Back to Journals » Drug Design, Development and Therapy » Volume 15
New Insights of Anti-Hyperglycemic Agents and Traditional Chinese Medicine on Gut Microbiota in Type 2 Diabetes
Received 14 August 2021
Accepted for publication 19 November 2021
Published 30 November 2021 Volume 2021:15 Pages 4849—4863
DOI https://doi.org/10.2147/DDDT.S334325
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Anastasios Lymperopoulos
Yanxia Chen, Mian Wang
Department of Endocrinology, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050051, People’s Republic of China
Correspondence: Mian Wang
Department of Endocrinology, The Second Hospital of Hebei Medical University, 215 Hepingxi Road, Shijiazhuang, Hebei, 050051, People’s Republic of China
Email [email protected]
Abstract: Type 2 diabetes mellitus (T2DM) is a widespread metabolic disease characterized by chronic hyperglycemia. Human microbiota, which is regarded as a “hidden organ”, plays an important role in the initiation and development of T2DM. In addition, anti-hyperglycemic agents and traditional Chinese medicine may affect the composition of gut microbiota and consequently improve glucose metabolism. However, the relationship between gut microbiota, T2DM and anti-hyperglycemic agents or traditional Chinese medicine is poorly understood. In this review, we summarized pre-clinical and clinical studies to elucidate the possible underlying mechanism. Some anti-hyperglycemic agents and traditional Chinese medicine may partly exert hypoglycemic effects by altering the gut microbiota composition in ways that reduce metabolic endotoxemia, maintain the integrity of intestinal mucosal barrier, promote the production of short-chain fatty acids (SCFAs), decrease trimethylamine-N-oxide (TMAO) and regulate bile acid metabolism. In conclusion, gut microbiota may provide some new therapeutic targets for treatment of patients with diabetes mellitus.
Keywords: gut microbiota, anti-hyperglycemic agents, traditional Chinese medicine, type 2 diabetes mellitus
Introduction
Diabetes mellitus (DM), which is contributed to genetic and/or environmental factors, is the most prevalent metabolic disease worldwide characterized by chronic hyperglycemia. Type 2 diabetes mellitus (T2DM), which results from insulin resistance and/or impaired insulin secretion, accounts for more than 90% of patients with DM. Long-term hyperglycemia can result in detrimental micro- and macrovascular complications, involving many organs and systems, especially kidneys, nervous system, eyes and blood vessel.1
Tens of trillions of microorganisms indigenously colonize the gastrointestinal tract immediately after birth, collectively termed as gut microbiota. Gut microbiota is a highly complex and dynamic microbial ecosystem and may be regarded as a “hidden organ”. Human microbiota, which is considered as a metabolic organ, can perform diverse physiological functions to maintain homeostasis in health.2 It is estimated that the number of microbial genes approximately exceeds 100-fold higher than that of human genome. The gut microbiota, also considered as the “second genome” of the host, provides humans with additional biological and metabolic functions that cannot be performed by the host.3 The composition of the gut microbiota is influenced by several variables including diet, sex, age, lifestyle, environment, geographical location and genetic background.4 The gut microbiota is dominated by four phyla: Firmicutes (Gram-positive, 60–65%), Bacteroidetes (Gram-negative, 20–25%), Proteobacteria (Gram-negative, 5–10%), Actinobacteria (Gram-positive, 3%).5 Growing evidences suggest that the perturbation of normal intestinal microbiota composition, also termed as dysbiosis, may break the homeostasis and result in a variety of common metabolic diseases, including diabetes and diabetic complications.6,7
Over the last few decades, a myriad of original research indicates that there is an association between gut microbiota and T2DM. In previous years, some reviews published have reported the interaction between anti-hyperglycemic agents and gut microbiota.8,9 The association between human microbiome and traditional Chinese medicine on healthcare and cancer treatment has gained attention in recent years,10,11 but reviews on the association between antidiabetic effect of the traditional Chinese medicine and gut microbiota are insufficient. Accordingly, we summarized the latest literatures and explored the potential mechanisms of how anti-hyperglycemic agents and traditional Chinese medicine influenced the gut microbiota, which might provide some new therapeutic targets of T2DM.
Gut Microbiota and T2DM
Increasing clinical and animal researches have revealed the important role of gut microbiota dysbiosis in the initiation and development of T2DM.7,12 Given that hyperglycemia also affect bacterial composition,13,14 the gut microbiota and diabetes have a reciprocally regulatory relationship.15
Metabolic Endotoxemia
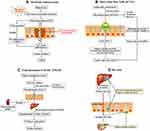
Several studies have determined that the gut microbiota dysbiosis leads to insulin resistance and T2DM through several mechanisms including lipopolysaccharide (LPS)-mediated metabolic endotoxemia (Figure 1A). LPS, which is a major component of the outer membrane of the Gram-negative bacteria, contributes to development of low-grade chronic inflammation known as metabolic endotoxemia.16 High-fat diet (HFD) may increase LPS-enriched intestinal microbiota and consequently elevate the plasma concentration of LPS.17 In the intestinal tract, LPS triggers the dysfunction of intestinal barrier and increases gut permeability. Excessive LPS enters the blood and translocates across the damaged intestinal barrier, leading to the low-grade inflammatory injury of intestinal epithelium.18 A great deal of researches have determined that the low-grade inflammation is associated with insulin resistance and T2DM.19 A typical LPS molecule is composed of three different constituents: a highly variable O-antigen constituted of repeating oligosaccharide units, a core oligosaccharide and lipid A.20 Lipid A is one of the core structures of bacterial LPS and is responsible for much of LPS’s toxicity. Lipid A, which is an amphipathic glycolipid domain, triggers strong immune and inflammatory responses by tightly binding to Toll-like receptors (TLRs).21 Most TLRs coordinate a series of proinflammatory signaling cascades through the Myeloid differentiation primary response 88 (MyD88)-dependent pathway.22–24 The activated MyD88 then stimulates the pro-inflammatory cytokines expression and secretion to induce the low-grade inflammatory, such as IL-1 receptor-associated kinase (IRAK), TNF receptor-associated factor (TRAF6), transforming growth factor B-associated kinase 1 (TAK1), Monocyte Chemoattractant Protein-1 (MCP-1), TNF-α and JNK and IKK complexes. JNK and IKK complexes, which can induce serine phosphorylation of insulin receptor substrate (IRS), inhibit insulin signaling and result in insulin resistance.25
Gut Permeability
An amount of investigation shows that the intestinal mucosal barrier, which can prevent bacteria and toxins from reaching the circulation, plays an important role in maintaining intestinal homeostasis. The intestinal mucosal barrier is composed of the intestinal epithelium cells (IECs), the tight junction proteins between IECs and their protective mucous layer.26 Under the influence of HFD, inflammatory stimulation and other factors, the intestinal barrier would be destroyed and the intestinal mucosa permeability would be enhanced, which in turn increases excessive inflammatory cytokine and metabolic endotoxemia.27 Thereby, the disruption of the intestinal barrier or the imbalance of gut microbiota would aggravate the progress of diabetes and its complications.28 Intestinal permeability is usually regulated by tight junction proteins, such as zonula occludens-1 (ZO-1), occludin, and claudin-1. Recently, prebiotic or exogenous glucagon-like peptide-2 (GLP-2) treatment significantly improves tight junctions and reduces gut permeability by increasing intestinal GLP-2 production in obese and diabetic mice.29,30 GLP-2 is a 33-amino acid peptide derived from proteolytic cleavage of proglucagon in enteroendocrine L cells. Moreover, blockade of endocannabinoid (eCB) system could improve the gut barrier and reduce metabolic endotoxemia through increasing tight junction proteins.31
Short-Chain Fatty Acids (SCFAs)
Gut microbiota is able to hydrolyze and ferment the indigestible carbohydrates to yield important microbial products, such as SCFAs, trimethylamine N-oxide (TMAO), amino acids and bile acids (BAs).32 These metabolites, particularly SCFAs, are involved in the regulation of glucose and energy metabolism (Figure 1B). SCFAs, such as acetate, propionate and butyrate, have considerable effects on regulating pancreatic β cell proliferation, insulin biosynthesis,33 maintaining gut epithelial barrier function,34 anti-inflammatory functions and so on. The principal receptors of gut microbiota-derived SCFAs are G-protein coupled receptors 43 and 41 (GPR43/41), which are expressed in the islets of Langerhans, enteroendocrine and intestinal epithelial cells.33 Activation of G protein-coupled receptors by SCFAs stimulates the production of glucagon-like peptide-1 (GLP-1) and the intestinal peptide YY (PYY).35 GLP-1 and PYY are both secreted from the enteroendocrine L-cells, predominantly distributed in the ileum and colon.36 GLP-1 regulates glucose, lipid and energy metabolism by binding to GLP-1 receptor, including stimulating glucose-dependent insulin secretion from pancreatic β cells, reducing glucagon secretion from pancreatic α cells,37 inhibiting the production of lipid proteins,38 slowing down gastric emptying,39 increasing energy expenditure and losing body weight.40 PYY participates in suppressing appetite and slowing gastric emptying.41 Butyrate, the main energy source of the colonic epithelium cell, can protect intestinal barrier through increasing tight junction protein expression (such as ZO-1 and occludin) and reducing intestinal permeability.42
Trimethylamine-N-Oxide (TMAO)
High consumption of meats contributes to undesirable health outcomes through the choline/carnitine-TMAO-pathway (Figure 1C). Choline, which is a water-soluble nutrient, is essential for a wide range of biological activities. Evidences suggest that gut microbiota can convert beneficial dietary choline into the production that are detrimental to human health.43 Choline is metabolized to produce a precursor trimethylamine (TMA) by gut microbiota, primarily the Firmicutes and Proteobacteria phyla.44 TMA is a gas that readily passes the intestinal wall. After being absorbed, TMA is oxidized to form TMAO by flavin monooxygenase 3 (FMO3) in live.45 Choline may escape microbial degradation and be converted into betaine and further metabolites (eg, dimethylglycine (DMG)) which have detrimental osmotic effects by mammalian mitochondrial pathways in kidney.46 High circulating level of TMAO can promote insulin resistance by forming N-Nitroso compounds and is a potent novel risk factor of T2DM.47 In addition, elevated circulating TMAO level independently predicts an increased risk of cardiovascular disease in clinical study.48–50 A study showed that TMAO could inhibit hepatic bile acid synthesis via activating the nuclear farnesoid X receptors (FXR) and small heterodimer partner (SHP) to accelerate aortic lesion formation in apoE−/− mice.51 Another study showed that TMAO induced oxidative stress, inflammation and endothelial dysfunction via activating ROS-TXNIP-NLRP3 inflammasome in human umbilical vein endothelial cells, which was involved in development of atherosclerosis.52 Furthermore, another study suggested that TMAO may induce synaptic plasticity deficits by promoting endoplasmic reticulum stress-mediated PERK signaling pathway.53 TMAO may be identified a potential new therapeutic target for diabetes and other metabolic disorders.
Bile Acids (BAs)
BAs, as one of the gut microbiota producing metabolites, also participate in lipid and glucose metabolism and are directly or indirectly related to insulin resistance (Figure 1D).54 Cumulative data demonstrate that manipulation of BAs via regulating the composition of gut microbiota could help glycemic control and prevent metabolic memory for early-onset T2DM subjects.55 Primary bile acids (eg, chenodeoxycholic acid (CDCA) and cholic acid (CA)) are synthesized from cholesterol in hepatocytes and stored in the gallbladder. After a meal, bile acids are excreted into the duodenum to facilitate the absorption of dietary lipids. After they complete their job, most bile acids (more than 95%) are efficiently reabsorbed in terminal ileum and recirculated to the liver via enterohepatic circulation. The remaining 5% are excreted via feces.56 Secondary bile acids (eg, deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA)) are derived from primary bile acids, mainly by the biosynthesis of few gut microbes. Both primary bile acids and their respective secondary bile acids act as key pleiotropic signaling mediators in glucose metabolism through activating FXR and/or the membrane Takeda G-protein-coupled receptor 5 (TGR5).57 FXR is abundantly expressed in liver and intestine.58 The relative binding affinity of primary and secondary bile acids to FXR is variable (CDCA > DCA > LCA > CA > UDCA).59,60 TGR5 is highly expressed in liver, gastrointestinal tract, immune cells, gallbladder and pancreas.61 Like FXR, its relative binding affinity to primary and secondary bile acids varies substantially (LCA > DCA > CDCA > CA > UDCA).59 Changes of FXR and/or TGR5 regulate the expression of GLP-1 and PYY, hepatic gluconeogenesis, glycogen synthesis and energy expenditure. FXR deficiency increases the secretion of GLP-1 to improve glucose metabolism.60 Activation of TGR5 potently stimulates GLP-162 and PYY63 secretion.
Impact of Anti-Hyperglycemic Agents on the Gut Microbiome Composition
Owing to the importance of gut microbiome in T2DM, we discuss the interaction between gut microbiome and anti-hyperglycemic agents and how do they take effect.
Metformin
Metformin has been the first-line medication for T2DM more than 60 years worldwide. Metformin can effectively control hyperglycemia through decreasing hepatic gluconeogenesis, reducing hepatic glucose output, and increasing glucose uptake and utilization in muscle cells and adipocytes.64 Metformin is usually administered through orally. The oral bioavailability is about 30–60%, and up to 30% of an ingested dose is eliminated through the feces.65 As a result, multiple evidences suggest that the anti-diabetic effect of metformin is at least partly mediated by gut microbiota.66 In a study of HFD-induced obese mice, when treated with metformin, the abundance of Akkermansia muciniphila (12.44%±5.26%) and Clostridium cocleatum (0.10%±0.09%) was significantly increased in the mice.67 An exploratory longitudinal study in healthy volunteers of Latvian was performed to observe the short-term effects of metformin on the gut microbiome.68 Participants were treated with metformin 850 mg twice daily for 7 days. After metformin administration, the inner diversity of gut microbiota was significantly reduced. Meanwhile, the families Peptostreptococcaceae (M24h vs M7d = 0.72% vs 0.18%) and Clostridiaceae_1 (M24h vs M7d = 0.49% vs 0.10%) and four genera within these families were significantly decreased. In another study in healthy young Danish men, the participants were given metformin up to 1 g twice daily.69 The abundance of Intestinibacter spp. and Clostridium spp. was reduced, while the abundance of Escherichia/Shigella spp. and Bilophila wadsworthia was increased. After discontinuation of metformin, these changes were reversed. This study also demonstrated that the relative abundance at baseline of 12 bacterial genera could predict the risk of gastrointestinal adverse effects of metformin.
The effects of metformin in newly diagnosed T2DM patients were different from healthy individuals.70 The alpha diversity of microbiota was reduced after metformin treatment, but not in T2DM. At the species level, the abundance of Clostridium bartlettii and Barnesiella intestinihominis was reduced, while the abundance of Parabacteroides distasonis and Oscillibacter was increased. Baseline gut microbiome composition predicted the different efficacy of metformin therapy (changes in HbA1c level) and the incidence of side effects. Another study of patients with T2DM in Japan, the ratio of Firmicutes/ Bacteroidetes was significantly decreased (before: 2.72±1.45; after four weeks: 2.26±1.09; p = 0.04) after four weeks of metformin treatment.71 The decrease of the genus Parabacteroides was associated with abdominal pain (r = −0.56, p = 0.008). The decrease of Parabacteroides (r = −0.53, p = 0.01) and Bifidobacterium (r = −0.56, p = 0.008) might be a predictor of abdominal pain and reflux.
Accumulating evidences suggested that metformin improved glucose homeostasis by several underlying mechanism in diabetes. A study in Colombian found that metformin helped ameliorating hyperglycemia and was positively associated with the abundance of SCFA-producing bacteria, including Akkermansia muciniphila, Butyrivibrio, Bifidobacterium bifidum, Megasphaera, and an operational taxonomic unit of Prevotella.72 In db/db mice, metformin treatment had been shown to inhibit bacterial TMA production and decrease TMAO availability.73 The effect of metformin on glucose metabolism was also linked to bile acid metabolism.74 In the individuals with newly diagnosed T2DM, metformin treatment could decrease Bacteroides fragilis and increase the bile acid glycoursodeoxycholic acid (GUDCA) via inhibiting intestinal FXR signaling, thereby improving glucose metabolism.75 However, the gut microbiota composition after metformin treatment may differ from these results due to different species, individuals and experimental design.
All above, we concluded that metformin exerted the glucose-lowering effect at least partly through affecting microbiome composition, and an individual’s tolerance or intolerance to metformin may also be influenced by the baseline composition of gut microbiome.65 Firstly, Forslund et al found that the increased abundance of Escherichia was consistent with well-known gastrointestinal side effects of metformin treatment.76 Secondly, in healthy volunteers of Latvian, an increased initial presence of gut opportunistic pathogen Escherichia-Shigella spp. before metformin administration was associated with the severity of gastrointestinal side effects.68 Lastly, another study in healthy young Danish men also indicated that the relative abundance at baseline of 12 bacterial genera might be a determinant for predicting gastrointestinal adverse effects following metformin intake.69
α-Glucosidase Inhibitor
The α-glucosidase inhibitors, including acarbose, voglibose and miglitol, are commonly used oral hypoglycemic agents in Asian, since their diet contains high content of carbohydrates. Under normal condition, the carbohydrates are digested and absorbed in the small intestine. The administration of α-glucosidase inhibitors specifically inhibite the α-glucosidase in the brush border of the small intestine, resulting in a delay of the absorption of carbohydrates in the small intestine and reduction of postprandial glucose concentration.77 When passing through the colon, the undigested carbohydrates are fermented by gut bacteria, and the microbial ecosystem changes to some degree.78 There are diverse influences on the abundance of gut microbiota after different glycosidase inhibitors.79
In mice fed with either a high-starch or high-fiber diet, acarbose treatment changed gut community, including an increase of the Bacteroidaceae and Bifidobacteriaceae and a decrease of the Verrucomicrobiaceae (such as Akkermansia muciniphila) and the Bacteroidales S24-7, but not irreversibly.80 These changes resulted in an increase of beneficial SCFA, especially butyrate. A recent human study of prediabetic patients also found that acarbose increased the abundance of SCFA-producing taxa, such as Faecalibacterium, Prevotella, and Lactobacillus.81 Acarbose treatment maybe increased median lifespan through increasing fecal SCFA concentrations in mice.78 In another cohort of Chinese patients with T2DM, the Bifidobacterium longum (8.49±1.00 vs 7.92±1.18 lgcopies/g, P=0.005) and Enterococcus faecalis (6.61±1.27 vs 8.08±1.20 lgcopies/g, P=0.001) were significantly increased after 4 weeks of acarbose treatment.82 These changes of gut microbiota were negatively correlated with inflammatory cytokines, including LPS, PAI-1 and MCP-1. Acarbose treatment also altered the composition of gut microbiome and bile acid profiles, resulting in the improvement of glucose metabolism.83
Thiazolidinediones (TZDs)
Thiazolidinediones (TZDs), also termed as selective peroxisome proliferator-activated receptor-γ (PPAR)-γ agonists, are potent oral insulin sensitizers used for T2DM treatment.84 This class of drugs once fell into disuse because of serious side effects. Thiazolidinediones reduce hepatic glucose production and increase peripheral utilization of glucose and lipid metabolism by increasing the transactivation activity of PPARs, resulting in improved glycemic control.85 PPAR-γ is predominantly expressed at high levels in adipose tissue, and is also abundantly expressed in colonic epithelial cells.86 Thiazolidinediones may influence gut microbiota.
In diabetic db/db mice, rosiglitazone treatment significantly improved insulin sensitivity and glucose homeostasis without altering gut bacterial composition.87 A global gene expression analysis in rosiglitazone-treated db/db mice showed substantial changes in colon and ileum, with no change in duodenum. Rosiglitazone treatment stimulated fatty acid metabolism by regulating the gene markers of intestinal fatty uptake, transport and disposal in the colon and ileum. The genes involved in gluconeogenesis were upregulated in colon, while the genes involved in glycolysis were downregulated in cecum and ileal. These effects were consistent with the fact that PPAR-γ being abundantly expressed in colonic epithelial cells.87 Another study found that Danshensu Bingpian Zhi (DBZ), a unique PPAR-γ partial agonist, prevented HFD-induced obesity and insulin resistance through modulating gut microbiota dysbiosis.88 DBZ treatment restored intestinal barrier integrity and reversed gut dysbiosis by increasing the ratio of Bacteroidetes to Firmicutes, the relative abundance of Akkermansia, and reducing the level of HFD-induced pernicious bacteria (such as Helicobacter marmotae, Odoribacter, and Anaerotruncus). DBZ was effective for metabolic syndrome without side effects, which is a promising approach novel therapeutic agent for metabolic diseases. The gut microbiota composition of girls with polycystic ovary syndrome (PCOS) was different from those in healthy controls, including a reduction of alpha diversity, a reduction of Prevotellaceae, Prevotella and Senegalimassilia and an increase of Bacillales Family XI.89 Spironolactone-pioglitazone-metformin (SPIOMET) treatment normalized the abundance of Family XI.
Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist and Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
GLP-1, an incretin hormone secreted by enteroendocrine L cells in the distal ileum and colon, can stimulate glucose-dependent insulin secretion and suppress glucagon secretion.90 GLP-1 is degraded within 2~3 mins in the circulation via enzymatic inactivation by DPP-4.90 At present, various GLP-1 receptor agonists (GLP-1RAs) and DPP-4 inhibitors (DPP-4i) have been developed and widely used in T2DM treatment.91 GLP-1RAs include the short-acting compounds (exenatide and lixisenatide) and the long-acting compounds (liraglutide, dulaglutide, albiglutide). The short-acting GLP-1RAs, which are resistant to the cleavage by DPP4, primarily lower postprandial blood glucose level through substantial retardation of gastric emptying. While the long-acting GLP-1RAs, binding to plasma albumin, have a stronger effect on fasting glucose level through their insulinotropic and glucagonostatic actions.92 Besides the glucose-lowering effect, GLP-1 can inhibit appetite and delay gastric emptying to reduce food intake. Therefore, GLP-1RAs are also recognized as a promising anti-obesity agent in simple obesity and diabetic obesity. However, recent studies indicated that GLP-1 had more beneficial effect on weight loss and glucose metabolism by modulating gut microbial composition than that by restricting food intake alone.93–95 A study provided evidence that in both simple obese and diabetic obese rats, liraglutide, as a GLP-1 analog, was able to attenuate blood glucose levels and suppress body weight gain via changing the structure of gut microbiota, including decreasing microflora community richness and diversity, increasing Bacteroidetes and decreasing Firmicutes phyla, decreasing the obesity-related phylotypes and increasing the lean-related phylotypes.93 Liraglutide effectively prevented the development of diabetes in rats through elevating SCFA-producing bacteria, including Bacteroides, Lachnospiraceae, and probiotic bacteria, Bifidobacterium.94 In patients with T2DM, liraglutide could reduce gut microbial alpha diversity and alter gut microbiota composition, including increasing Firmicutes and Bacteroidetes and reducing Ruminococcus (Firmicutes) and Actinomyces (Actinobacteria).95 Another study confirmed liraglutide was also associated with attenuating nonalcoholic fatty liver disease (NAFLD) through changing the structure of gut microbiota.96,97 Liraglutide could reduce the genus of Proteobacteria and increase the genus of Akkermansia muciniphila in HFD-fed mice.97 A recent research found that GLP-1/GLP-2 co-agonists, GUB09-145, had more effect on reducing food intake and improving glucose tolerance than GLP-1 receptor agonist.98 The biological actions of GLP-1 and GLP-2 receptor signaling on caloric intake and glucose metabolism in diet-induced obese mice were associated with gut bacterial compositional changes.99
DPP4i exerts glucose-lowering effect through inhibiting DPP4 activity and raising the plasma level of native GLP-1.100 In T2DM patients, treatment with sitagliptin, a DPP-4i, obviously increased the abundance of Bacteroidetes, especially the production of succinate.101 In this study, fecal microbiota transplantation (FMT) was further performed in germ-free mice to confirm the effect of sitagliptin on gut microbiota. These results indicated that the hypoglycemic effect of DPP-4i was at least partially mediated by the alterations of gut microbiota. An animal study found that sitagliptin treatment exerted beneficial effects on gut microbiota, especially increasing SCFA-producing bacteria, Blautia, Roseburia, and Clostridium, and probiotics Lactobacillus, Bifidobacterium.102 Another study in Western diet-fed mice, vildagliptin exerted beneficial effects by modulating of gut microbiota and increasing the amount of propionate, thus ameliorating gastrointestinal health. But there were no differences in total amount of SCFA, acetate or butyrate.103
Sodium Glucose Co-Transporter 2 Inhibitors (SGLT2i)
Sodium glucose co-transporter 2 (SGLT2) is expressed in the renal proximal tubule and accounts for reabsorbing approximately 90% of filtered glucose, whereas sodium glucose co-transporter 1 (SGLT1) is predominantly expressed in the small intestine mucosa and accounts for transporting glucose into intestinal enterocytes.104 SGLT2 inhibitors are oral anti-hyperglycemic medications for T2DM treatment, which can effectively improve glycemic control by blocking glucose reabsorption in the renal proximal tubule and increasing urinary glucose excretion.105 Even though the affinity to SGLT2 of SGLT2 inhibitors is significantly greater than SGLT1, SGLT2 inhibitors also have some affinity for SGLT1. Considering this, SGLT2 inhibitors potentially influence gut microbiota composition through reducing intestinal glucose uptake.106 Thus far, there are a few researches about the effects of SGLT2 inhibitors or SGLT1/2 dual inhibitors on gut microbiome composition, but the results are controversial. Early study found that dapagliflozin, a SGLT2 inhibitor, improved vascular dysfunction concomitantly subtly altering microbiota diversity in T2DM db/db mice.107 Dapagliflozin treatment could increase the abundance of beneficial bacterial species Akkermansia muciniphila and reduce Firmicutes/Bacteroidetes.107 However, whether these alterations of microbiota conclusively mediate the improvement on vascular function need to be further studied. Further preclinical studies about the effect of SGLT2 inhibitors on type 1 diabetes mellitus (T1DM) are currently underway. After dapagliflozin or empagliflozin treatment, the intermediate metabolite of gut metabolism known as succinate was significantly reduced in type 1 diabetic mice with diabetic retinopathy.108 Succinate was reported to promote the angiogenic factor VEGF expression in retinal ganglion cells and was shown to be a pathogenic factor in diabetic retinopathy.109 Another study in patients with T2DM, dapagliflozin treatment as add-on to metformin therapy had no effect on gut microbiome composition.110 The dosages of metformin were equal among intervention participants, but the effects of metformin on microbiome composition were very potent. Maybe metformin overshadowed the potential effect of dapagliflozin on microbiome composition. Unlike people, mice are cohousing animals. Then, it also may be a confounding factor. Above all, the effect of SGLT2 inhibitors on microbiome composition was slight. Potent SGLT1/2 dual inhibitor, which can reduce glucose uptake and increase urinary glucose excretion, was more powerful than SGLT2 inhibitor in lowering blood glucose.111 However, SGLT1/2 inhibitor alone did not alter gut microbiota composition in mice. If increasing glucose by high sucrose diet, SGLT1/2 inhibitor would alter the relative abundance of certain phyla to a certain extent.112
Traditional Chinese Medicine
Traditional Chinese medicine, which had recorded knowledge for more than 2000 years, could significantly improve glucose control and clinical indices through different molecular mechanisms, including simulating insulin secretion, enhancing insulin sensitivity and protecting β-cells.113 Accumulating evidences recently confirmed that traditional Chinese medicine alleviated diabetes at least partly by modulating the structure of the gut microbiota.114
Recently, studies indicated that polysaccharides from natural resources exerted beneficial effect on glucose metabolism. Polysaccharides, as macromolecular carbohydrates, are indigestible and fermentative in the intestinal tract after oral administration. So, polysaccharides would regulate gut microflora composition to alleviate diabetes. Holothuria leucospilota, as an edible tropical sea cucumber species, could improve glucose metabolism by regulating the structure of the gut microbiota on diabetic GK rats, including increasing the abundance of beneficial bacteria (such as Bacteroidetes, TM7, Cyanobacteria and Tenericutes) and SCFA-producing species of gut microbiota (such as Clostridium, Turicibacter, Allobaculum and Ruminococcus) and decreasing the conditional pathogenic bacteria (such as Anaerobiospirillum, Collinsella and Treponema).115 Pumpkin polysaccharide also could enrich key species of Bacteroidetes, Prevotella, Deltaproteobacteria, Oscillospira, Veillonellaceae, Phascolarctobacterium, Sutterella and Bilophila, leading to an increase of SCFA production in diabetic rats.116 Sargassum fusiforme fucoidan treatment alleviated streptozotocin-induced hyperglycemia in mice by increasing the relative abundances of SCFAs-producing bacteria, such as Bilophila, Oscillibacter, Mucispirillum, especially Alloprevotella.117 Mulberry fruit polysaccharides could efficiently alleviate hyperglycemia, dyslipidemia and oxidative stress partly by modulating gut microbiota.118 Mulberry fruit polysaccharides strongly increased the abundance of Akkermansia, resulting in the reduce of insulin resistance.118 In diabetic mice, polysaccharides from adlay seed increased the abundance of Lactobacillus spp. and Lactobacillus fermentum in diabetic mice, which may be associated with their beneficial role in T2DM. Furthermore, polysaccharides from adlay seed significantly increased the concentration of GLP-1 and reduced anti-amyloid beta (Aβ1–42) protein.119 Furthermore, long-term polysaccharides intake also have preventive and therapeutic effect on obesity120 and TMAO-induced cardiac dysfunction121 by targeting gut microbiota. In HFD-induced obese rats, a mixture of lentinan and Flos Lonicera polysaccharide (LF) could reduce TMAO in the urine, suggesting that LF could have a potential role in reducing the risk of atherosclerosis.120 Ganoderma lucidum spore (GS) polysaccharides could alleviate TMAO-induced cardiac dysfunction in rats by targeting gut microbiota, such as increasing the abundance of Firmicutes and Proteobacteria and reducing the abundance of Actinobacteria and Tenericutes.121 The flavonoids in Scutellaria baicalensis and the alkaloids in Coptis chinensis are a classic herbal pair for ameliorating diabetes and various intestinal diseases by inhibiting inflammation and modulating gut microbiota. Zhang et al initially found that Scutellaria–coptis exhibited the effect of relieving insulin resistant by regulating intestinal flora and inhibiting inflammatory reaction.122 Moreover, it was demonstrated that Scutellaria–coptis obviously improved metabolic disorders by increasing SCFAs-producing bacteria and decreasing secondary BAs-producing bacteria.123 Furthermore, the hypoglycaemic effects of Scutellaria-Coptis were involved in regulating gut microbiota and alleviating intestinal mucosal barrier damage.124 Scutellaria-Coptis significantly inhibited some potential enteropathogenic bacteria and LPS-producing bacteria, such as Proteobacteria, Enterobacteriaceae, Enterobacter, Escherichia-Shigella, and Enterococcus, and increased the abundances of butyrate-producing bacteria, such as Lachnospiraceae and Prevotellaceae.124 The change of gut microbiota resulted in alleviating intestinal mucosa barrier damage by preventing the leakage of LPS and increasing the intestinal tight junction proteins claudin-1, occluding and ZO-1 in diabetic rats.124 Their anti-inflammation effect of Scutellaria–coptis was involved in TLR signalling pathway,122 TLR-4/TRIF and TNFR-1/NF-κB signaling pathways.124 In a clinical study, the T2DM patients who had already been prescribed metformin and were allotted to additional Scutellaria baicalensis, significantly reduced the TNF-α expression and influenced gut microbiota, especially Lactobacillus and Akkermansia.125 Furthermore, both in vivo and in vitro experiments indicated that nobiletin effectively prevented TMAO-stimulated vascular inflammation via inhibiting NF-κB/MAPK signaling pathways.126
Berberine, an isoquinoline alkaloid extracted from Chinese traditional herb Coptis chinensis, was used to treat bacterial diarrhea in China for thousands of years.127 Berberine has poor oral bioavailability and leads to changes in the intestinal bacterial composition. Berberine exerts multiple beneficial effect against metabolic disease by modulating gut microbiota.128 Berberine significantly increased the abundance of Akkermansia spp., resulting in alleviating HFD-induced atherosclerosis.129 Furthermore, berberine could inhibit TMA/TMAO production by modulating gut microbiota composition.130 The anti-atherosclerotic effect of berberine may be partly attributed to the reduction of TMAO production.131 Berberine may be a better modulator to inhibit the development of atherosclerotic plaque. Recently, a large number of researches have demonstrated that berberine exhibited clinically beneficial effect in alleviating T2DM and could be possibly developed into a promising anti-diabetes candidate.132,133 Berberine administration enriched SCFA-producing bacteria, including Blautia and Allobaculum, and elevated fecal SCFA concentrations in HFD-fed rats, which may help to alleviate inflammation.134 Another study indicated that berberine inhibited LPS-induced TLR4/TNF-α activation.135 These findings suggested that berberine prevented insulin resistance at least partially attributable to structural modulation of the gut microbiota. Berberine could reduce TLR-4, NF-kB and TNF-a expression, increase GLP-2 secretion and improve intestinal permeability by regulating gut microbiota in impaired glucose tolerance (IGT) rats, which may slow the progression of prediabetes to T2DM in ZDF rats.136 Berberine compounds could increase microbiome mediated deoxycholic acid (DCA) production and up-regulate colonic TGR5 expression and GLP secretion in db/db mice, which improved hyperglycemia.137 In China, a clinical trial in newly diagnosed T2DM patients found that the hypoglycaemic effect of berberine was associated with the inhibition of DCA biotransformation by Ruminococcus bromii and the reduction of the microbial production of secondary BA.138
Conclusion and Future Perspectives
So far, increasing researches have demonstrated that the alteration of gut microbiota composition and function played pivotal roles in the pathogenesis and treatment of T2DM. While animal and human studies in this field are limited in size. In this review, we summarized the effects of anti-hyperglycemic agents and traditional Chinese medicine on gut microbiota dysbiosis (summarized in Table 1). The potential gut-targeting glucose-lowering treatment strategies are now emerging, so further longitudinal and interventional studies in preclinical and clinical are needed to fully elucidate the potential mechanism of gut microbiota in T2DM and its potential use in the prevention and treatment of T2DM. We expect in-depth studies providing the basis for the development of novel antidiabetic drugs that target gut microbiota in the future. In conclusion, the modulation of gut microbiota is increasingly regarded as promising targets to improve glucose metabolism and treat T2DM. The development of novel drug presumably requires long and painstaking work with no scope for shortcuts.
 |
Table 1 The Interaction Between Anti-Hyperglycemic Agents, Traditional Chinese Medicine and Specific Gut Microbiota Composition Changes |
Acknowledgments
This study was funded by Natural Science Foundation of Hebei Province (CN) 2020206108.
Disclosure
The authors declare no conflicts of interest.
References
1. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
2. Pascale A, Marchesi N, Marelli C, et al. Microbiota and metabolic diseases. Endocrine. 2018;61(3):357–371. doi:10.1007/s12020-018-1605-5
3. Grice EA, Segre JA. The human microbiome: our second genome. Annu Rev Genomics Hum Genet. 2012;13:151–170. doi:10.1146/annurev-genom-090711-163814
4. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi:10.1126/science.1110591
5. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–1836. doi:10.1042/BCJ20160510
6. Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015;16(1):55–65. doi:10.1007/s11154-015-9309-0
7. Kikuchi K, Saigusa D, Kanemitsu Y, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10(1):1835. doi:10.1038/s41467-019-09735-4
8. Montandon SA, Jornayvaz FR. Effects of antidiabetic drugs on gut microbiota composition. Genes. 2017;8(10):250. doi:10.3390/genes8100250
9. Kyriachenko Y, Falalyeyeva T, Korotkyi O, Molochek N, Kobyliak N. Crosstalk between gut microbiota and antidiabetic drug action. World J Diabetes. 2019;10(3):154–168. doi:10.4239/wjd.v10.i3.154
10. Zhang R, Gao X, Bai H, Ning K. Traditional Chinese medicine and gut microbiome: their respective and concert effects on healthcare. Front Pharmacol. 2020;11:538. doi:10.3389/fphar.2020.00538
11. Wang -R-R, Zhang L, Xu -J-J, et al. Human microbiome brings new insights to traditional Chinese medicine. J Bio-X Res. 2018;1(1):41–44. doi:10.1097/JBR.0000000000000007
12. Zhong H, Ren H, Lu Y, et al. Distinct gut metagenomics and metaproteomics signatures in prediabetics and treatment-naïve type 2 diabetics. EBioMedicine. 2019;47:373–383. doi:10.1016/j.ebiom.2019.08.048
13. Gao B, Zhong M, Shen Q, et al. Gut microbiota in early pregnancy among women with Hyperglycaemia vs. Normal blood glucose. BMC Pregnancy Childbirth. 2020;20(1):284. doi:10.1186/s12884-020-02961-5
14. Cao M, Peng Y, Lu Y, et al. Controls of hyperglycemia improves dysregulated microbiota in diabetic mice. Transplantation. 2021;105(9):1980–1988. doi:10.1097/TP.0000000000003603
15. Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology. 2017;152(7):1671–1678. doi:10.1053/j.gastro.2016.12.048
16. Fuke N, Nagata N, Suganuma H, Ota T. Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients. 2019;11(10):2277. doi:10.3390/nu11102277
17. Gomes JMG, Costa JA, Alfenas RCG. Metabolic endotoxemia and diabetes mellitus: a systematic review. Metabolism. 2017;68:133–144. doi:10.1016/j.metabol.2016.12.009
18. Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi:10.2337/db07-1403
19. Allin KH, Nielsen T, Pedersen O. Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2015;172(4):R167–177. doi:10.1530/EJE-14-0874
20. Gao J, Guo Z. Progress in the synthesis and biological evaluation of lipid A and its derivatives. Med Res Rev. 2018;38(2):556–601. doi:10.1002/med.21447
21. Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3(4):279–288. doi:10.4161/gmic.19625
22. Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology. 2016;31(4):283–293. doi:10.1152/physiol.00041.2015
23. Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42. doi:10.1186/s13073-016-0303-2
24. Alkanani AK, Hara N, Lien E, et al. Induction of diabetes in the RIP-B7.1 mouse model is critically dependent on TLR3 and MyD88 pathways and is associated with alterations in the intestinal microbiome. Diabetes. 2014;63(2):619–631. doi:10.2337/db13-1007
25. Kim JJ, Sears DD. TLR4 and insulin resistance. Gastroenterol Res Pract. 2010;2010:212563.
26. Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16(8):457–470. doi:10.1038/s41579-018-0036-x
27. Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20(1):40–54. doi:10.1038/s41577-019-0198-4
28. Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376–1383. doi:10.1126/science.aar3318
29. Maruta K, Takajo T, Akiba Y, et al. GLP-2 acutely prevents endotoxin-related increased intestinal paracellular permeability in rats. Dig Dis Sci. 2020;65(9):2605–2618. doi:10.1007/s10620-020-06097-6
30. Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi:10.1136/gut.2008.165886
31. Cani PD. Crosstalk between the gut microbiota and the endocannabinoid system: impact on the gut barrier function and the adipose tissue. Clin Microbiol Infect. 2012;18(Suppl 4):50–53.
32. Heiss CN, Olofsson LE. Gut microbiota-dependent modulation of energy metabolism. J Innate Immun. 2018;10(3):163–171. doi:10.1159/000481519
33. Priyadarshini M, Navarro G, Layden BT. Gut microbiota: FFAR reaching effects on islets. Endocrinology. 2018;159(6):2495–2505. doi:10.1210/en.2018-00296
34. Yang Q, Ouyang J, Sun F, Yang J. Short-chain fatty acids: a soldier fighting against inflammation and protecting from tumorigenesis in people with diabetes. Front Immunol. 2020;11:590685. doi:10.3389/fimmu.2020.590685
35. Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–g65. doi:10.1152/ajpgi.00346.2017
36. Xie C, Jones KL, Rayner CK, Wu T. Enteroendocrine hormone secretion and metabolic control: importance of the region of the gut stimulation. Pharmaceutics. 2020;12(9):790. doi:10.3390/pharmaceutics12090790
37. Hare KJ, Vilsbøll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59(7):1765–1770. doi:10.2337/db09-1414
38. Qin X, Shen H, Liu M, et al. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288(5):G943–949. doi:10.1152/ajpgi.00303.2004
39. Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab. 2010;95(1):215–221. doi:10.1210/jc.2009-1503
40. Wu T, Rayner CK, Horowitz M. Incretins. Handb Exp Pharmacol. 2016;233:137–171.
41. Moran TH, Smedh U, Kinzig KP, et al. (3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R384–388. doi:10.1152/ajpregu.00535.2004
42. Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol Biochem. 2018;49(1):190–205. doi:10.1159/000492853
43. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi:10.1038/nm.3145
44. Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6(2):e02481. doi:10.1128/mBio.02481-14
45. Chen Y, Weng Z, Liu Q, et al. FMO3 and its metabolite TMAO contribute to the formation of gallstones. Biochim Biophys Acta Mol Basis Dis. 2019;1865(10):2576–2585. doi:10.1016/j.bbadis.2019.06.016
46. Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem. 2010;43(9):732–744. doi:10.1016/j.clinbiochem.2010.03.009
47. Lent-Schochet D, Silva R, McLaughlin M, Huet B, Jialal I. Changes to trimethylamine-N-oxide and its precursors in nascent metabolic syndrome. Horm Mol Biol Clin Investig. 2018;35(2). doi: 10.1515/hmbci-2018-0015.
48. Randrianarisoa E, Lehn-Stefan A, Wang X, et al. Relationship of serum trimethylamine N-Oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep. 2016;6:26745. doi:10.1038/srep26745
49. Li XS, Obeid S, Klingenberg R, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38(11):814–824. doi:10.1093/eurheartj/ehw582
50. Li J, Tan Y, Zhou P, et al. Association of Trimethylamine N-Oxide levels and calcification in culprit lesion segments in patients with ST-segment-elevation myocardial infarction evaluated by optical coherence tomography. Front Cardiovasc Med. 2021;8:628471. doi:10.3389/fcvm.2021.628471
51. Ding L, Chang M, Guo Y, et al. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018;17(1):286. doi:10.1186/s12944-018-0939-6
52. Sun X, Jiao X, Ma Y, et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481(1–2):63–70. doi:10.1016/j.bbrc.2016.11.017
53. Govindarajulu M, Pinky PD, Steinke I, et al. Gut metabolite TMAO induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front Mol Neurosci. 2020;13:138. doi:10.3389/fnmol.2020.00138
54. McGlone ER, Bloom SR. Bile acids and the metabolic syndrome. Ann Clin Biochem. 2019;56(3):326–337. doi:10.1177/0004563218817798
55. Rajani C, Jia W. Bile acids and their effects on diabetes. Front Med. 2018;12(6):608–623. doi:10.1007/s11684-018-0644-x
56. van de Peppel IP, Verkade HJ, Jonker JW. Metabolic consequences of ileal interruption of the enterohepatic circulation of bile acids. Am J Physiol Gastrointest Liver Physiol. 2020;319(5):G619–g625. doi:10.1152/ajpgi.00308.2020
57. González-Regueiro JA, Moreno-Castañeda L, Uribe M, Chávez-Tapia NC. The role of bile acids in glucose metabolism and their relation with diabetes. Ann Hepatol. 2017;16(Suppl. 1: s3–105.):16–21. doi:10.5604/01.3001.0010.5672
58. Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3(1):5. doi:10.3978/j.issn.2305-5839.2014.12.06
59. Xie C, Huang W, Young RL, et al. Role of bile acids in the regulation of food intake, and their dysregulation in metabolic disease. Nutrients. 2021;13(4):1104. doi:10.3390/nu13041104
60. Trabelsi MS, Daoudi M, Prawitt J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun. 2015;6:7629. doi:10.1038/ncomms8629
61. Deutschmann K, Reich M, Klindt C, et al. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1319–1325. doi:10.1016/j.bbadis.2017.08.021
62. Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329(1):386–390. doi:10.1016/j.bbrc.2005.01.139
63. Yang H, Liu H, Jiao Y, Qian J. Roux-en-Y gastrointestinal bypass promotes activation of TGR5 and peptide YY. Endocr Metab Immune Disord Drug Targets. 2020;20(8):1262–1267. doi:10.2174/1871530320666200628024500
64. Duca FA, Côté CD, Rasmussen BA, et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21(5):506–511. doi:10.1038/nm.3787
65. McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59(3):426–435. doi:10.1007/s00125-015-3844-9
66. Hur KY, Lee MS. New mechanisms of metformin action: focusing on mitochondria and the gut. J Diabetes Investig. 2015;6(6):600–609. doi:10.1111/jdi.12328
67. Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol. 2014;80(19):5935–5943. doi:10.1128/AEM.01357-14
68. Elbere I, Kalnina I, Silamikelis I, et al. Association of metformin administration with gut microbiome dysbiosis in healthy volunteers. PLoS One. 2018;13(9):e0204317. doi:10.1371/journal.pone.0204317
69. Bryrup T, Thomsen CW, Kern T, et al. Metformin-induced changes of the gut microbiota in healthy young men: results of a non-blinded, one-armed intervention study. Diabetologia. 2019;62(6):1024–1035. doi:10.1007/s00125-019-4848-7
70. Elbere I, Silamikelis I, Dindune II, et al. Baseline gut microbiome composition predicts metformin therapy short-term efficacy in newly diagnosed type 2 diabetes patients. PLoS One. 2020;15(10):e0241338. doi:10.1371/journal.pone.0241338
71. Nakajima H, Takewaki F, Hashimoto Y, et al. The effects of metformin on the gut microbiota of patients with type 2 diabetes: a two-center, Quasi-Experimental Study. Life. 2020;10(9). doi:10.3390/life10090195
72. de la Cuesta-zuluaga J, Mueller NT, Corrales-Agudelo V, et al. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40(1):54–62. doi:10.2337/dc16-1324
73. Kuka J, Videja M, Makrecka-Kuka M, et al. Metformin decreases bacterial trimethylamine production and trimethylamine N-oxide levels in db/db mice. Sci Rep. 2020;10(1):14555. doi:10.1038/s41598-020-71470-4
74. Sansome DJ, Xie C, Veedfald S, Horowitz M, Rayner CK, Wu T. Mechanism of glucose-lowering by metformin in type 2 diabetes: role of bile acids. Diabetes Obes Metab. 2020;22(2):141–148. doi:10.1111/dom.13869
75. Sun L, Xie C, Wang G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24(12):1919–1929. doi:10.1038/s41591-018-0222-4
76. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi:10.1038/nature15766
77. Liu Z, Ma S. Recent advances in synthetic α-glucosidase inhibitors. ChemMedChem. 2017;12(11):819–829. doi:10.1002/cmdc.201700216
78. Smith BJ, Miller RA, Ericsson AC, Harrison DC, Strong R, Schmidt TM. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019;19(1):130. doi:10.1186/s12866-019-1494-7
79. Xu GD, Cai L, Ni YS, et al. Comparisons of effects on intestinal short-chain fatty acid concentration after exposure of two glycosidase inhibitors in mice. Biol Pharm Bull. 2018;41(7):1024–1033. doi:10.1248/bpb.b17-00978
80. Baxter NT, Lesniak NA, Sinani H, Schloss PD, Koropatkin NM. The glucoamylase inhibitor acarbose has a diet-dependent and reversible effect on the murine gut microbiome. mSphere. 2019;4(1):e00528–18.
81. Zhang X, Fang Z, Zhang C, et al. Effects of acarbose on the gut microbiota of prediabetic patients: a randomized, double-blind, controlled crossover trial. Diabetes Ther. 2017;8(2):293–307. doi:10.1007/s13300-017-0226-y
82. Su B, Liu H, Li J, et al. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7(5):729–739. doi:10.1111/1753-0407.12232
83. Gu Y, Wang X, Li J, et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8(1):1785. doi:10.1038/s41467-017-01682-2
84. Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20(4):573–591. doi:10.1016/j.cmet.2014.08.005
85. Nanjan MJ, Mohammed M, Prashantha Kumar BR, Chandrasekar MJN. Thiazolidinediones as antidiabetic agents: a critical review. Bioorg Chem. 2018;77:548–567. doi:10.1016/j.bioorg.2018.02.009
86. Fajas L, Auboeuf D, Raspé E, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272(30):18779–18789. doi:10.1074/jbc.272.30.18779
87. Madsen MSA, Grønlund RV, Eid J, et al. Characterization of local gut microbiome and intestinal transcriptome responses to rosiglitazone treatment in diabetic db/db mice. Biomed Pharmacother. 2021;133:110966. doi:10.1016/j.biopha.2020.110966
88. Xu P, Hong F, Wang J, et al. DBZ is a putative PPARγ agonist that prevents high fat diet-induced obesity, insulin resistance and gut dysbiosis. Biochim Biophys Acta Gen Subj. 2017;1861(11 Pt A):2690–2701. doi:10.1016/j.bbagen.2017.07.013
89. Garcia-Beltran C, Malpique R, Carbonetto B, et al. Gut microbiota in adolescent girls with polycystic ovary syndrome: effects of randomized treatments. Pediatr Obes. 2021;16(4):e12734. doi:10.1111/ijpo.12734
90. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
91. Gilbert MP, Pratley RE. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol (Lausanne). 2020;11:178. doi:10.3389/fendo.2020.00178
92. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. doi:10.1038/nrendo.2012.140
93. Zhao L, Chen Y, Xia F, et al. A glucagon-like peptide-1 receptor agonist lowers weight by modulating the structure of gut microbiota. Front Endocrinol (Lausanne). 2018;9:233. doi:10.3389/fendo.2018.00233
94. Zhang Q, Xiao X, Zheng J, et al. Featured article: structure moderation of gut microbiota in liraglutide-treated diabetic male rats. Exp Biol Med (Maywood). 2018;243(1):34–44. doi:10.1177/1535370217743765
95. Shang J, Liu F, Zhang B, et al. Liraglutide-induced structural modulation of the gut microbiota in patients with type 2 diabetes mellitus. PeerJ. 2021;9:e11128. doi:10.7717/peerj.11128
96. Zhang N, Tao J, Gao L, et al. Liraglutide attenuates nonalcoholic fatty liver disease by modulating gut microbiota in rats administered a high-fat diet. Biomed Res Int. 2020;2020:2947549. doi:10.1155/2020/2947549
97. Moreira GV, Azevedo FF, Ribeiro LM, et al. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. 2018;62:143–154. doi:10.1016/j.jnutbio.2018.07.009
98. Wismann P, Pedersen SL, Hansen G, et al. Novel GLP-1/GLP-2 co-agonists display marked effects on gut volume and improves glycemic control in mice. Physiol Behav. 2018;192:72–81. doi:10.1016/j.physbeh.2018.03.004
99. Madsen MSA, Holm JB, Pallejà A, et al. Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice. Sci Rep. 2019;9(1):15582. doi:10.1038/s41598-019-52103-x
100. Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16(11):642–653. doi:10.1038/s41574-020-0399-8
101. Liao X, Song L, Zeng B, et al. Alteration of gut microbiota induced by DPP-4i treatment improves glucose homeostasis. EBioMedicine. 2019;44:665–674. doi:10.1016/j.ebiom.2019.03.057
102. Yan X, Feng B, Li P, Tang Z, Wang L. Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: an Animal Study. J Diabetes Res. 2016;2016:2093171. doi:10.1155/2016/2093171
103. Olivares M, Neyrinck AM, Pötgens SA, et al. The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia. 2018;61(8):1838–1848. doi:10.1007/s00125-018-4647-6
104. Chen J, Williams S, Ho S, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. 2010;1(2):57–92. doi:10.1007/s13300-010-0006-4
105. van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol. 2017;12(4):700–710. doi:10.2215/CJN.06080616
106. Poulsen SB, Fenton RA, Rieg T. Sodium-glucose cotransport. Curr Opin Nephrol Hypertens. 2015;24(5):463–469. doi:10.1097/MNH.0000000000000152
107. Lee DM, Battson ML, Jarrell DK, et al. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17(1):62. doi:10.1186/s12933-018-0708-x
108. Herat LY, Ward NC, Magno AL, et al. Sodium glucose co-transporter 2 inhibition reduces succinate levels in diabetic mice. World J Gastroenterol. 2020;26(23):3225–3235. doi:10.3748/wjg.v26.i23.3225
109. Matsumoto M, Suzuma K, Maki T, et al. Succinate increases in the vitreous fluid of patients with active proliferative diabetic retinopathy. Am J Ophthalmol. 2012;153(5):896–902.e891. doi:10.1016/j.ajo.2011.10.006
110. van Bommel EJM, Herrema H, Davids M, Kramer MHH, Nieuwdorp M, van Raalte DH. Effects of 12-week treatment with dapagliflozin and gliclazide on faecal microbiome: results of a double-blind randomized trial in patients with type 2 diabetes. Diabetes Metab. 2020;46(2):164–168. doi:10.1016/j.diabet.2019.11.005
111. Kuo GH, Gaul MD, Liang Y, et al. Synthesis and biological evaluation of benzocyclobutane-C-glycosides as potent and orally active SGLT1/SGLT2 dual inhibitors. Bioorg Med Chem Lett. 2018;28(7):1182–1187. doi:10.1016/j.bmcl.2018.02.057
112. Du F, Hinke SA, Cavanaugh C, et al. Potent sodium/glucose cotransporter SGLT1/2 dual inhibition improves glycemic control without marked gastrointestinal adaptation or colonic microbiota changes in rodents. J Pharmacol Exp Ther. 2018;365(3):676–687. doi:10.1124/jpet.118.248575
113. Tian J, Jin D, Bao Q, et al. Evidence and potential mechanisms of traditional Chinese medicine for the treatment of type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(8):1801–1816. doi:10.1111/dom.13760
114. Zheng Y, Ding Q, Wei Y, et al. Effect of traditional Chinese medicine on gut microbiota in adults with type 2 diabetes: a systematic review and meta-analysis. Phytomedicine. 2020;88:153455. doi:10.1016/j.phymed.2020.153455
115. Zhao F, Liu Q, Cao J, et al. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food Chem Toxicol. 2020;135:110886. doi:10.1016/j.fct.2019.110886
116. Liu G, Liang L, Yu G, Li Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int J Biol Macromol. 2018;115:711–717. doi:10.1016/j.ijbiomac.2018.04.127
117. Cheng Y, Sibusiso L, Hou L, et al. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int J Biol Macromol. 2019;131:1162–1170. doi:10.1016/j.ijbiomac.2019.04.040
118. Chen C, You LJ, Huang Q, et al. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018;9(7):3732–3742. doi:10.1039/C7FO01346A
119. Chen LC, Fan ZY, Wang HY, Wen DC, Zhang SY. Effect of polysaccharides from adlay seed on anti-diabetic and gut microbiota. Food Funct. 2019;10(7):4372–4380. doi:10.1039/C9FO00406H
120. Chen M, Lu B, Li Y, et al. Metabolomics insights into the modulatory effects of long-term compound polysaccharide intake in high-fat diet-induced obese rats. Nutr Metab (Lond). 2018;15:8. doi:10.1186/s12986-018-0246-2
121. Liu Y, Lai G, Guo Y, et al. Protective effect of Ganoderma lucidum spore extract in trimethylamine-N-oxide-induced cardiac dysfunction in rats. J Food Sci. 2021;86(2):546–562. doi:10.1111/1750-3841.15575
122. Zhang CH, Sheng JQ, Sarsaiya S, et al. The anti-diabetic activities, gut microbiota composition, the anti-inflammatory effects of Scutellaria-coptis herb couple against insulin resistance-model of diabetes involving the toll-like receptor 4 signaling pathway. J Ethnopharmacol. 2019;237:202–214. doi:10.1016/j.jep.2019.02.040
123. Xiao S, Liu C, Chen M, et al. Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl Microbiol Biotechnol. 2020;104(1):303–317. doi:10.1007/s00253-019-10174-w
124. Zhang B, Yue R, Chen Y, et al. The herbal medicine scutellaria-coptis alleviates intestinal mucosal barrier damage in diabetic rats by inhibiting inflammation and modulating the gut microbiota. Evid Based Complement Alternat Med. 2020;2020:4568629. doi:10.1155/2020/4568629
125. Shin NR, Gu N, Choi HS, Kim H. Combined effects of Scutellaria baicalensis with metformin on glucose tolerance of patients with type 2 diabetes via gut microbiota modulation. Am J Physiol Endocrinol Metab. 2020;318(1):E52–e61. doi:10.1152/ajpendo.00221.2019
126. Yang G, Lin CC, Yang Y, et al. Nobiletin prevents trimethylamine oxide-induced vascular inflammation via inhibition of the NF-κB/MAPK pathways. J Agric Food Chem. 2019;67(22):6169–6176. doi:10.1021/acs.jafc.9b01270
127. Wang K, Feng X, Chai L, Cao S, Qiu F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab Rev. 2017;49(2):139–157. doi:10.1080/03602532.2017.1306544
128. Habtemariam S. Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol Res. 2020;155:104722. doi:10.1016/j.phrs.2020.104722
129. Zhu L, Zhang D, Zhu H, et al. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe(-/-) mice. Atherosclerosis. 2018;268:117–126. doi:10.1016/j.atherosclerosis.2017.11.023
130. Li X, Su C, Jiang Z, et al. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes. 2021;7(1):36. doi:10.1038/s41522-021-00205-8
131. Wu M, Yang S, Wang S, et al. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE-/- Mice. Front Pharmacol. 2020;11:223. doi:10.3389/fphar.2020.00223
132. Dou Y, Huang R, Li Q, et al. Oxyberberine, an absorbed metabolite of berberine, possess superior hypoglycemic effect via regulating the PI3K/Akt and Nrf2 signaling pathways. Biomed Pharmacother. 2021;137:111312. doi:10.1016/j.biopha.2021.111312
133. Zhang W, Xu JH, Yu T, Chen QK. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed Pharmacother. 2019;118:109131. doi:10.1016/j.biopha.2019.109131
134. Zhang X, Zhao Y, Zhang M, et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7(8):e42529. doi:10.1371/journal.pone.0042529
135. Liu D, Zhang Y, Liu Y, et al. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp Clin Endocrinol Diabetes. 2018;126(8):513–520. doi:10.1055/s-0043-125066
136. Wang Y, Liu H, Zheng M, et al. Berberine slows the progression of prediabetes to diabetes in Zucker diabetic fatty rats by enhancing intestinal secretion of glucagon-like peptide-2 and improving the gut microbiota. Front Endocrinol (Lausanne). 2021;12:609134. doi:10.3389/fendo.2021.609134
137. Li M, Zhou W, Dang Y, Li C, Ji G, Zhang L. Berberine compounds improves hyperglycemia via microbiome mediated colonic TGR5-GLP pathway in db/db mice. Biomed Pharmacother. 2020;132:110953. doi:10.1016/j.biopha.2020.110953
138. Zhang Y, Gu Y, Ren H, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11(1):5015. doi:10.1038/s41467-020-18414-8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.