Back to Journals » Cancer Management and Research » Volume 12
Neutrophil-to-Lymphocyte Ratio and Its Changes are Related to Grade II–IV Glioma Recurrence
Received 23 June 2020
Accepted for publication 8 September 2020
Published 30 September 2020 Volume 2020:12 Pages 9429—9434
DOI https://doi.org/10.2147/CMAR.S267523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Lianghua Ma,1 Guang Li,1 Minjie Wei2
1Department of Radiation Oncology, The First Affiliated Hospital of China Medical University, Shenyang 110001, People’s Republic of China; 2Department of Pharmacology, School of Pharmaceutical Science, China Medical University, Shenyang 110122, People’s Republic of China
Correspondence: Guang Li
Department of Radiation Oncology, The First Affiliated Hospital of China Medical University, Heping District, Shenyang 110001, Liaoning, People’s Republic of China
Email [email protected]
Minjie Wei
Department of Pharmacology, School of Pharmaceutical Science, China Medical University, Shenyang North New Area, Shenyang 110122, Liaoning, People’s Republic of China
Email [email protected]
Objective: To explore whether the neutrophil-to-lymphocyte ratio (NLR) and its changes are related to tumor recurrence in grade II–IV glioma patients.
Methods: One hundred patients who underwent two surgeries (first for diagnosis and the second for recurrence) were retrospectively analyzed. Complete blood count was obtained preoperatively before any treatment. Basic NLR (before the first surgery) and NLR changes were calculated. Tumor recurrence was evaluated by progression-free survival (PFS) using the Kaplan–Meier method. Univariate and multivariate Cox regression analyses were used to determine the potential prognostic factors for PFS.
Results: The PFS of patients with high basic NLR (≥ 4) (median 9 months) was shorter than that of patients with low basic NLR (< 4) (median 23 months) (P = 0.004). Univariate and multivariate analyses both showed that basic NLR (before the first surgery) (≥ 4 vs < 4) was an independent predictor of PFS (P = 0.011). The PFS is also varied with NLR changes before two surgeries (P < 0.05). The PFS of patients with two low NLR (< 4) at both initial surgical resection and section for tumor recurrence had the longest PSF. The patients with two high NLR (≥ 4) at both initial surgical resection and section for tumor recurrence had the shortest PSF. The patients with one high NLR (≥ 4) at initial surgical resection or section for tumor recurrence had an average PSF. Multivariate analysis showed that the change of NLR was of prognostic significance independent of glioma grade.
Conclusion: We showed both basic NLR and NLR changes could predict the recurrence of glioma, but the change of NLR is more accurate than that of basic NLR. The current research not only provides a simple and feasible method for clinical judgment of glioma recurrence but also provides a new idea for exploring the mechanism of glioma recurrence.
Keywords: neutrophil-to-lymphocyte ratio, glioma, prognosis, recurrence, PFS
Introduction
Glioma is one of the most common malignant brain tumors in adults.1 It is usually classified into I to IV grades depending on its severity based on features of cellular atypia, cell proliferation, angiogenesis, and necrosis.2 Despite receiving all kinds of treatments (surgery, radiotherapy, chemotherapy), the prognosis of patients with glioma remains poor. Almost all glioma will eventually relapse. It will be of practical clinical significance to search for the prognostic factors of glioma.
Nowadays, the neutrophil-to-lymphocyte ratio (NLR) in peripheral blood, a marker reflecting the innate immune system, showed the prognostic value in glioma. Some studies showed that patients with high NLR in glioblastoma (grade IV) have a short survival time.3,4 Although these studies have suggested that NLR can predict the prognosis of glioma patients, there are still several problems to be further investigated: (1) the significance of NLR in all gliomas (not limited in grade IV)? (2) the relationship between NLR and recurrence? (3) whether NLR changes at the time of recurrence? To solve these problems, we have carried out the following research.
Methods
Patients
We reviewed the electronic medical records of patients with histopathology diagnosed glioma based on the 2007 World Health Organization (WHO) criteria in the first affiliated hospital of china medical university between January 2011 and December 2018. Those patients who meet the following criteria were included in this study: (1) underwent two surgeries. (2) the first surgery for pathological diagnosis and the second surgery for the first recurrence of the tumor. (3) NLR could be obtained before each surgery; (3) without underlying factors (diabetes mellitus, metabolic syndrome, heart disease, hypertension, severe hepatic or renal dysfunction, inflammatory diseases, previous history of infection within three months and any medication usage related to inflammatory conditions) that could significantly affect NLR.4
Data Collection
The following data were collected from medical records: sex, age at diagnosed, ECOG PS, and tumor grade. All patients underwent routine blood testing (complete blood count was obtained preoperatively before any treatment (eg, steroids)) using an automated complete blood count analyzer (Sysmex XE-2100, Sysmex, Kobe, Japan) as previously described.4 The NLR was defined by dividing neutrophil count by lymphocyte count. Since two NLRs (before the first and second surgery) are involved in this study, for the sake of distinguishing, NLR in the following articles refers to basic NLR (before the first surgery) unless otherwise specified. This study was approved by the institutional review board of the first affiliated hospital of china medical university and each glioma patient signed an informed consent to authorize their clinical data to be used in future studies. This study was in compliance with the Declaration of Helsinki.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as frequencies and percentages. The chi-square or ANOVA tests were used to determining statistical significance. Spearman correlation was used to examine the association between different groups. Tumor recurrence was evaluated by progression-free survival (PFS). PFS was defined as the interval from histopathology diagnosis at the first surgery until recurrence received the second surgery. The comparison of PFS among different groups was based on the Kaplan–Meier method and the Log rank test. Univariate and multivariate Cox regression analyses were used to determine the potential prognostic factors for PFS. All analyses were performed using SPSS 13.0 (SPSS Ins., Chicago, IL, USA). A P-value <0.05 (two-tailed) was considered statistically significant.
Results
The present study finally included 100 glioma patients who met the screening criteria. Clinical data of these patients are summarized in Table 1. Fifty-two (52%) patients were male, and 48 (48%) patients were female. Eighty-two (82%) patients were under 60, and 18 (18%) patients were over 60 years old. The ECOG PS was 0–1 in 80 (80%) patients and 2–4 in 20 (20%) patients. According to the WHO classification system, 33 (33%) patients had grade II glioma, 27 (27%) patients had grade III gliomas, and 40 (40%) patients had grade IV glioma. For all patients, the median PFS was 17 months (95% confidence interval (CI) 12.101–21.899 months). The median PFS for glioma patients with II, III, and IV grades was 38, 15, and 12 months, respectively (P < 0.001).
 |
Table 1 Clinical Characteristics According to Basic NLR in 100 Glioma Patients |
In the present study, the mean neutrophil and lymphocyte counts before the first surgery were 4.906 ± 0.269 x 109/L (range, 1.400–15.240 x 109/L) and 1.894 ± 0.066 x 109/L (range, 0.440–3.950 x 109/L). The mean NLR was 3.165 ± 0.365 (median, 2.199; range, 0.780–34.200). A total of 76 patients (76%) had NLR <4 and 24 patients (24%) had NLR ≥4. As shown in Table 1, the NLR did not vary significantly with sex, age, and ECOG PS. But NLR varied in different grades of glioma. The percentage of patients with low NLR (<4) for II, III, and IV grades was 97%, 74.1%, and 60%, respectively. The percentage of patients with high NLR (≥4) for II, III, and IV grades was 3%, 25.9%, and 40%, respectively. The NLR was significantly positively correlated with grades (P < 0.001). Spearman correlation coefficient was 0.365.
Next, we examined the survival function of NLR in glioma patients. As shown in Table 2 and Figure 1, the PFS of patients with high NLR (≥4) (median 9 months, 95% CI 7.817–10.183 months) was shorter than that of patients with low NLR (<4) (median 23 months, 95% CI 13.389–32.611 months) (P = 0.004). Univariate and multivariate analyses both showed that NLR was an independent predictor of PFS (multivariate hazard ratio (HR) = 1.855, 95% CI 1.155–2.979, P = 0.011, Table 3).
 |
Table 2 The PFS Stratified by Basic NLR Levels |
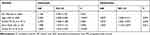 |
Table 3 Univariate and Multivariate Analyses of Factors Affecting PSF in 100 Glioma Patients |
 |
Figure 1 Kaplan–Meier survival curves stratified by basic NLR levels. |
We further examined the influence of changes in NLR during two surgeries on PFS (Table 4 and Figure 2). We found that the PFS is also varied with different NLR changes before two surgeries (P < 0.05). In patients with grade II–IV gliomas, those with two low NLR (<4) at both initial surgical resection and section for tumor recurrence had the longest PSF (median 24 months, 95% CI 14.000–34.000 months). The patients with two high NLR (≥4) at both initial surgical resection and section for tumor recurrence had the shortest PSF (median 7 months 95% CI 4.737–9.263 months). The patients with one high NLR (≥4) at initial surgical resection or section for tumor recurrence had an average PSF (median 14 months, 95% CI 9.135–18.865 months). In patients with grade III–IV gliomas, those with two low NLR (<4) at both initial surgical resection and section for tumor recurrence had the longest PSF (median 15 months, 95% CI 11.636–18.364 months). The patients with two high NLR (≥4) at both initial surgical resection and section for tumor recurrence had the shortest PSF (median 7 months 95% CI 4.737–9.263 months). The patients with one high NLR (≥4) at initial surgical resection or section for tumor recurrence had an average PSF (median 11 months, 95% CI 7.936–14.064 months). Univariate and multivariate analyses both showed that the NLR change was an independent predictor of PFS (multivariate hazard ratio (HR) = 1.501, 95% CI 1.060–2.125, P = 0.022, Table 5).
 |
Table 4 The PFS Stratified by NLR Changes |
 |
Table 5 Univariate and Multivariate Analyses of Factors Affecting PSF in 67 Patients with Grade III–IV Glioma |
Discussion
With recent advances in cancer research, inflammation has been identified to be a hallmark of cancer.5 The critical role of inflammation components became a well-established concept in glioma.6,7 Infiltrative inflammation cells, including neutrophils and lymphocytes, which could promote or suppress tumor progression, played a critical role in the glioma microenvironment.8–11 NLR, a marker reflecting inflammation, has attracted more and more attention in glioma research because of its easy access.
Although many scholars have studied NLR and glioma before, most of them focus on glioblastoma, and the main finding is the effect of NLR on overall survival (OS). The impact of NLR on local recurrence in all gliomas is not clear.
Because most of the previous studies about NLR were limited to glioblastoma (grade IV), to increase the representativeness of cases, this study included multiple levels (II–IV) of glioma patients. In the current study, we found that the proportion of high NLR (≥4) increased with the grade of glioma, and there was a positive correlation between NLR and glioma grades. Our finding is consistent with the results of other studies.12,13 Some researchers found that with the increase of glioma grade, the infiltration of granulocytes increases, and the two are positively correlated.12 Also, the infiltration of granulocytes and lymphocytes in local tumor inflammation was related to NLR, which represents systemic inflammation.13 Our study further confirmed the correlation between local inflammation and systemic inflammation in glioma.
Previous studies suggest that NLR is related to the prognosis of patients with glioma. Han et al analyzed 217 cases of glioblastoma and found that high NLR (≥4 vs <4) could predict poor OS (mean, 10.6 vs 17.9 months, P < 0.001).4 Bambury et al found similar results in another study, which included 137 patients of glioblastoma. In their cohort patients with NLR > 4 had a worse median OS at 7.5 months versus 11.2 months in patients with NLR ≤ 4 (hazard ratio 1.6, 95% CI 1.00–2.52, P = 0.048).3 Although these studies show that NLR can affect the survival of patients, the relationship between NLR and glioma recurrence, especially at all grades (not limited to grade IV), is still unclear. The current study confirmed the correlation between them. We found that basic NLR (before the first surgery) was an independent prognostic factor for tumor recurrence. The PFS of patients with basic NLR greater than 4 was shorter than that of patients with basic NLR less than 4 (median, 9 vs 23 months, P = 0.004). These results suggest that basic NLR can predict the recurrence of glioma.
Since previous studies only used single NLR, basic NLR (before the first surgery), it is not known whether NLR changes when gliomas recur. Therefore, we compared the NLR before the first surgery (diagnosis) and the second surgery (recurrence). We found that the changes in NLR were different when gliomas recur, and these changes were closely related to the relapse time. The details are as follows: (1) If the NLR is less than 4 before diagnosis and at the time of recurrence, then the recurrence interval of these patients is the longest. (2) On the contrary, if the NLR is greater than four before diagnosis. At the time of recurrence, the recurrence interval of these patients is the shortest. (3) There are two kinds of cases in patients with average relapse time. In group A, the initial NLR is less than 4, and the NLR increases to more than 4 when relapse occurs. The most interesting is the other group B, the initial NLR is more than 4, but the NLR decreases to less than 4 when relapse occurs. Although NLR fell at the time of recurrence in group B, patients in group B and group A had similar recurrence time (details not shown).
In the current study, we investigated the relationship between NLR and glioma recurrence. We found that patients with different basic NLR had different relapse times. Since NLR is related to glioma grade, glioma grade is not included in multivariate analysis to avoid statistical error. To further verify the relationship between NLR and glioma recurrence, we analyzed the data in detail. We were surprised to find that NLR changed during glioma recurrence, and the change of NLR was closely related to PFS. Because only one patient with grade II glioma had a NLR greater than 4, and there were differences in treatment methods between grade II and grade III–IV gliomas, so grade II glioma was not included in univariate and multivariate analysis. Multivariate analysis showed that the change of NLR was of prognostic significance independent of glioma grade. Since grade II–IV gliomas are included in the current study, we conducted the relevant analysis in order to exclude the influence of different treatments (radiotherapy, temozolomide, etc.) on NLR changes due to different tumor grades. As shown in Table S1, we found that there was no difference in all conditions leading to NLR changes in grade II–IV gliomas. These results suggest that the change of NLR in recurrent gliomas may not be related to the treatment or drug use. In conclusion, both basic NLR and NLR changes can predict the recurrence of glioma, but the change of NLR is more accurate than that of basic NLR.
Of course, there are inevitable limitations in this study. First of all, this is a single-center retrospective study with a small number of cases and a certain selection bias. We only included patients with recurrence who underwent the second operation and did not score all patients with recurrence. We did not find NLR and its changes associated with OS, possibly due to the selected patients (results not shown). Secondly, the current study has not obtained data on IDH1 or MGMT status, so the relationship between NLR and them could not be analyzed. The relevant data will be further improved in the follow-up study to make the specific statistical analysis based on the molecular characteristics of glioma. Finally, although we found the relationship between NLR and the recurrence of gliomas, the exact mechanism remains unclear. As a marker reflecting the innate immune system, how NLR affects glioma recurrence remains to be explored.
Conclusions
We showed both basic NLR and NLR changes could predict the recurrence of glioma, but the change of NLR is more accurate than that of basic NLR. The current research not only provides a simple and feasible method for clinical judgment of glioma recurrence but also provides a new idea for exploring the mechanism of glioma recurrence.
Acknowledgment
The authors thank Dr Guangxiao Li for his assistance in data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McNeill KA. Epidemiology of brain tumors. Neurol Clin. 2016;34:981–998.
2. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi:10.1007/s00401-007-0243-4
3. Bambury RM, Teo MY, Power DG, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114:149–154.
4. Han S, Liu Y, Li QC, et al. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15. doi:10.1186/s12885-015-1629-7
5. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi:10.1093/carcin/bgp127
6. Galvao RP, Zong H. Inflammation and gliomagenesis: bi-directional communication at early and late stages of tumor progression. Curr Pathobiol Rep. 2013;1:19–28. doi:10.1007/s40139-012-0006-3
7. Michelson N, Rincon-Torroella J, Quinones-Hinojosa A, Greenfield JP. Exploring the role of inflammation in the malignant transformation of low-grade gliomas. J Neuroimmunol. 2016;297:132–140. doi:10.1016/j.jneuroim.2016.05.019
8. Han S, Zhang C, Li Q, et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110:2560–2568. doi:10.1038/bjc.2014.162
9. Rutledge WC, Kong J, Gao J, et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013;19:4951–4960.
10. Liang J, Piao Y, Holmes L, et al. Neutrophils promote the malignant glioma phenotype through S100A4. Clin Cancer Res. 2014;20:187–198.
11. Massara M, Persico P, Bonavita O, et al. Neutrophils in gliomas. Front Immunol. 2017;8:1349. doi:10.3389/fimmu.2017.01349
12. Wang ZL, Zhang CB, Liu YQ, Wang Z, Jiang T. Peripheral blood test provides a practical method for glioma evaluation and prognosis prediction. CNS Neurosci Ther. 2019;25:876–883.
13. Fossati G, Ricevuti G, Edwards SW, et al. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98:349–354. doi:10.1007/s004010051093
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

