Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Neutrophil-to-Lymphocyte Ratio on Admission is an Independent Risk Factor for the Severity of Neurological Impairment at Disease Onset in Patients with a First Episode of Neuromyelitis Optica Spectrum Disorder
Authors Zhou Y, Xie H, Zhao Y, Zhang J, Li Y, Duan R, Yao Y, Jia Y
Received 19 March 2021
Accepted for publication 29 April 2021
Published 18 May 2021 Volume 2021:17 Pages 1493—1503
DOI https://doi.org/10.2147/NDT.S311942
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Yongyan Zhou, Haojie Xie, Yi Zhao, Jinwei Zhang, Yanfei Li, Ranran Duan, Yaobing Yao, Yanjie Jia
Department of Neurology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Correspondence: Yanjie Jia
Department of Neurology, The First Affiliated Hospital of Zhengzhou University, Erqi District, Zhengzhou City, Henan Province, People’s Republic of China
Tel +861 351 380 2916
Email [email protected]
Purpose: To investigate the relationship between the neutrophil-to-lymphocyte ratio (NLR) and the severity of neurological impairment at disease onset in patients with a first episode of neuromyelitis optica spectrum disorder (NMOSD).
Patients and Methods: This retrospective study included 259 patients with newly diagnosed NMOSD who were hospitalized at our institution between January 2013 and January 2020 (NMOSD group) and 169 healthy control subjects who underwent a physical examination at our hospital during the same period (control group). The clinical data collected included general information, past medical history, biochemical test results, imaging findings, NLR, AQP-4 antibody status, and initial Expanded Disability Status Scale score. A logistic regression model was used to analyze NLR as an independent risk factor for the severity of neurological impairment at disease onset in the NMOSD group. Receiver-operating characteristic curve analysis was used to evaluate the ability of the NLR to predict the severity of neurological impairment at disease onset in the NMOSD group and to determine its critical value.
Results: The NLR was significantly higher in the NMOSD group than in the control group (P< 0.001). In the NMOSD group, neurological impairment at disease onset was more severe in those with a high NLR than in those with a low NLR (P< 0.001). At onset of disease, patients with severe neurological impairment had a more significant increase in NLR than those with mild-to-moderate neurological impairment (P< 0.001). Both univariate (OR 1.180, 95% CI 1.046– 1.331, P=0.007) and multivariate (OR 1.146, 95% CI 1.003– 1.308, P=0.044) logistic regression analyses showed that the NLR was positively correlated with the severity of neurological impairment at onset of disease in the NMOSD group. The area under the receiver-operating characteristic curve was 0.687.
Conclusion: The NLR is an independent risk factor for the severity of neurological impairment at disease onset in patients with a first episode of NMOSD.
Keywords: neuromyelitis optica spectrum disorder, neutrophil-to-lymphocyte ratio, first episode, severity, Expanded Disability Status Scale score
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a primary inflammatory demyelinating disease of the central nervous system (CNS) that mainly involves the optic nerve and spinal cord and characterized by antiaquaporin-4 (AQP4) antibody-mediated autoimmune astrocytopathy.1 Recent worldwide epidemiological surveys showed differences in the regional and ethnic prevalence of NMOSD and a higher prevalence in Africans and non-whites.2,3 Most patients with NMOSD have a relapsing-remitting course, with an increase in the number of relapses, a decrease in the recovery rate, an increase in the degree of disability, and, eventually, severe visual or motor impairment with progression of the disease.4 Diagnosis of NMOSD is based mainly on clinical manifestations, AQP4 antibody status in serum or cerebrospinal fluid (CSF), and manifestations in the spinal cord or optic nerve seen on magnetic resonance imaging (MRI). In recent years, many researchers have focused on biological indicators of the severity of neurological impairment in patients with NMOSD. However, little progress has been made in terms of identification of cost-effective, easily accessible, and reliable biological indicators that would assist in early detection of patients with NMOSD to improve the prognosis.
The neutrophil-to-lymphocyte ratio (NLR) is a novel inflammatory index that has been used to predict the prognosis of non-small cell lung cancer, squamous cell carcinoma of the lung, colorectal cancer, liver cancer, esophageal and gastric cancer, kidney cancer, bladder cancer, melanoma and other malignant tumors, as well as cerebral hemorrhage, cerebral infarction, and cardiovascular disease.5–15 The NLR has also been associated with the activity of multiple sclerosis, systemic lupus erythematosus, Sjogren’s syndrome, Behçet’s disease, and other autoimmune diseases.16–19 However, there are few studies on the NLR in patients with NMOSD and the relationship between the severity of neurological impairment and the NLR in these patients. This study evaluated the relationship between NLR and the severity of neurological impairment at onset of disease in patients with a first episode of NMOSD.
Patients and Methods
Study Population
Clinical data were collected retrospectively for the 336 patients with newly diagnosed NMOSD who were admitted as inpatients to the First Affiliated Hospital of Zhengzhou University from January 2013 to January 2020 (NMOSD group). All patients met the diagnostic criteria for NMOSD established by the International Panel for NMO Diagnosis in 2015 and were diagnosed by an experienced neurologist. The following exclusion criteria were applied: 1) not a first episode of NMOSD; 2) other diseases affecting the Expanded Disability Status Scale (EDSS) score; 3) hematological disease, the active period of other chronic infectious diseases or other conditions that could affect the blood count; 4) hormone therapy, immunosuppressive treatment, or other agents that can affect the blood count within the previous 6 months; and 5) incomplete data. A total of 259 patients were finally included in the study. One hundred and sixty-nine healthy individuals who underwent a physical examination at our institution during the same period and were matched to the NMOSD group for age, sex, smoking history, and history of alcohol consumption were selected as a control group.
The study was reviewed and approved by the Ethics Committee of Zhengzhou University (review number: 2021-KY-0038-002). The requirement for informed consent was waived in view of the retrospective design of the study, the risk of this study is not greater than the minimum risk, and the exemption of informed consent does not have an adverse effect on the rights or welfare of patients. We strictly complied with the Declaration of Helsinki and have anonymized all patient data.
Research Methods
Clinical Data
The medical records of all patients were reviewed. Information on patient age and sex, smoking history, history of alcohol consumption, past medical history (hypertension, diabetes, autoimmune disease, mental disease), and EDSS scores was collected. The initial EDSS score were obtained by either of two experienced neurologists and used to assess the severity of neurological impairment at disease onset in the NMOSD group.
Auxiliary Examinations
All patients in the NMOSD group were fasted for at least 12 hours after admission to hospital and before the start of treatment, blood samples were taken from the cubital vein for a complete blood count. The enrolled patients underwent CSF cytology and MRI (cranial, spinal cord, and optic nerves). The normal reference ranges for relevant indicators established by our hospital laboratory are as follows: white blood cells (3.5–9.5 × 109/L), red blood cells (3.8–5.1 × 1012/L), platelets (125–350 × 109/L), neutrophils (1.8–6.3 × 109/L), lymphocytes (1.1–3.2 × 109/L), monocytes (0.1–0.6 × 109/L), eosinophils (0.02–0.52 × 109/L), and basophils (0–0.06 × 109/L); white blood cells in CSF (0–5 × 106/L), and CSF lymphocyte ratio (60–70%). The NLR was calculated as the absolute number of neutrophils to the absolute number of lymphocytes. MRI scans showing characteristic manifestations of optic nerve, spinal cord, and brain were defined as abnormal and scans showing unspecific lesions, normal brain, and brain demyelinating lesions were defined as normal. Spinal cord MRI scans showing the number of affected spinal cord segments was called spinal cord MRI. The Neurology Laboratory of the First Affiliated Hospital of Zhengzhou University uses live cells transfected with AQP4 to detect AQP-4 antibody status in blood or CSF.
All laboratory tests were performed using samples collected for the first time on admission, and the examiner was blinded to all clinical information.
Statistical Analysis
All statistical analyses were performed using SPSS version 26.0 software (IBM Corp, Armonk, NY, USA). The Kolmogorov–Smirnov test was used to test the normality of the continuous data. Continuous data conforming to a normal distribution were expressed as the mean ± standard deviation (SD); otherwise, they were expressed as the median (IQR). Categorical data were expressed as the percentage. When continuous data were normally distributed, between-group differences were examined using the t-test, and when not normally distributed, they were analyzed using the Mann–Whitney U-test. The chi-square test or Fisher’s exact test was used for between-group comparisons of categorical variables. Single-factor logistic regression analysis was used to identify potential factors affecting the severity of neurological impairment at disease onset in the NMOSD group. Multivariate logistic regression analysis was used to determine the independent effect of the NLR on the severity of neurological impairment at disease onset in the NMOSD group. Receiver-operating characteristic (ROC) curve analysis was used to evaluate the diagnostic value of the NLR in predicting the severity of neurological impairment at disease onset in the NMOSD group and to determine its critical value. A P-value <0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
Table 1 shows the demographic and clinical characteristics of the two study groups. There was no significant difference in sex, age, smoking history, or history of alcohol consumption between the two groups (all P>0.05). White blood cell, neutrophil, and monocyte counts and the NLR were significantly higher (all P<0.001) and the lymphocyte (P=0.005) and eosinophil (P<0.001) counts were significantly lower in the NMOSD group than in the control group. There was no significant between-group difference in the red blood cell, platelet, or basophil count (all P>0.05).
 |
Table 1 Demographic and Clinical Characteristics of Patients in the NMOSD Group and Healthy Subjects in the Control Group |
The median NLR was 2.54 in the 259 patients in the NMOSD group. To evaluate the impact of the NLR on the severity of neurological impairment at disease onset in this group, the patients were divided into a low NLR group (NLR <2.54, n=128) and a high NLR group (NLR ≥2.54, n=131). Table 2 shows the demographic and clinical characteristics of patients in the NMOSD group according to the NLR. There was no significant between-group difference in sex, age, smoking history, history of alcohol consumption, or past medical history of hypertension, diabetes, autoimmune disease, or mental disease (all P>0.05). The initial EDSS score was significantly higher in the high NLR group than in the low NLR group (P<0.001). As well as differences in the neutrophil and lymphocyte counts, we found significantly higher white blood cell (P<0.001) and platelet (P=0.001) counts and significantly lower eosinophil (P<0.001), and basophil (P=0.011) counts in the high NLR group; there was no significant between-group difference in the red blood cell or monocyte count (all P>0.05). We also evaluated the AQP4 antibody status in serum and CSF, white blood cells in CSF, CSF lymphocyte ratio, and abnormalities on MRI, brain lesions, spinal cord MRI between the two groups. Compared with the low NLR group, there was a significant increase in the number of white blood cells in the CSF (P=0.030) and the number of spinal cord segments affected (P=0.042) in the high NLR group. There was no significant difference in the CSF lymphocyte ratio, abnormalities on MRI or brain lesions between these groups (all P>0.05). Only 224 patients in the study were tested for AQP4 antibody status, and there was no significant difference between the high NLR and low NLR groups (P>0.05).
 |
Table 2 Demographic and Clinical Characteristics of Patients Admitted with a First Episode of NMOSD According to the NLR at the Time of Admission |
Patients in the NMOSD group were divided into mild-to-moderate and severe groups according to whether their initial EDSS score was ≤3 or >3 to identify factors that may affect the severity of neurological impairment at disease onset in patients with a first episode of NMOSD. Table 3 compares the demographic and clinical features of these patients according to their initial EDSS score. Compared with patients in the mild-to-moderate group, those in the severe group were significantly older (P=0.041), were significantly more likely to have hypertension (P=0.011) and had significantly higher neutrophil counts (P=0.001), NLR values (P<0.001), and number of spinal cord segments affected (P<0.001), as well as significantly more abnormalities on MRI (P=0.018); they also had significantly lower lymphocyte (P<0.001) and eosinophil (P=0.027) counts. There was no significant between-group difference in sex, past medical history (diabetes, autoimmune disease, or mental disease), blood cell count (white blood cells, red blood cells, platelets, monocytes, basophils), AQP4 antibody status, white blood cells in CSF, CSF lymphocyte ratio, brain lesions (all P>0.05).
 |
Table 3 Demographic and Clinical Characteristics of Patients with a First Episode of NMOSD According to the Initial EDSS Score |
Influence of Clinical Parameters on Severity of Neurological Impairment at Disease Onset in Patients Admitted with a First Episode of NMOSD
As shown in Table 4, single factor logistic regression analysis showed that age (OR 1.017, 95% CI 1.001–1.034, P=0.040), hypertension (OR 4.373, 95% CI 1.278–14.963, P=0.019), abnormalities on MRI (OR 2.328, 95% CI 1.141–4.751, P=0.020), spinal cord MRI (OR 1.016, 95% CI 1.044–1.173, P=0.001), the neutrophil count (OR 1.145, 95% CI 1.027–1.277, P=0.015), and the NLR (OR 1.180, 95% CI 1.046–1.331, P=0.007) were positively correlated with the initial EDSS score in the NMOSD group. Patient sex, smoking history, history of alcohol consumption, past medical history (diabetes, autoimmune disease, or psychiatric illness), AQP4 antibody status, white blood cells in CSF, CSF lymphocyte ratio, brain lesions, and blood cell count (white blood cells, red blood cells, platelets, lymphocytes, monocytes, eosinophils, and basophils) were not significantly correlated with the initial EDSS score (all P>0.05).
 |
Table 4 Single-Factor Logistic Regression Analysis of Factors Related to the Severity of Neurological Impairment at Disease Onset in Patients with a First Episode of NMOSD |
NLR at Admission is an Independent Risk Factor for Severity of Neurological Impairment at Disease Onset in Patients with a First Episode of NMOSD
We consider that the neutrophil count has a serious impact on the NLR. All factors (except for neutrophils) that were analyzed in the univariate analysis and found to have a P-value <0.2 or >0.2 but believed to have a clinical impact on the initial EDSS, including age, sex, hypertension, diabetes, white blood cells in CSF, and abnormalities on MRI, spinal cord MRI, and white blood cells, NLR, eosinophils were included in the multivariate logistic regression model. As shown in Table 5, the NLR (OR 1.146, 95% CI 1.003–1.308, P=0.044) and spinal cord MRI (OR 1.084, 95% CI 1.012–1.161, P=0.022) were independent risk factors for the severity of neurological impairment at disease onset in the NMOSD group.
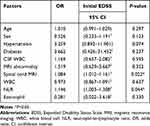 |
Table 5 Multivariate Logistic Regression Analysis of Factors Related to the Severity of Neurological Impairment at Disease Onset in Patients with a First Episode of NMOSD |
ROC Curve for NLR at Admission Predicts Severity of Neurological Impairment at Disease Onset in Patients with a First Episode of NMOSD
As shown in Figure 1, the ROC curve for NLR predicted the severity of neurological impairment at disease onset in the NMOSD group. Table 6 shows that the area under the ROC curve was 0.687 (95% CI 0.618–0.755). The critical value was 2.754, the sensitivity was 57.5%, and the specificity was 76.5%.
 |
Table 6 Ability of the ROC Curve for NLR to Predict the Severity of Neurological Impairment at Disease Onset in Patients with a First Episode of NMOSD |
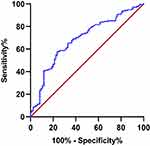 |
Figure 1 The ROC curve of NLR predicts the severity of neurological impairment at disease onset in patients with a first episode of NMOSD. |
Discussion
To the best of our knowledge, this is the first study to investigate the relationship between the NLR at the time of admission and the severity of neurological impairment at disease onset in patients with a first episode of NMOSD. We found that the NLR was significantly higher in these patients than in healthy controls. In the NMOSD group, neurological impairment at disease onset was significantly more severe in patients with a high NLR than in those with a low NLR. Moreover, the NLR was significantly higher in the group with severe neurological impairment than in the group with mild-to-moderate neurological impairment at disease onset. Both univariate and multivariate regression analyses revealed a significant positive correlation of the NLR with the severity of neurological impairment at disease onset in the NMOSD group. Therefore, the NLR at the time of admission is an independent risk factor for the severity of neurological impairment at disease onset in patients with a first episode of NMOSD. The results of the ROC curve analysis indicated that the NLR on admission may also be a valuable predictor of the severity of neurological impairment at disease onset in these patients.
It is currently believed that the main pathogenic mechanism of NMOSD involves antibody-mediated complement-dependent cytotoxic pathways that cause damage to astrocytes, leading to infiltration of inflammatory cells and release of inflammatory mediators followed by para-inflammatory mechanisms that damage oligodendrocytes and cause demyelination.20 The main cytokines and inflammatory cells involved in the pathogenesis of NMOSD are interleukin (IL)-17, IL-8, IL-6, IL-1β, IL-10, IL-3, IL-4, neutrophils, T-cells, macrophages, and eosinophils.21–25 Neutrophils are recognized as an indicator of acute systemic inflammation. In the acute phase of inflammation, neutrophils proliferate and become activated rapidly, and then migrate to the site of inflammation.26 Studies have found a higher proportion of TH17 cells and higher concentrations of TH17-related cytokines, such as IL-17, IL-6, IL-21, IL-23, and TGF-β, in the serum of patients with NMOSD and that these cells and cytokines promote recruitment of neutrophils.27 Research in a mouse model of NMOSD found that neutrophil colony-stimulating factor aggravated the disorder whereas neutrophil protease inhibitors reduced its severity. Therefore, neutrophils are likely to play an important role in the pathogenesis of NMOSD.28 A variety of agents targeting neutrophils are presently under investigation in clinical trials, and it is anticipated that clinical application of some of these agents will reduce the degree of disability in patients with NMOSD.29 Lymphocytes are also an important component of white blood cells and participate in humoral and cellular immunity. B lymphocytes produce pro-inflammatory cytokines and antibodies that are involved in the pathogenesis of inflammatory diseases of the CNS. They also produce anti-inflammatory cytokines that play a role in autoimmune regulation.30 Treatment that targets B lymphocytes significantly reduces disease activity and improves the prognosis of NMOSD but, for various reasons, including high cost, is not widely used in clinical practice. Autoreactive T-cells also contribute to the pathogenesis of NMOSD by regulating the passage of antibodies across the blood-brain barrier and helping to create a local inflammatory environment.31
The blood cell count alone is readily affected by many physiological factors, including pressure and stress, and cannot be used alone to predict inflammation. However, the NLR is not easily affected by other factors in the body and reflects the inflammatory state and extent of the associated damage more reliably than the blood cell count. A recent study showed that the NLR was higher during acute episodes of NMOSD than during periods of remission, indicating that there is a relationship between the NLR and activity of NMOSD.32 Another study in pediatric patients with demyelinating disease found an increase in NLR in children during an acute episode of myelin oligodendrocyte glycoprotein antibody disease and that the NLR was related to disease activity.33 Previous studies of inflammatory diseases of the CNS have rarely investigated the relationship between the NLR and the severity of disease. In our present study, the NLR at the time of admission was an independent risk factor for severity of neurological impairment at disease onset in patients with a first episode of NMOSD. The area under the ROC curve was 0.687, we can use NLR as a predictor of the severity of neurological impairment at disease onset in patients with first-onset NMOSD. In actual clinical work, the NLR may aid early detection and active treatment of first-onset NMOSD patients with a high degree of disability, which may improve the prognosis in these patients.
A further finding in this study was of more abnormalities on MRI and a greater number of spinal cord segments affected in patients with severe NMOSD than in those with mild-to-moderate NMOSD, and the number of spinal cord segments affected is an independent risk factor for the severity of neurological impairment at disease onset in patients with a first episode of NMOSD. NMOSD is an inflammatory disease of the CNS characterized by optic neuritis and longitudinally extensive transverse myelitis. It manifests on MRI as inflammatory demyelinating lesions of the long spinal cord and inflammation of the optic nerve, which is important for the diagnosis of NMOSD. Studies have shown that abnormalities of the brain on MRI are common in patients with NMOSD. In the absence of optic nerve and spinal cord involvement, the characteristic manifestations of abnormalities on brain MRI are essential for early detection of the disease and improvement of the prognosis.34,35 At present, there are few studies on MRI manifestations in patients with NMOSD according to severity.
Of note, a recent cohort study by Carnero Contentti et al concluded that NLR is not an independent outcome predictor of LATAM patients with AQP4-IgG-positive NMOSD, which seems to be slightly different from our finding.36 There are several possible explanations for the discrepant finding. First, their study only included patients with NMOSD who were AQP4 antibody-positive whereas our study included patients with NMOSD who were AQP4 antibody-positive, AQP4 antibody-negative, and untested. A previous study has shown that AQP4 antibody status affects the EDSS, course and prognosis of NMOSD patients.37 Second, their study participants came from three different countries in Latin America whereas most of our study population came from a central region of China. The influence of regional and ethnic differences on the degree of disability cannot be ignored. Third, the purpose of the study by Carnero Contentti et al was mainly to assess the influence of NLR on the prognosis of patients with first-onset NMOSD whereas we mainly investigated the relationship between the NLR and the severity of neurological impairment at disease onset in patients with a first episode of NMOSD. The difference in research purposes may also be one of the reasons for the slightly different conclusions.
A recent study showed that hypertension is a common comorbidity in patients with NMOSD. The prevalence of hypertension was significantly higher in patients with highly active NMOSD than in controls.38 Another study found that hypertension was more common and that blood pressure levels were higher in female patients with acute NMOSD than in those with multiple sclerosis.39 Other researchers have found that hypertension is associated with progression and prognosis of multiple sclerosis.40 Our present study shows that patients with severe NMOSD have a higher incidence of hypertension than those with mild-to-moderate NMOSD, which is similar to previous studies.
The discovery of the AQP-4 antibody in patients with NMOSD has further deepened our understanding of this clinical entity and provided clinicians with more diagnostic evidence. Previous studies have shown that patients with NMOSD have a longer duration of symptoms, a polyphasic course, and a poorer prognosis if they are AQP-4 antibody-positive than if they are AQP-4 antibody-negative.37 However, our analysis did not find a correlation between the AQP-4 antibody and the severity of neurological impairment at disease onset in patients with a first episode of NMOSD. This finding may reflect the fact that our hospital cannot detect AQP-4 status in CSF or serum in the early years. However, although AQP4 antibody status is unrelated to disability in these patients, there may be a relationship with the AQP4 titer. Given that detection of the AQP4 antibody at our hospital is mainly qualitative, few patients have been tested for their AQP4 antibody titer, so a more detailed analysis cannot be performed. This is one of the shortcomings of this research. Larger cohort studies are needed to confirm the relationship between AQP4 antibody status and the severity of neurological dysfunction in patients with NMOSD.
Conclusion
In summary, the NLR at the time of admission is an independent risk factor for the severity of neurological impairment at disease onset in patients with a first episode of NMOSD. However, given the single-center retrospective nature of this study, our findings await confirmation in larger multicenter cohort studies.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol. 2010;6(7):383–392. doi:10.1038/nrneurol.2010.72
2. Papp V, Magyari M, Aktas O, et al. Worldwide incidence and prevalence of neuromyelitis optica: a systematic review. Neurology. 2021;96(2):59–77. doi:10.1212/WNL.0000000000011153
3. Viswanathan S, Wah LM. A nationwide epidemiological study on the prevalence of multiple sclerosis and neuromyelitis optica spectrum disorder with important multi-ethnic differences in Malaysia. Mult Scler. 2019;25(11):1452–1461. doi:10.1177/1352458518792430
4. Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. doi:10.1186/1742-2094-9-14
5. Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer. JAMA Oncol. 2019;5(10):1481–1485. doi:10.1001/jamaoncol.2019.1747
6. Kadota K, Nitadori JI, Ujiie H, et al. Prognostic impact of immune microenvironment in lung squamous cell carcinoma: tumor-infiltrating CD10+ neutrophil/CD20+ lymphocyte ratio as an independent prognostic factor. J Thorac Oncol. 2015;10(9):1301–1310. doi:10.1097/JTO.0000000000000617
7. Dell’Aquila E, Cremolini C, Zeppola T, et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: a retrospective analysis of the TRIBE study by GONO. Ann Oncol. 2018;29(4):924–930. doi:10.1093/annonc/mdy004
8. Pinato DJ, Stebbing J, Ishizuka M, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol. 2012;57(5):1013–1020. doi:10.1016/j.jhep.2012.06.022
9. Grenader T, Waddell T, Peckitt C, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol. 2016;27(4):687–692. doi:10.1093/annonc/mdw012
10. Templeton AJ, Knox JJ, Lin X, et al. Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol. 2016;70(2):358–364. doi:10.1016/j.eururo.2016.02.033
11. Viers BR, Boorjian SA, Frank I, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66(6):1157–1164. doi:10.1016/j.eururo.2014.02.042
12. Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732–738. doi:10.1093/annonc/mdw016
13. Gusdon AM, Gialdini G, Kone G, et al. Neutrophil-lymphocyte ratio and perihematomal edema growth in intracerebral hemorrhage. Stroke. 2017;48(9):2589–2592. doi:10.1161/STROKEAHA.117.018120
14. Goyal N, Tsivgoulis G, Chang JJ, et al. Admission neutrophil-to-lymphocyte ratio as a prognostic biomarker of outcomes in large vessel occlusion strokes. Stroke. 2018;49(8):1985–1987. doi:10.1161/STROKEAHA.118.021477
15. Kim S, Eliot M, Koestler DC, Wu WC, Kelsey KT. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson Heart Study and modification by the Duffy antigen variant. JAMA Cardiol. 2018;3(6):455–462. doi:10.1001/jamacardio.2018.1042
16. D’Amico E, Zanghì A, Romano A, et al. The neutrophil-to-lymphocyte ratio is related to disease activity in relapsing remitting multiple sclerosis. Cells. 2019;8(10):1114. doi:10.3390/cells8101114
17. Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26(3):372–376. doi:10.3109/14397595.2015.1091136
18. Hu ZD, Sun Y, Guo J, et al. Red blood cell distribution width and neutrophil/lymphocyte ratio are positively correlated with disease activity in primary Sjögren’s syndrome. Clin Biochem. 2014;47(18):287–290. doi:10.1016/j.clinbiochem.2014.08.022
19. Lee YH, Song GG. Neutrophil-to-lymphocyte ratio, mean platelet volume and platelet-to-lymphocyte ratio in Behçet’s disease and their correlation with disease activity: a meta-analysis. Int J Rheum Dis. 2018;21(12):2180–2187. doi:10.1111/1756-185X.13404
20. Levy M, Wildemann B, Jarius S, et al. Immunopathogenesis of neuromyelitis optica. Adv Immunol. 2014;121:213–242.
21. Ishizu T, Osoegawa M, Mei FJ, et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128(Pt 5):988–1002. doi:10.1093/brain/awh453
22. Uzawa A, Mori M, Sawai S, et al. Cerebrospinal fluid interleukin-6 and glial fibrillary acidic protein levels are increased during initial neuromyelitis optica attacks. Clin Chim Acta. 2013;421:181–183. doi:10.1016/j.cca.2013.03.020
23. Uzawa A, Mori M, Arai K, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. 2010;16(12):1443–1452. doi:10.1177/1352458510379247
24. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125(Pt 7):1450–1461. doi:10.1093/brain/awf151
25. Uzawa A, Mori M, Kuwabara S. Cytokines and chemokines in neuromyelitis optica: pathogenetic and therapeutic implications. Brain Pathol. 2014;24(1):67–73. doi:10.1111/bpa.12097
26. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi:10.1038/nri3399
27. Wang HH, Dai YQ, Qiu W, et al. Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci. 2011;18(10):1313–1317. doi:10.1016/j.jocn.2011.01.031
28. Saadoun S, Waters P, MacDonald C, et al. Neutrophil protease inhibition reduces neuromyelitis optica-immunoglobulin G-induced damage in mouse brain. Ann Neurol. 2012;71(3):323–333. doi:10.1002/ana.22686
29. Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19(4):253–275.
30. Klein da Costa B, Brant de Souza Melo R, Passos G, et al. Unraveling B lymphocytes in CNS inflammatory diseases: distinct mechanisms and treatment targets. Neurology. 2020;95(16):733–744. doi:10.1212/WNL.0000000000010789
31. Fitzgerald D, Laurent M, Funaro M, et al. Defining the role of T lymphocytes in the immunopathogenesis of neuromyelitis optica spectrum disorder. Discov Med. 2020;29(157):91–102.
32. Lin J, Xue B, Li J, et al. Neutrophil to lymphocyte ratio may be a helpful marker to evaluate disease activity in NMOSD. Neurol Sci. 2017;38(10):1859–1863. doi:10.1007/s10072-017-3068-5
33. Benetou C, Berti F, Hemingway C, Hacohen Y, Lim M. Neutrophil-to-lymphocyte ratio correlates with disease activity in myelin oligodendrocyte glycoprotein antibody associated disease (MOGAD) in children. Mult Scler Relat Disord. 2020;45:102345. doi:10.1016/j.msard.2020.102345
34. Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84(11):1165–1173. doi:10.1212/WNL.0000000000001367
35. Carnero Contentti E, Daccach Marques V, Soto de Castillo I, et al. Frequency of brain MRI abnormalities in neuromyelitis optica spectrum disorder at presentation: a cohort of Latin American patients. Mult Scler Relat Disord. 2018;19:73–78. doi:10.1016/j.msard.2017.11.004
36. Carnero Contentti E, Delgado-García G, Criniti J, et al. An abnormally high neutrophil-to-lymphocyte ratio is not an independent outcome predictor in AQP4-IgG-positive NMOSD. Front Immunol. 2021;12:628024. doi:10.3389/fimmu.2021.628024
37. Wang X, Chen X, Zhu C, et al. A multi-facet comparative analysis of neuromyelitis optica spectrum disorders in patients with seropositive and seronegative AQP4-IgG. Medicine. 2018;97(48):e13100. doi:10.1097/MD.0000000000013100
38. Ajmera MR, Boscoe A, Mauskopf J, Candrilli SD, Levy M. Evaluation of comorbidities and health care resource use among patients with highly active neuromyelitis optica. J Neurol Sci. 2018;384:96–103. doi:10.1016/j.jns.2017.11.022
39. Chen X, Fan R, Peng F, et al. Blood pressure and body fat percent in women with NMOSD. Brain Behav. 2019;9(9):e01350. doi:10.1002/brb3.1350
40. Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041–1047. doi:10.1212/WNL.0b013e3181d6b125
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
