Back to Journals » OncoTargets and Therapy » Volume 12
Neutrophil–lymphocyte ratio (NLR) predicted prognosis for advanced non-small-cell lung cancer (NSCLC) patients who received immune checkpoint blockade (ICB)
Authors Ren F, Zhao T, Liu B, Pan L
Received 21 December 2018
Accepted for publication 12 March 2019
Published 29 May 2019 Volume 2019:12 Pages 4235—4244
DOI https://doi.org/10.2147/OTT.S199176
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Fangping Ren,1,* Tian Zhao,2,* Bing Liu,3 Lei Pan1
1Department of Respiratory, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, People’s Republic of China; 2Department of Clinical Laboratory, The 161st Hospital of PLA, Wuhan, 430010, People’s Republic of China; 3Department of Disease Control and Prevention, PLA Rocket Force Characteristic Medical Center, Beijing, People’s Republic of China
*These authors contributed equally to this work
Purpose: The aim of this study was to identify the prognostic value of blood neutrophil–lymphocyte ratio (NLR) in patients with advanced non-small-cell lung cancer (NSCLC) who received immune checkpoint blockade (ICB) therapy.
Materials and methods: 147 advanced NSCLC patients were enrolled in this study from June 30, 2013, to August 30, 2017. Survival analysis used the Kaplan and Meier methodology. The mean follow-up time was 2.6 years. The phenotypic T cells subtypes were evaluated by flow cytometry.
Results: Of these patients, receiver operating characteristic (ROC) curves analysis were used to confirm the cut-off value, and patients were stratified into NLR>2.5 (n=88) and NLR≤2.5 (n=59) groups. Survival analysis showed that patients with NLR≤2.5 had significantly favorable overall survival (OS) and progression-free survival (PFS) compared with patients with NLR>2.5. After stratified with the tumor mutational burden (TMB), we further found that patients with NLR≤2.5 had significantly favorable OS and PFS compared with patients with NLR>2.5 in the group of patients with TMB>10, while in group patients with TMB≤10, patients with NLR≤2.5 had no significantly favorable OS and PFS compared with patients with NLR>2.5. The CD3+ and CD8+/CD28+ T cell subsets were significantly increased in patients with NLR≤2.5 (P<0.05), while the CD8+/CD28− and CD4+/CD25+ cell subsets were significantly decreased in patients with NLR≤2.5 (P<0.05).
Conclusion: High NLR value independently predicted poorer survival in advanced NSCLC patients received ICB therapy. The NLR may help oncologists to predict outcomes of patients received ICB and choose alternative therapies for patients with high NLR value.
Keywords: NLR, NSCLC, ICB
Introduction
Lung cancer, one of the most common cancers with high degree of malignancy, is a devastating disease with a poor prognosis worldwide.1–4 Approximately 85% of the lung cancers are diagnosed as non-small-cell lung cancer (NSCLC), which composed of 2 major histology subtypes: squamous (SQ) and non-squamous (non-SQ) carcinoma. A large proportion of patients are diagnosed at advanced-stage in this world, which would be the leading cause of cancer-related mortality. Newer therapeutic modalities for NSCLC have focused on targeting the immune system.5
Immune checkpoint blockade (ICB), which has been approved as second-line treatment of advanced NSCLC, has led to paradigm shifts in the use of manipulation of immune cells to control tumor growth. Therapy targeting the programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) checkpoint has produced an impressive response rate in patients with multiple treatment-refractory metastatic diseases.6 Although high levels of PD-L1 expression may indicate a greater possibility of response to anti-PD-1/PD-L1 treatment of ICB, PD-L1 expression detected by immunohistochemistry (IHC) is based on tumor histology and space changes and temporal factors (for example, the time and location frame of the biopsy). In addition, it has been reported that some patients with little or no PD-L1 expression also benefit from treatment of ICB.7
Recent studies have shown that the status of tumor-infiltrating lymphocyte (TIL) could predict response to anti-PD-1 or PD-L1 treatment.8 Patients with high T effect or interferon-related gene expression showed improved both progressive PFS and OS with anti-PD-1 or PD-L1 therapy.9 A predictive model based on the expression of CD8 was established by detecting tumor biopsies before and during anti-PD-1 treatment against melanoma.10 In a mouse model, Tang et al showed that adequate T cell infiltration but not PD-L1 expression is more significant and critical for tumor response to checkpoint blockade.11 Moreover, tumor mutation burden (TMB) has become a potential measure of genomic instability and neoantigens used to predict tumor response to immunotherapy, including ICB and adoptive cell therapy. Previous studies have shown that the higher non-synonymous mutation burden assessed by whole exome sequencing is objectively related to patients with NSCLC treated with ICB.12 In addition to whole exon sequencing, the prediction accuracy of the tumor mutation burden was reported using a comprehensive genomic analysis in NSCLC.13
Increasing evidence showed that systemic inflammatory activation in cancer cells predicted tumor progression by inducing cancer proliferation and metastasis or promoting angiogenesis.14 The neutrophil–lymphocyte ratio (NLR), which has been considered as a member of the marker of the systemic inflammation response, is valuable for predicting the prognosis of various cancers.15,16 Clearly, challenges remain to identify reliable, cost-effective biomarkers to determine which patients are most likely to benefit from ICB and avoid unnecessary immune-related adverse events.
In the present study, we evaluated the prognostic value of NLR in advanced NSCLC patients received treatment of anti-PD-1 inhibitors. The relationship between the NLR and immune cells phenotypes and other clinical characteristics was further analyzed.
Patients and methods
Study design and participants
The cohort included 147 consecutive NSCLC patients between June 30, 2013, and August 30, 2017, retrospectively. The study was approved by the Regional Ethics Review Committee of the Cancer Center of the Capital Medical University. Patients were treated according to the ethical principles of the Helsinki Declaration concerning medical research in human subjects. All patients provided informed written consent prior to entering the study. Patients need to meet the following inclusion criteria: Participants are between 18 and <80 years old; Eastern Cooperative Oncology Group performance status (ECOG-PS)17≤2; Histology or cytology confirmed unresectable, locally advanced or metastatic NSCLC; No immunotherapy was performed prior to this study. If the patient has a concurrent malignancy other than NSCLC, a severe, uncontrolled disease or psychiatric disorder that limits the ability to meet the research requirements, then the patient is excluded.
Pretreatment evaluation
Each patient recorded a medical history and physical examination results. Each patient also had an electrocardiogram, a computed tomography scan of the abdomen and pelvis (as well as the chest, if needed), serum chemistry and CBC, and urine analysis.
Procedures
Receipt of radiotherapy, adjuvant chemotherapy, and number of previous lines of palliative intent chemotherapy preceding PD-1 antibody treatment were recorded. The chemotherapy choices were following platinum-based chemotherapy regimens for 4 to 6 cycles: carboplatin plus pemetrexed, cisplatin plus pemetrexed, carboplatin plus gemcitabine, cisplatin plus gemcitabine, or carboplatin plus paclitaxel. PD-1 inhibitors were administered according to standard dosing: nivolumab 3 mg/kg body weight every 2 weeks and pembrolizumab 2 mg/kg body weight every 3 weeks. Adverse events were assessed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0 (NCI-CTCAE v4.0) and response to treatment was assessed by the Response Evaluation Criteria in Solid Tumors (
Tissue IHC
Tissue staining was performed retrospectively on a subset of 86 patients with IHC-accessible tissue. All of these patients were treated with an immunological checkpoint blockade. Of all patients treated with immunological checkpoint block, 61 were excluded due to inaccessible pathology or insufficient IHC samples (eg, samples obtained by fine needle aspiration biopsy). Tissue staining was performed on PD-L1 (tumor), PD-L1 (Immunomicroenvironment), CD3, CD4, CD8, CD25, and CD56. The primary antibody against PD-L1 (SP263, Ventana) was used according to the manufacturer instructions. primary antibody of anti-CD3-FITC/anti-CD56-RPE (Dako), anti-CD3-FITC (fluorescein isothiocyanate), anti-CD4-RPE, anti-CD8-RPE, anti-CD45RO, and anti-CD4-FITC/anti-CD25-PE (BD Biosciences) were used. The sample was read and explained by the attending pathologist. The data report the percentage of cells stained for each particular marker.
Definition of TMB
TMB is determined by Foundation Medicine. Calculate TMB by including all substitutions and insertions in the entire somatic cell, coding, and sequencing length.18,19 Synonymous mutations were included given their potential to promote genomic instability. Noncoding alterations were excluded. TMB was calculated in mutations per megabase pair. TMB categories were determined by Foundation Medicine on the basis of the following ranges, rounded to the nearest whole number: low (0–10), intermediate (11–19), high (20–34), and very high (35 and above).
Analysis of the circulating immune response
Peripheral venous blood was obtained from each patient at various time points. Whole blood (100 mL) was incubated with primary antibody in the dark for 15 mins at 4°C. anti-CD3-FITC/anti-CD56-RPE (Dako), anti-CD3-FITC (fluorescein isothiocyanate), anti-CD4-RPE, anti-CD8-RPE, anti-CD45RO and anti-CD4-FITC/anti-CD25-PE (BD Biosciences). After 10 mins of hemolysis, the samples were centrifuged at 1,500 rpm for 10 mins at room temperature, then washed twice in PBS and subjected to flow cytometry analysis. Three-color flow cytometry analysis was performed using FC500 (Beckman-Coulter) and CXP analysis software (Beckman-Coulter) to determine the cell phenotype. Lymphocytes are gated by forward scatter and side scatter. The analysis will collect 5,000 gated events.
Statistical methods
Continuous variables were expressed as mean±SD (standard deviation) and compared using a two-tailed unpaired Student’s t-test; categorical variables were compared using χ2 or Fisher analysis. The predictive performance of NLR was measured using the area under receiver operating characteristic (ROC) curve (AUC) .20 Survival analysis used the Kaplan and Meier methodology.21 A Cox proportional hazards regression approach22 was used for evaluating PFS and OS, which was the primary end-point. Univariate analysis of possible prognostic variables, using a factor at a time, followed by a multivariate model combining all factors. Results were reported as hazard ratio (HR) and 95% confidence interval (CI). HR >1 indicates an increase in risk relative to the reference category. A confidence interval that does not include a value of 1 indicates a statistical significance of 5% level. All statistical evaluations were performed using SPSS software (Statistical Package for the Social Science, version 15.0, SPSS Inc, Chicago, IL). Values of P<0.05 were considered to be statistically significant in all analyses.
Results
Patients’ characteristics
The 147 patients in this study were treated with immune checkpoint blockade including nivolumab and pembrolizumab. We then performed ROC curves analysis to stratify all patients into NLR>2.5 (n=88) and NLR≤2.5 (n=59) groups according to the prognosis of patients with NSCLC. Characteristics of all patients are detailed in Table 1. There were no significant differences in relevant baseline characteristics of age, gender, histologic subtypes, PD-1 antibodies, TMB, presence of EGFR/KRAS Mutations, No. prior lines of treatment, TNM stages, and site of metastasis between the two groups divided by NLR, as shown in Table 1. There were significant differences in ECOG-PS status (P=0.008), smoking status (P=0.024), and expression of PD-L1 (P=0.048) between the two groups divided by NLR.
 | Table 1 Demographics and baseline characteristics of patients |
Survival analysis of patients with NSCLC with respect to NLR
In this study, we performed ROC analysis to confirm 2.5 as the cut-off value of NLR according to the survival of patients with NSCLC (Figure 1). After then, we performed survival analysis to identify the factors associated with prognosis of patients with NSCLC received PD-1 antibodies. The results showed that patients with NLR≤2.5 had significantly favorable OS (P=0.009, Figure 2A) and PFS (P=0.017, Figure 2B) compared with patients with NLR>2.5. Patients with TMB>10 had significantly favorable OS (P=0.003, Figure 2C) and PFS (P=0.001, Figure 2D) compared with patients with TMB≤10. After stratified with the variable of TMB, we further found that patients with NLR≤2.5 had significantly favorable OS (P=0.003, Figure 3A) and PFS (P=0.001, Figure 3B) compared with patients with NLR>2.5 in the group of patients with TMB>10, while in group patients with TMB≤10, patients with NLR≤2.5 had no significantly favorable OS (P=0.251, Figure 3C) and PFS (P=0325, Figure 3D) compared with patients with NLR>2.5.
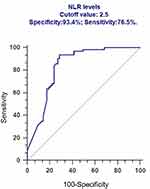 | Figure 1 ROC analysis to confirm 2.5 as the cut-off value of NLR according to the survival of patients with NSCLC. |
Predictors associated with clinical outcomes
Cox proportional hazards models were then used to quantify the prognostic significance of risk factors after multivariable adjustment. A multivariable analysis was performed to assess the factors that demonstrated significant effects in univariate analysis. After adjusting for competing risk factors, NLR≤2.5 (HR: 2.165, 95% CI: 1.392–5.201, P=0.012), TMB≤10 (HR: 2.439, 95% CI: 1.243–5.493, P=0.007) and No. of sites metastasis (HR: 3.154, 95% CI: 1.229–6.593, P=0.002) remained independent predictors of PFS and OS. The details are shown in Figure 4.
 | Figure 4 Cox proportional hazards models were used to quantify the prognostic significance of risk factors after multivariable adjustment. |
Phenotypic analysis of peripheral blood immune cells
Phenotypic analysis of peripheral blood mononuclear cells before the treatment and at the end of the first cycle of therapy demonstrated that the CD3+ and CD8+/CD28+ T cell subsets were significantly increased in patients with NLR≤2.5 (P<0.05), while the CD8+/CD28− and CD4+/CD25+ cell subsets were significantly decreased in patients with NLR≤2.5 (P<0.05) (Figure 4). However, only CD3+ T cell subsets were significantly increased in patients with NLR>2.5 (P<0.05) (Figure 5).
Treatment toxicity
Among both hematologic and nonhematologic adverse events, no significant differences were shown in various groups. Moreover, there were no significant differences in adverse events between the groups of patients with NLR≤2.5 and NLR>2.5, as shown in Table 2.
 | Table 2 Hematological and nonhematologic adverse events |
Discussion
Although PD-1 checkpoint inhibitors improved both OS and PFS for patients with NSCLC have been reported in several clinical trials,23,24 SQ-NSCLC and non-SQ-NSCLC showed differences in anti-PD-1 efficacy and response.25 Compared with chemotherapy, ICBs demonstrated a delayed OS benefit but no statistically significant improvement in PFS for non-SQ-NSCLC. Moreover, treatment-related adverse events of grade 3 or 4 were acceptable in the immunotherapy group compared with the chemotherapy group.26,27 ICBs plus chemotherapy are emerging as effective first-line treatment in advanced NSCLC, but little is known about the magnitude of benefits and potential clinical predictors. pembrolizumab monotherapy has replaced chemotherapy as the first-line treatment for patients with PD-L1 tumor proportion score (TPS) of at least 50%,28,29 and pembrolizumab plus platinum and pemetrexed for those with nonsquamous histology irrespective of PD-L1 expression.30,31 Nevertheless, little is known about the magnitude of benefits and potential clinical predictors. Moreover, new data from anti-PD treatment clinical trials show that only about 15–25% of the NSCLC patients respond to immune checkpoint block therapy, and most patients have primary resistance.32
This study showed that assessment of the NLR calculated from complete blood counts before treatments predicted survival of patients with advanced NSCLC independently. Patients with NLR≤2.5 had significantly favorable OS and PFS compared with patients with NLR>2.5. After stratified with the variable of TMB, we further found that patients with NLR≤2.5 had significantly favorable OS and PFS compared with patients with NLR>2.5 in the group of patients with TMB>10, while in group patients with TMB≤10, patients with NLR≤2.5 had no significantly favorable OS and PFS compared with patients with NLR>2.5. The result is consistent with previously published papers displaying that high NLR with poor outcome in patients with lung cancer.33–35 Yet, the cutoff value of the NLR is inconsistent in these above studies, which reduces its clinical applicability. From our perspective, we believe that the impact of the NLR has been explored as a continuous explanatory variable, and it is affected by the patients baselines and therapeutic approaches. Various treatment prior to immunotherapy is an important factor in the oncologic outcome. In this study, patients receive chemotherapy including platinum, paclitaxel, gemcitabine, docetaxel, and so on. Consequently, we explored the pretreatment value of 2.5 as the most appropriate cutoff, not only the statistical sensitivity and specificity were taken into account but also the clinical significance. Our data indicated that the median OS of patients in NLR>2.5 group was much shorter when compared with those in NLR≤2.5 group.
The mechanism underlying the potential prognostic value of NLR is mainly due to the significance of the infiltrated neutrophils and lymphocytes. The systemic inflammatory response from cancer cells promotes the infiltration of neutrophils, which benefits cancer progression via secreting interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor α (TNF-α), and vascular endothelial growth factor (VEGF).36 VEGF is a proangiogenic factor contributes to cancer development especially through angiogenesis. Moreover, increased TNF-α and IL-10 issue in lymphocyte count decrease and lymphocyte dysfunction also. It is well known that lymphocyte depletion is likely reflection of an impaired T-lymphocyte-mediated antitumor response, which represents an adverse prognostic trait. In general, the relative ratio of elevated neutrophils and decreased lymphocytes could be a scientific marker for evaluating the systemic inflammatory response and outcome of individuals. And so, NLR is valuable as a potential indicator of prognosis to some degree.
By the way, this study is in small size and retrospective design. On the other hand, the relationship between survival and change of NLR after treatment apart from pretreatment can be investigated in future studies.
Conclusion
A high NLR value independently predicts poor survival in advanced NSCLC patients received ICB. The NLR may help oncologists evaluate outcomes of patients received immunotherapy and choose alternative therapies for patients with high NLR value.
Disclosure
The authors who have taken part in this study declared that they have nothing to disclose regarding funding or conflict of interest with respect to this manuscript.
References
1. Camerini A, Banna GL, Cinieri S, et al. Metronomic oral vinorelbine for the treatment of advanced non-small cell lung cancer: a multicenter international retrospective analysis. Clin Transl Oncol. 2018. doi:10.1007/s12094-018-1989-y
2. Walia R, Jain D, Madan K, et al. p40 & thyroid transcription factor-1 immunohistochemistry: A useful panel to characterize non-small cell lung carcinoma-not otherwise specified (NSCLC-NOS) category. Indian J Med Res. 2017;146(1):42–48. doi:10.4103/ijmr.IJMR_1221_15
3. Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377(9):849–861. doi:10.1056/NEJMra1703413
4. Reck M, Rabe KF. Advanced non-small-cell lung cancer. N Engl J Med. 2017;377(20):1999. doi:10.1056/NEJMc1712794
5. Rangachari D, Brahmer JR. Targeting the immune system in the treatment of non-small-cell lung cancer. Curr Treat Options Oncol. 2013;14(4):580–594. doi:10.1007/s11864-013-0250-8
6. Kopalli SR, Kang TB, Lee KH, Koppula S. Novel small molecule inhibitors of programmed cell death (PD)-1, and its ligand, PD-L1 in cancer immunotherapy: a review update of patent literature. Recent Pat Anticancer Drug Discov. 2018. doi:10.2174/1574892813666181029142812
7. De Lima Lopes G, Wu YL, Sadowski S, et al. P2.43: pembrolizumab vs platinum-based chemotherapy for PD-L1+ NSCLC: phase 3, randomized, open-label KEYNOTE-042 (NCT02220894): track: immunotherapy. J Thorac Oncol. 2016;11(10s):S244–s5. doi:10.1016/j.jtho.2016.08.114
8. Bremnes RM, Busund LT, Kilvaer TL, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016;11(6):789–800. doi:10.1016/j.jtho.2016.01.015
9. Chen J, Chen Y, Feng F, et al. Programmed cell death protein-1/programmed death-ligand 1 blockade enhances the antitumor efficacy of adoptive cell therapy against non-small cell lung cancer. J Thorac Dis. 2018;10(12):6711–6721. doi:10.21037/jtd.2018.10.111
10. Wieland A, Kamphorst AO, Adsay NV, et al. T cell receptor sequencing of activated CD8 T cells in the blood identifies tumor-infiltrating clones that expand after PD-1 therapy and radiation in a melanoma patient. Cancer Immunol Immunother. 2018;67(11):1767–1776. doi:10.1007/s00262-018-2228-7
11. Tang H, Wang Y, Chlewicki LK, et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;30(3):500. doi:10.1016/j.ccell.2016.08.011
12. Endris V, Buchhalter I, Allgauer M, et al. Measurement of Tumor Mutational Burden (TMB) in routine molecular diagnostics: in-silico and real-life analysis of three larger gene panels. Int J Cancer. 2018. doi:10.1002/ijc.32002
13. Riediger AL, Dietz S, Schirmer U, et al. Mutation analysis of circulating plasma DNA to determine response to EGFR tyrosine kinase inhibitor therapy of lung adenocarcinoma patients. Sci Rep. 2016;6:33505. doi:10.1038/srep33505
14. Hainaut P, Plymoth A. Targeting the hallmarks of cancer: towards a rational approach to next-generation cancer therapy. Curr Opin Oncol. 2013;25(1):50–51. doi:10.1097/CCO.0b013e32835b651e
15. Jung MR, Park YK, Jeong O, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104(5):504–510. doi:10.1002/jso.21986
16. Zhang H, Xia H, Zhang L, Zhang B, Yue D, Wang C. Clinical significance of preoperative neutrophil-lymphocyte vs platelet-lymphocyte ratio in primary operable patients with non-small cell lung cancer. Am J Surg. 2015;210(3):526–535. doi:10.1016/j.amjsurg.2015.03.022
17. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655.
18. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi:10.1186/s13073-017-0424-2
19. Wang L, Li F, Sheng J, Wong ST. A computational method for clinically relevant cancer stratification and driver mutation module discovery using personal genomics profiles. BMC Genomics. 2015;16(Suppl 7):S6. doi:10.1186/1471-2164-16-s7-s6
20. Hanley JA. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging. 1989;29(3):307–335.
21. Kaplan EL, Meier P. Nonparametric estimations from incomplete observations. J Am Stat Assoc. 1958;53:457–481.
22. Cox DR. Regression models and life-tables. J Royal Stat Soc B. 1972;34:187–220.
23. Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18(12):1600–1609. doi:10.1016/s1470-2045(17)30690-3
24. Gadgeel SM, Stevenson JP, Langer CJ, et al. Pembrolizumab and platinum-based chemotherapy as first-line therapy for advanced non-small-cell lung cancer: phase 1 cohorts from the KEYNOTE-021 study. Lung Cancer. 2018;125:273–281. doi:10.1016/j.lungcan.2018.08.019
25. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865
26. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643
27. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced non-small cell lung cancer: checkMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019. doi:10.1016/j.jtho.2019.01.006
28. Schulze AB, Schmidt LH. PD-1 targeted immunotherapy as first-line therapy for advanced non-small-cell lung cancer patients. J Thorac Dis. 2017;9(4):E384–E6. doi:10.21037/jtd.2017.03.118
29. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
30. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi:10.1016/s1470-2045(16)30498-3
31. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
32. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi:10.1016/s0140-6736(16)32517-x
33. Lin GN, Peng JW, Liu PP, Liu DY, Xiao JJ, Chen XQ. Elevated neutrophil-to-lymphocyte ratio predicts poor outcome in patients with advanced non-small-cell lung cancer receiving first-line gefitinib or erlotinib treatment. Asia Pac J Clin Oncol. 2017;13(5):e189–e94. doi:10.1111/ajco.12273
34. Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin Chim Acta. 2018;484:272–277. doi:10.1016/j.cca.2018.05.059
35. Kang MH, Go SI, Song HN, et al. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer. 2014;111(3):452–460. doi:10.1038/bjc.2014.317
36. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/s0140-6736(00)04046-0
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



