Back to Journals » Cancer Management and Research » Volume 6
New modalities of cancer treatment for NSCLC: focus on immunotherapy
Authors Davies M
Received 14 November 2013
Accepted for publication 2 December 2013
Published 3 February 2014 Volume 2014:6 Pages 63—75
DOI https://doi.org/10.2147/CMAR.S57550
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Video abstract presented by Marianne Davies.
Views: 20974
Marianne Davies
Smilow Cancer Hospital at Yale-New Haven Hospital, New Haven, CT, USA
Abstract: Recent advances in the understanding of immunology and antitumor immune responses have led to the development of new immunotherapies, including vaccination approaches and monoclonal antibodies that inhibit immune checkpoint pathways. These strategies have shown activity in melanoma and are now being tested in lung cancer. The antibody drugs targeting cytotoxic T-lymphocyte-associated antigen-4 and programmed cell death protein-1 immune checkpoint pathways work by restoring immune responses against cancer cells, and are associated with unconventional response patterns and immune-related adverse events as a result of their mechanism of action. As these new agents enter the clinic, nurses and other health care providers will require an understanding of the unique efficacy and safety profiles with immunotherapy to optimize potential patient benefits. This paper provides a review of the new immunotherapeutic agents in development for lung cancer, and strategies for managing patients on immunotherapy.
Keywords: immunotherapy, lung cancer, vaccination, nivolumab, ipilimumab, nursing
Introduction
Lung cancer is the leading cause of cancer-related deaths in the US.1 Approximately 85% of cases are non-small-cell lung cancer (NSCLC), and the majority of patients are diagnosed at an advanced stage of disease.2 Unfortunately, current treatment options for NSCLC are limited. Historically, most patients in the US receive platinum-based chemotherapy as first-line treatment.3 A small population of patients are also candidates for treatment with targeted agents; however, most patients eventually develop resistance to targeted agents.3,4 Erlotinib (Tarceva®, OSI Pharmaceuticals, LLC, Farmingdale, NY, USA), afatinib (Gilotrif®, Boehringer Ingelheim Pharmaceuticals, Inc, Ridgefield, CT, USA), and crizotinib (Xalkori®, Pfizer, Inc, New York, NY, USA) are approved first-line therapies for patients expressing particular mutations in the epidermal growth factor (EGF) receptor, or with anaplastic lymphoma kinase (ALK)-positive tumors, respectively. Patients with non-squamous NSCLC without a recent history of hemoptysis may be treated with bevacizumab (Avastin®, Genentech, South San Francisco, CA, USA) in combination with doublet therapy. Bevacizumab is a monoclonal antibody that inhibits angiogenesis of the tumor, leading to tumor starvation. Docetaxel, pemetrexed, and erlotinib are approved second-line therapies.3
Even with treatment, 5-year survival rates average 17% for patients with early disease and 4% for patients diagnosed with metastatic disease.1,2 Current chemotherapies work by acting non-selectively, targeting cell division or cell suicide pathways to encourage tumor cell death.5 Targeted agents inactivate specific mutated proteins that confer growth advantages to the tumor; however, only a percentage of patients express these mutations, ie, 10%–15% for EGF receptor mutations and 2%–7% for ALK mutations.6–9 In contrast, immunotherapies are designed to restore, stimulate, or enhance the ability of the immune system to recognize and eliminate tumors, and in initial trials, immunotherapies have shown activity in NSCLC and other cancer types.10–14 Herein, new therapeutic approaches for NSCLC are reviewed, focusing on immunotherapy.
Tumor immunology
The immune system primarily functions to protect the body from damage caused by pathogens. However, the immune system also serves to detect and eliminate aberrant cells, including cancer cells, which could potentially cause harm.15 This is evidenced by the finding that patients with reduced immune function as a result of acquired immune deficiency syndrome or chronic immunosuppression have increased rates of malignancy.16 T-cells eliminate tumors by recognizing aberrant proteins presented by cancerous cells, and coordinating an immune response against them.15 Immune responses, whether against tumor cells, infected cells, or as a result of autoimmunity, can damage healthy tissue if left unchecked. To protect against this, the immune system has multiple mechanisms to downregulate immune responses – collectively known as immune checkpoint pathways.
The cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1) pathways are two of several immune checkpoint pathways that play critical roles in controlling T-cell immune responses. CTLA-4 and PD-1 are expressed by T-cells. When they bind their ligands (CD80 and CD86 [B7 molecules] for CTLA-4; programmed death ligand-1 [PD-L]1 and PD-L2 for PD-1), the T-cell effector functions are dampened or stopped, and the T-cell can become nonresponsive.17 CTLA-4 is thought to act in the early stages of an immune response, primarily to reduce T-cell responses to self antigens and prevent autoimmunity.17 In contrast, PD-1 functions in the later stages of an immune response to stop ongoing immune activity in tissues.17
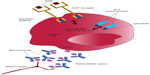
Some tumors evade immune responses by exploiting immune checkpoint pathways and other regulatory mechanisms (Figure 1).18–23 A better understanding of these immune evasion strategies has given rise to novel immunotherapies that can restore the patient’s own immune system to respond to and eliminate cancer cells. These immunotherapies re-engage and allow T-cells to function appropriately against tumors.
  | Figure 1 Immune evasion or immunosuppressive strategies used by tumor cells. |
Rationale for immunotherapy in NSCLC
Immunotherapeutic approaches have been effective in some cancers. Both sipuleucel-T (Provenge™, Dendreon Corporation, Seattle, WA, USA) and ipilimumab (Yervoy™, Bristol-Myers Squibb, Princeton, NJ, USA) were approved based on significantly improved patient survival versus control treatment in clinical trials.24–27 Sipuleucel-T, approved for asymptomatic or minimally symptomatic metastatic castrate-resistant (hormone-refractory) prostate cancer, is a vaccination approach in which a patient’s own immune cells are removed, primed specifically against a prostate cancer antigen, and reintroduced into the patient’s body.26,27 Ipilimumab, approved in the US for the treatment of unresectable or metastatic melanoma, is a human monoclonal antibody that directly modulates the immune system by preventing CTLA-4 from binding its ligands.24,25
Historically, NSCLC has not been considered sensitive to immune-based therapies; however, data show lung tumors are recognized by the immune system, and a more robust antitumor immune response is associated with better survival. In clinical studies, higher numbers of tumor-infiltrating CD4+ T-cells, CD8+ T-cells, natural killer cells, and/or dendritic cells have been associated with improved patient survival.28–33 Nevertheless, the poor outcomes in NSCLC indicate that in many cases, the immune system is ultimately unable to completely destroy cancerous cells. This may be due to an immunosuppressive environment created by lung tumors, that hinders the ability of the immune system to eliminate NSCLC.20,34–39 The observation that the immune system can recognize and respond to lung tumors, but is countered by immunosuppressive effects of the tumor, suggests that immunotherapies could work by harnessing the natural ability of immune cells to recognize and respond to lung cancer cells.
Immunotherapeutic approaches for NSCLC
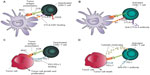
Standard therapies for NSCLC act directly on cancer cells to inhibit tumor growth or cause tumor cell death. The mechanisms of action for chemotherapeutic agents include interrupting proper DNA synthesis, replication, and repair, or inhibiting normal cell division.5,40,41 Erlotinib, afatinib, and crizotinib target proteins (the EGF receptor or ALK) that are aberrantly expressed or mutated in subsets of NSCLC cancers, predominantly in nonsquamous subtypes.42–44 These proteins confer a survival and growth advantage to the tumor; the targeted agents work to inactivate them and reduce the aggressiveness of the tumor.4,44,45 Bevacizumab is thought to restrict tumor cell growth by inhibiting angiogenesis, thereby limiting the tumor’s blood supply; it may also enhance delivery of chemotherapeutic drugs to the tumor (Figure 2).46
In contrast, immunotherapy acts on the patient’s own immune system, not the tumor. The therapeutic goals of immunotherapy are to modulate the immune system to recognize and attack the tumor. Strategies to actively enhance the immune response against NSCLC include vaccination to stimulate antibody and T-cell responses to cancer cells and use of immune checkpoint inhibitors to restart T-cell immune responses to NSCLC cells.
Vaccination strategies
Many vaccines for NSCLC have reached the Phase II or III trial stage of development, the majority of which pair cancer-associated antigen(s) with an immune-activating compound.
Melanoma-associated antigen A3
Melanoma-associated antigen A3 (MAGE-A3) is a tumor-associated antigen (ie, expressed on the surface of certain cancer cell types, but not normal cells), and is thus a good candidate for a vaccine.47 In a study of patients with stage 1 or 2 NSCLC, MAGE-A3 was expressed by 39% of tumors.47 A randomized, placebo-controlled Phase II trial tested a MAGE-A3 vaccine in patients with completely resected MAGE-A3-positive stage 1b–2 NSCLC.48 After a median of 44 months, disease recurrence was slightly reduced in patients receiving the vaccine versus placebo (35% versus 43%), but there were no significant differences in disease-free interval or survival between the groups.48 An ongoing Phase III trial in patients with completely resected MAGE-A3-positive stage 1b–3a NSCLC is evaluating disease-free survival after MAGE-A3 vaccination.49
Mucinous glycoprotein-1
Mucinous glycoprotein-1 (MUC1) is another tumor-associated antigen that is commonly expressed in NSCLC, and often aberrantly expressed or glycosylated.50 Two MUC1 vaccines, L-BLP25 and TG4010, have shown evidence of activity in clinical trials, and are being pursued in Phase III trials.
L-BLP25 (tecemotide, Stimuvax®) uses the BLP25 lipopeptide of MUC1 and an adjuvant in a liposomal delivery system.50,51 In a Phase IIb trial in patients with stage 3b or 4 NSCLC who were stable or had responded to first-line chemotherapy, L-BLP25 following a low dose of cyclophosphamide was associated with improved median and 2-year survival, particularly in the subset of patients with stage 3b locoregional disease.51 Two trials will evaluate overall survival in patients with nonresectable stage 3 NSCLC receiving best supportive care with L-BLP25 versus placebo, following a low dose of cyclophosphamide.52,53
TG4010 contains a genetically modified virus that expresses both MUC1 and interleukin-2, a cytokine which activates T-cells and natural killer cells.54 TG4010 was evaluated in patients with stage 3b or 4 MUC1-positive NSCLC in conjunction with chemotherapy (cisplatin plus gemcitabine) in a Phase IIb study.54 Six-month progression-free survival was higher with TG4010 versus chemotherapy alone (43% versus 35%). A Phase IIb/III trial of TG4010 (versus placebo) with first-line therapy in patients with stage 4 NSCLC is currently recruiting patients. Progression-free survival and overall survival will be evaluated.55
Belagenpumatucel-L
Belagenpumatucel-L (Lucanix™, NovaRx Corporation, San Diego, CA, USA) is a cellular vaccine composed of four types of NSCLC cell lines (two adenocarcinomas, one squamous carcinoma, and one large-cell carcinoma) that have reduced transforming growth factor-beta production as a result of genetic modification.56 Three different doses of belagenpumatucel-L using the same vaccination schedule were tested in patients with stage 2–4 NSCLC after prior chemotherapy.56 A dose-dependent survival advantage was seen, in that the estimated median survival time for patients receiving the two higher doses (combined) was significantly higher than for patients receiving the lowest dose (581 days versus 252 days; P=0.0186). The investigators reported that no significant adverse events were observed. A Phase III trial of the vaccine versus placebo in patients with advanced NSCLC who had previously received chemotherapy completed enrollment in 2012, but has not yet reported results.57
Epidermal growth factor
The EGF receptor is overexpressed in many tumor types, including NSCLC, and signaling through this receptor is associated with cell proliferation, decreased cell death, cell migration, and angiogenesis.4,58 A vaccine (CimaVax-EGF) was designed to elicit an antibody response against EGF, an important ligand for the EGF receptor, in order to reduce EGF receptor signaling and limit tumor growth.58 CimaVax-EGF was tested in patients with advanced NSCLC who had finished first-line therapy, and showed significant survival improvement in patients <60 years of age as compared with patients who did not receive the vaccine (median survival 11.6 months versus 5.3 months, P=0.0124). The vaccine was well tolerated, with no grade 3 or 4 adverse events reported.58 A Phase III trial is currently being conducted in the UK, with an estimated completion date in 2015.59 No US trials are ongoing.
Immune checkpoint inhibitors
Whereas vaccines are designed to stimulate tumor antigen-specific immune responses, immune checkpoint inhibitors, in theory, should “remove the brakes” on most T-cell-mediated immune responses. This mechanism of action has the risk of inducing immune-related reactions. In this section, the current data on the activity of immune checkpoint inhibitors in NSCLC are reviewed, followed by separate sections on the incidence and management of select adverse events (adverse events with potential immunologic etiologies that require more frequent monitoring and/or unique intervention) observed with these therapies.
Anti-CTLA-4
Ipilimumab is a fully human IgG1 monoclonal antibody that binds to CTLA-4 and prevents cytotoxic T-cell downregulation at early stages of T-cell activation. In patients with previously treated metastatic melanoma, ipilimumab therapy can improve survival.25 In the pivotal trial, one-year survival was estimated at 46% of patients versus 22%–38% for patients with similar disease receiving other treatment regimens using historical data.25 Approximately 24% of clinical trial patients with advanced melanoma treated with ipilimumab were alive at 2 years.25 Using real-world data, one-year survival rates for patients with advanced melanoma treated with ipilimumab were estimated to be 49%–60%.60,61
The activity of ipilimumab in combination with paclitaxel and carboplatin was evaluated in patients with chemotherapy-naïve advanced (stage 3b or 4) NSCLC.62 Patients were randomized to a concurrent ipilimumab regimen (four doses of ipilimumab plus paclitaxel and carboplatin followed by two doses of placebo plus paclitaxel and carboplatin), a phased ipilimumab regimen (two doses of placebo plus paclitaxel and carboplatin followed by four doses of ipilimumab plus paclitaxel and carboplatin), or a control regimen (up to six doses of placebo plus paclitaxel and carboplatin). Ipilimumab or placebo, paclitaxel, and carboplatin were administered intravenously once every 3 weeks for a maximum of 18 weeks. Patients without progression received maintenance treatment with either ipilimumab (ipilimumab arms) or placebo (control arm) once every 12 weeks until progression, death, or intolerance.
Antitumor responses were highest in the phased ipilimumab group (Table 1).62 Although the numbers were small in the trial, subset analyses suggested phased ipilimumab significantly improved the activity of chemotherapy in patients with squamous histology and no significant benefit for patients with nonsquamous histology.62 Based on the promising results, a Phase III study of phased ipilimumab with chemotherapy has been initiated in patients with stage 4/recurrent squamous NSCLC.63
  | Table 1 Clinical results of ipilimumab in combination with chemotherapy in patients with chemotherapy-naïve advanced (stage 3b or 4) NSCLC |
Anti-PD-1
Blocking the PD-1 pathway is thought to modulate the activity of T-cells that have become nonresponsive after encountering PD-1 ligands in the tumor microenvironment (Figure 3).17,64 There are currently two anti-PD-1 agents in advanced stages of development: nivolumab and MK-3475.
Nivolumab
Nivolumab (BMS-936558), a fully human IgG4 PD-1 immune checkpoint inhibitor, is currently being evaluated in two Phase III trials for NSCLC after promising Phase I data. In the initial trial, nivolumab monotherapy was administered to patients with several tumor types, including 129 patients with NSCLC, nearly all of whom had received prior treatment.12,65 Patients received one of five different nivolumab doses, ranging from 0.1 mg/kg to 10.0 mg/kg every 2 weeks for up to 12 8-week cycles. In NSCLC patients, objective responses were observed in evaluable patients across doses of 1–10 mg/kg and across histologic subtypes in the initial analysis (Table 2). With extended treatment, the median response duration for the 3 mg/kg dose had not been reached at the time of analysis (range 16.1+ weeks to 133.9+ weeks). Median overall survival across all dose cohorts was 9.2 months for patients with squamous NSCLC and 10.1 months for those with nonsquamous NSCLC. At the 3 mg/kg dose, the median overall survival was 14.9 months (9.5 months for squamous NSCLC; 18.2 months for nonsquamous NSCLC), and this dose has been selected for Phase III trials. Data on overall survival rates are limited. Overall survival for NSCLC patients at one and 2 years was 42% and 24%, respectively. One-year survival was 39% for patients with squamous NSCLC and 43% for patients with nonsquamous NSCLC across all doses.65
  | Table 2 Initial Phase I efficacy results for nivolumab monotherapy in evaluable patients with NSCLC |
The tolerability and activity seen with nivolumab in previously-treated NSCLC patients, including 54% of patients with three prior lines of therapy, was notable and served as the basis for pursuing Phase III trials. The trials will evaluate efficacy (response rate and overall survival) of nivolumab versus docetaxel as a second-line therapy in patients with previously treated advanced stage or metastatic squamous66 or nonsquamous NSCLC.67 Additional Phase II studies are also ongoing, with objective response rate as their primary endpoint, testing nivolumab monotherapy as third-line treatment in patients with advanced or metastatic squamous NSCLC,68 nivolumab plus ipilimumab in advanced or metastatic solid tumors, including NSCLC,69 and nivolumab following azacitidine and entinostat versus oral azacitidine in patients with recurrent metastatic NSCLC.70 A Phase I trial is testing nivolumab as monotherapy, maintenance therapy, or in combination with chemotherapy or targeted agents (erlotinib and bevacizumab), or with ipilimumab in patients with stage 3b/4 NSCLC.71
MK-3475
MK-3475 is a humanized IgG4 monoclonal antibody that binds PD-1. A Phase I dose-finding study of MK-3475 showed a high response rate and durable responses in patients with advanced melanoma.72 In patients with NSCLC who were previously treated with two systemic regimens, MK-3475 was administered at 10 mg/kg every 3 weeks. In an interim analysis of 38 patients, the objective response rate was 21%, and most responses had occurred by 9 weeks.73 A Phase II/III study comparing MK-3475 and docetaxel has been initiated in patients with NSCLC that has progressed after platinum-containing therapy. The trial is evaluating a low and high dose of MK-3475, with overall survival, progression-free survival, and safety as the primary endpoints.74
Anti-PD-L1
Anti-PD-L1 therapies also target the PD-1 pathway by binding one of its ligands, ie, PD-L1.
BMS-936559
BMS-936559 is a high-affinity, fully human, PD-L1-specific monoclonal antibody which showed activity against advanced NSCLC in a Phase I clinical trial that included multiple advanced tumor types.75 Five of 49 evaluable NSCLC patients had an objective response; response duration ranged from 2.3+ months to 16.6+ months. Six of 49 patients had stable disease lasting ≥24 weeks, and 31% of patients had progression-free survival at 24 weeks.
MPDL3280A
Another anti-PDL1 antibody is MPDL3280A, which is a human monoclonal antibody containing an altered nonbinding domain.76 A Phase I dose escalation/expansion study in multiple solid tumor types is being conducted to evaluate dose-limiting toxicities. In an interim analysis of 40 NSCLC patients who had received doses of 1–20 mg/kg, the response rate was 23%, and all responses were ongoing or improving at data cutoff (range 1+ to 214+ days). The rate of progression-free survival at 24 weeks was 46%.76 Two Phase II studies with MPDL3280A are ongoing. One trial is monitoring objective responses and safety in patients with PD-L1-positive locally advanced or metastatic NSCLC receiving MPDL3280A monotherapy.77 The other study is evaluating response rates and safety of MPDL3280A compared with docetaxel in patients with advanced or metastatic NSCLC in whom platinum therapy has failed.78
Immunotherapy combinations
Given the many agents with nonredundant mechanisms of action, immunotherapy combinations are being explored in cancer trials in the hope of superior efficacy over single agents. Preliminary evidence in advanced melanoma suggests that this might indeed be possible. A combination of ipilimumab and nivolumab in advanced melanoma showed that 53% of patients had an objective response, and these patients all had a reduction in tumor volume of 80% or more. In total, 65% of patients had evidence of clinical activity.13
Trials of ipilimumab and nivolumab are now underway in NSCLC, including the one in patients with stage 3b/4 NSCLC mentioned above.79 Two early (Phase I) trials in advanced tumors, including NSCLC, are evaluating nivolumab in combination with recombinant interleukin-21,80 an immune-stimulating cytokine that has shown clinical activity in melanoma and renal cell carcinoma, and in combination with lirilumab (a natural killer cell-activating antibody).81 Several approaches combining checkpoint inhibitors and vaccines are being investigated in numerous cancer types, but so far none are exclusively in NSCLC.82
Immune-mediated responses and treatment decisions
As compared with chemotherapy, unconventional treatment responses have been observed with immunotherapy. These are also likely due to the immune mechanism of action, and can be associated with improved long-term patient outcomes.83 In addition to shrinkage of baseline lesions in the absence of new lesions, the following immune-related response patterns have been described:
- durable stable disease (in some patients followed by a slow, steady decline in total tumor burden)
- reduced tumor burden after an increase in total tumor burden
- overall reduced tumor burden in the presence of new lesions.
Increased tumor volume followed by tumor shrinkage has been called pseudoprogression. The increased tumor size may be caused by higher numbers of immune cells infiltrating the tumor. Also, as compared with chemotherapy, delayed responses are not uncommon, and likely due to the time it takes to generate effective antitumor immune responses.25,84 The health care provider will need to consider these potential response patterns in patients on immunotherapy when assessing the effectiveness of therapy and making treatment decisions.
Safety profile of immunotherapy
Immunotherapies stimulate the immune system with the goal of eradicating tumors, and some side effects are likely related to this immunologic mechanism of action. Inhibiting the CTLA-4 and PD-1 pathways will lead to activation of T-cells specific to tumor antigens and potentially to self antigens.11 As such, certain adverse events in patients receiving checkpoint inhibitors differ from chemotherapy-related adverse events. These events are termed select adverse events, and may require more frequent monitoring and/or unique intervention.
The most common types of select adverse events reported with checkpoint pathway inhibitors are listed in Table 3,12,13,25,62,65,72,73,75,76,85,86 and include gastrointestinal, dermatologic, and endocrine side effects. In clinical studies, select adverse events were reported in approximately 40%–60% of patients, including 3%–20% patients with grades 3 or 4. Select adverse events were reversible in most cases; however, some adverse event-related deaths occurred.12,25,62,65,72,73,75 Differences in the incidence and type of grade 3–4 select adverse events across the different immunotherapy drugs were observed, and newer agents under investigation appear to have a better overall safety profile than ipilimumab. However, additional data are needed to confirm this observation.
  | Table 3 Examples of select adverse events reported with immune checkpoint inhibitors12,13,25,62,65,72,73,75,76,85,86 |
Overall, dermatologic select adverse events were the most frequently reported (12%–44%), followed by gastrointestinal events (9%–32%).12,25,65,73 Infusion-related reactions (3%–10%), endocrine disorders, including hypothyroidism, adrenal insufficiency, and hypophysitis (2%–8%), and pneumonitis (3%–6%) were also reported,12,25,65,72,75 as were isolated cases of uveitis (Table 3).13,75 In a pilot study of combination therapy with both ipilimumab and nivolumab in melanoma, select adverse events occurred at a higher frequency, with 53% of patients experiencing a grade 3–4 event.13 These select adverse events were generally reversible, although three patients (9%) discontinued therapy owing to treatment-related adverse events. No treatment-related deaths were reported in the study.13
Management of select adverse events
Onset of immune-mediated side effects is variable and appears to depend on the organ system affected, although many occur during the first treatment cycles. Some, however, occur after a patient has been treated with several cycles. This is in keeping with the immune mechanism of action, as the time it takes to recognize antigens and build a response is inconsistent and may be prolonged. Also, delayed select adverse events can develop after a patient has completed or discontinued treatment, as the immune responses may persist. Thus, it is imperative for clinicians to continue to monitor for potential side effects.
Although most select adverse events with immunotherapies are of low grade, select adverse events can occur with rapid onset, and prompt medical attention is critical to the management of immune-related events.25,87 This requires a joint effort between health care providers, patients, and caregivers. Key strategies for successful management of select adverse events include the following:
- early detection and continued screening for potential select adverse events
- utilization of treatment algorithms for immune-mediated diarrhea/colitis, pneumonitis, and endocrine disorders (hypothyroidism, hypophysitis, and adrenal insufficiency)
- ongoing education of staff nurses and support staff regarding the unique side effect profile
- continual education of family members and caregivers to the unique side effect profile and encouragement of early notification of side effects
- referral to specialists, ie, an endocrinologist, pulmonologist, and ophthalmologist.
Health care providers, patients, and caregivers should have a low threshold for acting on gastrointestinal symptoms for ipilimumab-treated patients, because the pathology and treatment are different than for chemotherapy-associated diarrhea.86 Similarly, any exacerbation in pulmonary symptoms, even in patients with lung cancer, should be evaluated in patients who have received nivolumab, MK-3475, or other immunotherapy. The evidence suggests that progression to severe events may be avoided through proactive monitoring and early and sustained intervention.86,87 Any potential select adverse event should be treated with a high level of concern.
Clinical trial protocols and reports, and the ipilimumab prescribing information offer guidelines for the management of select adverse events that occur in patients receiving immune checkpoint inhibitors, although it should be noted that, at present, only ipilimumab has an approved anticancer indication (advanced melanoma).12,13,24,25,72,75,86,87 These guidelines include:
- evaluation/monitoring of thyroid and liver function tests prior to each ipilimumab dose
- supportive antidiarrheals for grade 1 diarrhea
- administration of corticosteroids if diarrhea progresses to grade 2
- systemic high-dose corticosteroid treatment for severe, persistent, or recurring select adverse events; steroid taper is usually protracted to prevent rebound recurrence of select adverse events
- a delay in a scheduled dose for moderate select adverse events until complete recovery, or discontinuation of therapy for severe reactions
- use of replacement therapy for endocrine disorders, specifically thyroid replacement and steroid replacement.
Investigators in clinical trials used these strategies for effective management of select adverse events in most cases. In nivolumab trials, hepatic or gastrointestinal adverse events were managed with treatment interruption and, as necessary, with the administration of glucocorticoids. Also, early-grade pneumonitis in six nivolumab-treated patients was reversible with treatment discontinuation, glucocorticoid administration, or both. In three patients with pneumonitis, infliximab, mycophenolate, or both were used for additional immunosuppression, although the efficacy of this strategy was difficult to evaluate.12 Glucocorticoid administration for select adverse events is usually continued on a slow taper following recovery to reduce recurrence. For pneumonitis, diarrhea or colitis, or adrenal insufficiency of more than grade 2, patients may require hospitalization. Referral to specialists (eg, endocrinologist, ophthalmologist) is recommended for the management of select adverse events and ongoing management of patients on immunotherapy, as needed. In clinical trials, at the discretion of the treating physician, treatment with immunotherapy was typically reinitiated once the adverse event had been successfully managed.12,75,87
Role of the advanced practice registered nurse
It is essential to educate all members of the health care team regarding the introduction of new treatment regimens. The advance practice nurse is involved in the coordination of these education efforts. In our center, education sessions for physicians, advanced practice registered nurses, research nurses, and pharmacists are scheduled prior to the initiation of all new clinical trials to provide indepth education regarding the new therapy. Symptom management algorithms are reviewed during the new therapy planning sessions. Thereafter, the team works closely with the nursing leadership to provide education for the nursing staff, including the importance of frequent symptom monitoring. The advanced practice registered nurse is ideally placed to monitor the patient for physical side effects and to order appropriate diagnostic evaluations for suspected select adverse events. The advanced practice registered nurse is also involved in coordinating the consult services needed for the management of select adverse events.
The successful treatment of patients on immunotherapy depends on the diligence of ongoing assessment/identification and management of immune-mediated side effects. Unmanaged select adverse events can mean the discontinuation of potentially beneficial therapy, and in some cases, can be fatal.
Conclusion
Immunotherapies represent a novel approach to the treatment of NSCLC, and offer the potential for extended benefits, even in advanced disease. Unlike targeted agents, the efficacy of immunotherapy is not limited to patients with particular mutations, and may have broad applicability. As immunotherapies have novel antitumor effects, such as pseudoprogression, repeat biopsies and/or confirmatory scans may be necessary to inform treatment decisions. Also, unlike chemotherapy or targeted therapy, health care providers need to consider select adverse events for patients receiving immunotherapy. Although select adverse events may not be a familiar concept in oncology clinics, most select adverse events can be managed with awareness and action, and immunotherapy may be maintained or reinitiated following resolution of immune-related adverse events. To ensure that these therapies can be used to their fullest benefit, health care providers should be aware of the unique response patterns and immune-mediated side effects of these agents so that they can make appropriate treatment decisions. Nurses will play a key role as immunotherapies become more common in the clinic, both in treating patients and in the education of patients, family members, and other health care providers.
Disclosure
The author takes full responsibility for the content of this publication and confirms that it reflects her viewpoint and medical expertise. The author also wishes to acknowledge StemScientific, funded by Bristol-Myers Squibb, for writing and editorial support. Neither Bristol-Myers Squibb nor StemScientific influenced the content of the manuscript, nor did the author receive financial compensation for authoring the manuscript.
References
American Cancer Society. Cancer facts and figures 2013. Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfacts figures2013/index. Updated 2013. Accessed October 8, 2013. | |
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. | |
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-small cell lung cancer. Version 2. 2013. Available from: http://www.nccn.com. Updated 2013. Accessed October 8, 2013. | |
Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J Clin Oncol. 2005;23(14):3227–3234. | |
Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Pérez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem. 2007;7(1):3–18. | |
Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. | |
Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. | |
Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346. | |
Thunnissen E, Bubendorf L, Dietel M, et al. EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch. 2012;461(3):245–257. | |
Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16(1):5–24. | |
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. | |
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. | |
Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. | |
Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. | |
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. | |
Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. | |
Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. | |
Baba T, Hanagiri T, Ichiki Y, et al. Lack and restoration of sensitivity of lung cancer cells to cellular attack with special reference to expression of human leukocyte antigen class I and/or major histocompatibility complex class I chain related molecules A/B. Cancer Sci. 2007;98(11):1795–1802. | |
Fukuyama T, Ichiki Y, Yamada S, et al. Cytokine production of lung cancer cell lines: Correlation between their production and the inflammatory/ immunological responses both in vivo and in vitro. Cancer Sci. 2007;98(7):1048–1054. | |
Jadus MR, Natividad J, Mai A, et al. Lung cancer: a classic example of tumor escape and progression while providing opportunities for immunological intervention. Clin Dev Immunol. 2012;2012: 160724. | |
Niehans GA, Brunner T, Frizelle SP, et al. Human lung carcinomas express Fas ligand. Cancer Res. 1997;57(6):1007–1012. | |
Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107(12):2866–2872. | |
Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5(5):585–590. | |
Yervoy® (ipilimumab) [package insert]. Princeton, NJ, USA: Bristol-Myers Squibb Company; 2013. | |
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. | |
Provenge® (ipilimumab) [package insert]. Seattle, WA, USA: Dendreon Corporation; 2011. | |
Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. | |
Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–5227. | |
Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–4417. | |
Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94(2):275–280. | |
Zhuang X, Xia X, Wang C, et al. A high number of CD8+ T cells infiltrated in NSCLC tissues is associated with a favorable prognosis. Appl Immunohistochem Mol Morphol. 2010;18(1):24–28. | |
Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2001;121(6):1058–1063. | |
Villegas FR, Coca S, Villarrubia VG, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35(1):23–28. | |
Dasanu CA, Sethi N, Ahmed N. Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert Opin Biol Ther. 2012;12(7):923–937. | |
Platonova S, Cherfils-Vicini J, Damotte D, et al. Profound coordinated alterations of intratumoral natural killer cell phenotype and function in lung carcinoma. Cancer Res. 2011;71(16):5412–5422. | |
Schneider T, Hoffmann H, Dienemann H, et al. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J Thorac Oncol. 2011;6(7):1162–1168. | |
Schneider T, Kimpfler S, Warth A, et al. Foxp3(+) regulatory T cells and natural killer cells distinctly infiltrate primary tumors and draining lymph nodes in pulmonary adenocarcinoma. J Thorac Oncol. 2011;6(3):432–438. | |
Wang R, Lu M, Zhang J, et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res. 2011;30:62. | |
Zeni E, Mazzetti L, Miotto D, et al. Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J. 2007;30(4):627–632. | |
Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589–1597. | |
Lyseng-Williamson KA, Fenton C. Docetaxel: a review of its use in metastatic breast cancer. Drugs. 2005;65(17):2513–2531. | |
Xalkori® (crizotinib) [package insert]. New York, NY, USA: Pfizer; 2013. | |
Pallis AG, Fennell DA, Szutowicz E, Leighl NB, Greillier L, Dziadziuszko R. Biomarkers of clinical benefit for anti-epidermal growth factor receptor agents in patients with non-small-cell lung cancer. Br J Cancer. 2011;105(1):1–8. | |
Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. | |
Bang YJ. The potential for crizotinib in non-small cell lung cancer: a perspective review. Ther Adv Med Oncol. 2011;3(6):279–291. | |
Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. | |
Sienel W, Varwerk C, Linder A, et al. Melanoma associated antigen (MAGE)-A3 expression in Stages I and II non-small cell lung cancer: results of a multi-center study. Eur J Cardiothorac Surg. 2004;25(1):131–134. | |
Vansteenkiste JF, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol. 2013;43:Abstr 7103. | |
GlaxoSmithKline. GSK1572932A Antigen-Specific Cancer Immunotherapeutic as Adjuvant Therapy in Patients With Non-Small Cell Lung Cancer. Available from: http://clinicaltrials.gov/show/NCT00480025. NLM identifier NCT00480025. Accessed December 11, 2013. | |
Reck M, Vansteenkiste J, Brahmer JR. Targeting the immune system for management of NSCLC: the revival? Curr Respir Care Rep. 2013;2(1):22–39. | |
Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23(27):6674–6681. | |
EMD Serono. Cancer Vaccine Study for Unresectable Stage III Non-small Cell Lung Cancer (START). Available from: http://clinicaltrials.gov/ct2/show/NCT00409188. NLM identifier: NCT00409188. Accessed December 11, 2013. | |
Merck KGaA. Cancer Vaccine Study for Stage III, Unresectable, Non-small Cell Lung Cancer (NSCLC) in the Asian Population (INSPIRE). Available from: http://clinicaltrials.gov/ct2/show/NCT01015443. NLM identifier: NCT01015443. Accessed December 11, 2013. | |
Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12(12):1125–1133. | |
Transgene. Phase IIB/III of TG4010 Immunotherapy In Patients With Stage IV Non-Small Cell Lung Cancer (TIME). Available from: http://clinicaltrials.gov/show/NCT01383148. NLM identifier: NCT01383148. Accessed December 11, 2013. | |
Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24(29):4721–4730. | |
NovaRx Corporation. Phase III Lucanix™ Vaccine Therapy in Advanced Non-small Cell Lung Cancer (NSCLC) Following Front-line Chemotherapy (STOP). Available from: http://clinicaltrials.gov/show/NCT00676507. NLM identifier: NCT00676507. Accessed December 11, 2013. | |
Neninger Vinageras E, de la Torre A, Osorio Rodríguez M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(9):1452–1458. | |
Bioven Europe. A Randomized Trial to Study the Safety and Efficacy of EGF Cancer Vaccination in Late-stage (IIIB/IV) Non-small Cell Lung Cancer Patients (NSCLC). Available from: http://clinicaltrials.gov/show/NCT01444118. NLM identifier: NCT01444118. Accessed December 11, 2013. | |
Margolin KA, Wong SL, Penrod JR, et al. Effectiveness and safety of first-line ipilimumab 3 mg/kg therapy for advanced melanoma: evidence from a US multisite retrospective chart review. Poster 3742 presented at the European Cancer Congress, September 27 to October 1, 2013, Amsterdam, The Netherlands. | |
Patt D, Juday T, Penrod JR, Chen C, Wong SL. A community-based, real-world, study of treatment-naïve advanced melanoma (AM) patients treated with 3 mg/kg ipilimumab (IPI) in the United States. Poster 3751 presented at the European Cancer Congress, September 27 to October 1, 2013, Amsterdam, The Netherlands. | |
Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–2054. | |
Bristol-Myers Squibb. Trial in Squamous Non Small Cell Lung Cancer Subjects Comparing Ipilimumab Plus Paclitaxel and Carboplatin Versus Placebo Plus Paclitaxel and Carboplatin. Available from: http://clinicaltrials.gov/show/NCT01285609. NLM identifier: NCT01285609. Accessed December 11, 2013. | |
Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther. 2013;13(6):847–861. | |
Brahmer JR, Horn L, Antonia SJ, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with non-small cell lung cancer (NSCLC): overall survival and long-term safety in a phase 1 trial. Abstract MO18.03 presented at the 15th International Association for the Study of Lung Cancer World Conference on Lung Cancer, October 27–30, 2013, Sydney, Australia. | |
Bristol-Myers Squibb. Study of BMS-936558 (Nivolumab) Compared to Docetaxel in Previously Treated Advanced or Metastatic Squamous Cell Non-small Cell Lung Cancer (NSCLC) (CheckMate 017). Available from: http://clinicaltrials.gov/show/NCT01642004. NLM identifier: NCT01642004. Accessed December 11, 2013. | |
Bristol-Myers Squibb. Study of BMS-936558 (Nivolumab) Compared to Docetaxel in Previously Treated Metastatic Non-squamous NSCLC (CheckMate 057). Available from: http://clinicaltrials.gov/show/NCT01673867. NLM identifier: NCT01673867. Accessed December 11, 2013. | |
Bristol-Myers Squibb. Study of Nivolumab (BMS-936558) in Subjects With Advanced or Metastatic Squamous Cell Non-Small Cell Lung Cancer Who Have Received At Least Two Prior Systemic Regimens (CheckMate 063). Available from: http://clinicaltrials.gov/show/NCT01721759. NLM identifier: NCT01721759. Accessed December 11, 2013. | |
Bristol-Myers Squibb. A Phase 1/2, Open-label Study of Nivolumab Monotherapy or Nivolumab Combined With Ipilimumab in Subjects With Advanced or Metastatic Solid Tumors. Available from: http://clinicaltrials.gov/show/NCT01928394. NLM identifier: NCT01928394. Accessed December 11, 2013. | |
Sidney Kimmel Comprehensive Cancer Center. Phase II Anti-PD1 Epigenetic Priming Study in NSCLC. (NA_00084192). Available from:http://clinicaltrials.gov/show/NCT01928576. NLM identifier: NCT01928576. Accessed December 11, 2013. | |
Bristol-Myers Squibb. Study of Nivolumab (BMS-936558) in Combination With Gemcitabine/Cisplatin, Pemetrexed/Cisplatin, Carboplatin/Paclitaxel, Bevacizumab Maintenance, Erlotinib, Ipilimumab or as Monotherapy in Subjects With Stage IIIB/IV Non-small Cell Lung Cancer (NSCLC) (CheckMate 012). Available from: http://clinicaltrials.gov/show/NCT01454102. NLM identifier: NCT01454102. Accessed December 11, 2013. | |
Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. | |
Garon EB, Balmanoukian A, Hamid O, et al. Preliminary clinical safety and activity of MK-3475 monotherapy for the treatment of previously treated patients with non-small cell lung cancer (NSCLC). Abstract MO18.02 presented at the 15th International Association for the Study of Lung Cancer World Conference on Lung Cancer, October 27–30, 2013, Sydney, Australia. | |
Merck. Study of Two Doses of MK-3475 Versus Docetaxel in Previously-Treated Participants With Non-Small Cell Lung Cancer (MK-3475-010). Available from: http://clinicaltrials.gov/show/NCT01905657. NLM identifier: NCT01905657. Accessed December 11, 2013. | |
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. | |
Horn L, Herbst RS, Spigel DR, et al. An analysis of the relationship of clinical activity to baseline EGFR status, PD-L1 expression and prior treatment history in patients with non-small cell lung cancer (NSCLC) following PD-L1 blockade with MPDL3280A (anti-PDL1). Abstract MO18.01 presented at the 15th International Association for the Study of Lung Cancer World Conference on Lung Cancer, October 27–30, 2013, Sydney, Australia. | |
Genentech. A Study Of MPDL3280A in Patients With PD-L1-Positive Locally Advanced or Metastatic Non-Small Cell Lung Cancer. Available from: http://clinicaltrials.gov/show/NCT01846416. NLM identifier: NCT01846416. Accessed December 11, 2013. | |
Hoffmann-La Roche. A Study of MPDL3280A Compared With Docetaxel in Patients With Non-Small Cell Lung Cancer After Platinum Failure. Available from: http://clinicaltrials.gov/show/NCT01903993. NLM identifier: NCT01903993. Accessed December 11, 2013. | |
Bristol-Myers Squibb. Study of Nivolumab (BMS-936558) in Combination With Gemcitabine/Cisplatin, Pemetrexed/Cisplatin, Carboplatin/Paclitaxel, Bevacizumab Maintenance, Erlotinib, Ipilimumab or as Monotherapy in Subjects With Stage IIIB/IV Non-small Cell Lung Cancer (NSCLC) (CheckMate 012). Available from: http://clinicaltrials.gov/show/NCT01454102. NLM identifier: NCT01454102. Accessed December 11, 2013. | |
Bristol-Myers Squibb. Safety Study of IL-21/Anti-PD-1 Combination in the Treatment of Solid Tumors. Available from: http://clinicaltrials.gov/show/NCT01629758. NLM identifier: NCT01629758. Accessed December 11, 2013. | |
Bristol-Myers Squibb. A Phase I Study of an Anti-KIR Antibody in Combination With an Anti-PD1 Antibody in Patients With Advanced Solid Tumors. Available from: http://clinicaltrials.gov/show/NCT01714739. NLM identifier: NCT01714739. Accessed December 11, 2013. | |
ClinicalTrials.gov. [homepage on the Internet]. US National Institutes of Health. Updated 2013. Available from: http://clinicaltrials.gov/. Accessed October 8, 2013. | |
Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. | |
Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18(7):2039–2047. | |
Herbst RS, Gordon MS, Fine GD, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol. 2013;31 Suppl:Abstr 3000. | |
Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. | |
Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12(7):864–872. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.