. Thus, this study may contribute to filling the gaps in the current guidelines on management of POAF.7,8 Our findings also contribute to the discussion of transient use of OAC, especially, since patients with POAF and early recurrence have a high risk of late recurrence of AF.
Our results are in line with other studies which also suggest a high rate of recurrence of AF.3,10 According to Ahlsson et al3 in their cohort of 571 CABG-operated patients with no history of AF, postoperative AF occurred in 28.9%, of whom 25.4% had AF at follow-up after a median follow-up of 6.9 years. This is further supported by recent data from the Framingham Heart Study, where almost two-thirds of the patients are initially considered to have AF secondary to cardiac surgery recurred within 15 years.10
In our study, we found no significant difference in mortality which may be due to the limited number of patients in the cohort. Indeed, El-Chami et al11 have analyzed the effect of new-onset AF in patients undergoing CABG and identified that postoperative onset AF predicted long-term mortality with a 21% relative increase in mortality. Importantly, use of warfarin was associated with improved survival in postoperative onset AF patients.11 This could again suggest a degree of recurrence.
In our study, we found a high risk of POAF associated with advanced age, which is consistent with findings from other studies.12,13 We also found that the need for the treatment with RAS blockers was associated with late recurrence of POAF, consistent with hypertension and heart failure as the known risk factors for AF.2
Interestingly, we found LAACS to significantly reduce the risk of postoperative AF, although this was not the aim of the study. However, it has been claimed that ligation of the left atrium appendage could maintain sinus rhythm in patients with AF through electrical remodeling.14
Study limitations
A limitation to this study was that AF diagnosis is mainly based on clinical outcomes recorded during clinical setting rather than a continuous ECG monitoring in all patients. In addition, the study focuses on the recurrence of AF, and not on its substrate, which is important to assess the tendency to AF.15
The relatively low absolute event rate in mortality could have led to underpowered analysis, notwithstanding this would not have altered the main findings of this study.
The management of AF was not homogeneous which could have influenced the outcomes of recurrence of AF, and finally, there is a possible selection bias in the capacity of monitoring the weakest patients opting out of physical follow-up.
Conclusion
Our results show that POAF is likely to recur beyond the first 3 months after the surgery. Thus, challenging the concept that POAF is a transient phenomenon secondary to surgery.
Clinical implications
A typical everyday scenario of dealing with postoperative AF is the attempt to restore sinus rhythm in patients within 24–48 hours after onset with the use of antiarrhythmic drugs or by electrical cardioversion. Patients who are discharged in AF are maintained on anticoagulant therapy and referred for cardioversion in 4–6 weeks. Patients discharged on antiarrhythmic drugs are followed up in the cardiology clinic after 3 months. In the absence of evident AF, their antiarrhythmic drugs are usually stopped. The decision to stop anticoagulant therapy is in these cases left to the cardiologist’s discretion.11 With our data in mind, we believe that patients with POAF, and especially those with early recurrence, should be treated as other patients with AF, according to the current guidelines for the management of AF. This includes use of OAC medicines dependent on their risk factors for thromboembolism.
Acknowledgment
The authors would like to acknowledge Dr Mads Liljekvist for help on Holter monitoring of the patients in the study, and Dr Sabrina Nolan for help on the presentation of the results.
Disclosure
Cortrium ApS is a partner in other projects and provided Holter monitors to the current study. The authors report no other conflicts of interest in this work.
References
Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2005;(3):CD001927. | ||
Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. Europace. 2012;14(2):159–174. | ||
Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37(6):1353–1359. | ||
Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720–1729. | ||
Greve AM, Dalsgaard M, Bang CN, et al. Stroke in patients with aortic stenosis: the simvastatin and ezetimibe in aortic stenosis study. Stroke. 2014;45(7):1939–1946. | ||
Frendl G, Sodickson AC, Chung MK, et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg. 2014;148(3):e153–e193. | ||
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Rev Esp Cardiol (Engl Ed). 2017;70(1):50. | ||
Writing Committee M, January CT, Wann LS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–e267. | ||
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. | ||
Lubitz SA, Yin X, Rienstra M, et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131(19):1648–1655. | ||
El-Chami MF, Kilgo P, Thourani V, et al. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55(13):1370–1376. | ||
Rocha AS, Pittella FJ, Lorenzo AR, et al. Age influences outcomes in 70-year or older patients undergoing isolated coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2012;27(1):45–51. | ||
Wang WH, Hsiao SH, Lin KL, Wu CJ, Kang PL, Chiou KR. Left atrial expansion index for predicting atrial fibrillation and in-hospital mortality after coronary artery bypass graft surgery. Ann Thorac Surg. 2012;93(3):796–803. | ||
Kawamura M, Scheinman MM, Lee RJ, Badhwar N. Left atrial appendage ligation in patients with atrial fibrillation leads to a decrease in atrial dispersion. J Am Heart Assoc. 2015;4(5):e001581. | ||
Gasparova I, Kubatka P, Opatrilova R, et al. Perspectives and challenges of antioxidant therapy for atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol. 2017;390(1):1–14. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.