Back to Journals » Open Access Journal of Clinical Trials » Volume 8
Neuropsychiatric symptoms in dementia and the effects of Ginkgo biloba extract EGb 761® treatment: additional results from a 24-week randomized, placebo-controlled trial
Received 1 August 2015
Accepted for publication 16 December 2015
Published 18 January 2016 Volume 2016:8 Pages 1—6
DOI https://doi.org/10.2147/OAJCT.S93531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Greg Martin
Anatol Nacu,1 Robert Hoerr2
1Clinical Psychiatry Hospital, State University of Medicine and Pharmacy Nicolae Testemiţanu, Chişinău, Republic of Moldova; 2Clinical Research Department, Dr Willmar Schwabe GmbH and Co. KG, Karlsruhe, Germany
Background: In randomized controlled trials, Ginkgo biloba extract EGb 761® has proven to be effective in the treatment of dementia.
Trial registration: Clinical-Trials.ru 390378.
Patients and methods: In pre-specified descriptive analyses of data from a recently published trial, we examined the effects of EGb 761® on specific neuropsychiatric symptoms. In a 24-week, double-blind, multi-center trial, 410 outpatients with mild to moderate dementia and clinically significant neuropsychiatric symptoms were enrolled and randomized to receive 240 mg/day EGb 761® or placebo. The Neuropsychiatric Inventory composite score and the SKT short cognitive performance test were prospectively defined as co-primary outcomes; caregiver distress scores and single item scores were prospectively defined as secondary outcomes.
Results: Post-baseline efficacy data were available for 402 patients included in the full analysis set. Neuropsychiatric Inventory composite and caregiver distress scores improved significantly more under EGb 761® treatment than under placebo (P<0.001). Composite and caregiver distress scores of anxiety, apathy, and disturbances of sleep and nighttime behavior, as well as caregiver distress scores of depression and aberrant motor behavior, were improved most markedly by EGb 761® (P<0.05 vs placebo).
Conclusion: EGb 761® at daily doses of 240 mg alleviated neuropsychiatric symptoms of dementia and reduced related caregiver distress.
Keywords: dementia, neuropsychiatric symptoms, Ginkgo biloba, EGb 761®, randomized controlled trial
Introduction
Neuropsychiatric symptoms (NPS) are common manifestations of Alzheimer’s disease (AD) and other forms of dementia; they contribute to patients’ suffering, caregiver distress, and cost of care.1 In a longitudinal cohort study involving 362 patients with dementia, apathy (35.9%), depression (32.3%), agitation/aggression (30.3%), aberrant sleep and nighttime behavior (27.4%), and irritability (27.0%) were the most prevalent symptoms.2 Such symptoms were classified as merely “consistent” with a diagnosis of AD or vascular dementia (VaD) in older diagnostic systems,3,4 however, they are now listed among the “core clinical criteria” for all-cause dementia.5 NPS are therefore a major target for treatment in patients with dementia.
The defined, quantified Ginkgo biloba extract EGb 761® has been found to be effective in improving cognition, NPS, functional abilities, and overall condition in patients with mild to moderate dementia.6,7 Recently, the main results from a large randomized controlled trial of EGb 761® in patients with dementia, which are in line with those of preceding trials, were published.8 Here, we report the findings from prospectively planned secondary analyses focusing on the effects of EGb 761® on specific NPS.
Patients and methods
The randomized, placebo-controlled, double-blind, multi-center, parallel-group trial was performed in accordance with the provisions for Good Clinical Practice set by the International Conference on Harmonization9 and the World Medical Association’s Declaration of Helsinki. Approval by regulatory bodies and the National Ethics Committee of the Republic of Moldova, the Ethics Committee of the Republican Clinical Mental Hospital Minsk, Republic of Belarus, and the Ethics Committee under the Federal Service on Surveillance in Healthcare and Social Development of the Russian Federation was obtained and patients were enrolled only after written informed consent had been obtained. The trial was registered with Clinical-Trials.ru under number 390378 and in the public register of clinical trials of the Russian drug agency (Roszdravnadzor) under number 2008/281.
The methods and main results have been reported elsewhere.8 Briefly, we enrolled patients with mild to moderate dementia (AD, VaD or AD with cerebrovascular disease) who scored 35 or less in the Test for the Early Detection of Dementia with Differentiation from Depression10 and 9 to 23 on the SKT short cognitive performance test11 and had clinically significant NPS, evidenced by a composite score of at least 6 on the Neuropsychiatric Inventory (NPI),12 with at least one item score (other than delusions or hallucinations) of at least 4. Diagnoses of probable AD, probable VaD or possible AD with cerebrovascular disease were established using the research diagnostic criteria of McKhann et al3 and/or Román et al,4 as applicable.
In accordance with random allocation, patients received G. biloba extract EGb 761® (Dr Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany [EGb 761® is a registered trademark of Dr Willmar Schwabe GmbH & Co. KG]) at a daily dose of 240 mg or matching placebo tablets for 24 weeks. EGb 761® is a dry extract from G. biloba leaves (35–67:1), extraction solvent: acetone 60% (w/w). The extract is adjusted to 22.0%–27.0% ginkgo flavonoids calculated as ginkgo flavone glycosides and 5.0%–7.0% terpene lactones consisting of 2.8%–3.4% ginkgolides A, B, C, and 2.6%–3.2% bilobalide, and contains less than 5 ppm ginkgolic acids.
Changes from baseline in the SKT and NPI total scores were defined prospectively as primary outcome measures. Changes from baseline to week 24 in NPI caregiver distress and in all single items of the NPI were specified as secondary outcomes. Our report here mainly concerns the findings for the composite (frequency × severity) and caregiver distress scores of the single NPI items.
The statistical analysis was primarily based on the full analysis set, which included all patients who received randomized clinical trial medication at least once and who had at least one measurement of the primary efficacy parameters during the randomized treatment period. Missing data were handled by the last observation carried forward method. Details of the statistical analysis are described elsewhere.8
The changes of the composite and caregiver distress scores of the single NPI items between baseline and week 24 were compared between the treatment groups using two-sided Student’s t-tests.
Results
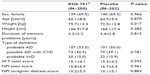
Of 410 randomized patients, eight (EGb 761®, five; placebo, three) had no efficacy data after baseline due to dropout before the assessment at week 12. The remaining 402 patients (EGb 761®, 200; placebo, 202) were included in the full analysis set for efficacy evaluation. The treatment groups were similar with respect to demographic characteristics and disease severity at baseline (Table 1).
Before treatment, anxiety, apathy/indifference, irritability/lability, depression/dysphoria, and sleep/nighttime behavior were the most prevalent symptoms; they were also rated highest in terms of frequency and severity and perceived as most distressing by caregivers, with no noticeable differences between the treatment groups (Figures 1 and 2).
  | Figure 1 Neuropsychiatric Inventory composite scores by item at baseline; means and 95% confidence intervals. |
  | Figure 2 Neuropsychiatric Inventory caregiver distress scores by item at baseline; means and 95% confidence intervals. |
During the 24-week treatment, the NPI (total) composite score improved by 4.6±7.1 (mean, standard deviation) in the EGb 761®-treated patients and by 2.1±6.5 in the placebo group (P<0.001 for between-group difference, analysis of covariance with factors for treatment and centers and the baseline value as covariate), while the caregiver distress score improved by 2.4±4.5 and 0.5±4.0, respectively (P<0.001, two-sided Student’s t-test). Improvements in both composite and caregiver distress scores under EGb 761® treatment were most pronounced in symptoms with the highest baseline severity. Anxiety, apathy/indifference, and sleep/nighttime behavior improved significantly more in patients treated with EGb 761® than in those receiving placebo (Table 2, Figure 3). Similarly, significantly larger reductions in caregiver distress related to depression/dysphoria, anxiety, apathy/indifference, aberrant motor behavior, and sleep/nighttime behavior were found under EGb 761® treatment (Table 2, Figure 4).
Discussion
In this randomized, placebo-controlled, 24-week trial, the efficacy of the defined G. biloba extract EGb 761® was tested and confirmed in patients suffering from dementia with clinically significant NPS (NPI composite score of at least 6 and at least one item score of 4 or higher). EGb 761® treatment alleviated dementia-related NPS, as indicated by significant drug-placebo differences in the total (composite) score, the caregiver distress score, and several symptom scores of the NPI. The pattern of effects was remarkably consistent with those found in previous studies that included similar patients.13,14
Our findings are also in line with those reported from former studies of EGb 761® in dementia and vascular cognitive impairment that did not specifically enroll patients with NPS, but accepted those with mild NPS.15,16 Similar to the study reported here, depression, anxiety, irritability, emotional lability, and motivation were improved by EGb 761® in these earlier studies.
Taking into account that NPS have a strong impact on patients’ and caregivers’ quality of life,17,18 considerably increase the caregivers’ burden in terms of hours of active help and hours of supervision, and predict faster progression to severe dementia in AD,19,20 the importance of targeting NPS in the treatment of patients with dementia is evident.
By involving 17 clinical sites in three different countries and enrolling patients with NPS, who make up the vast majority of patients with dementia, the generalizability of our findings suggest that there is good reason to assume it is increased and may therefore be considered as a strength of our study. Of note, four of the five NPS that were most frequent in our trial were among the symptoms found to be most prevalent in a population-based cohort.2 The frequency and severity of delusions, hallucinations, and elation/euphoria were very low, thus, no conclusions can be drawn with regard to potential effects of EGb 761® on these symptoms, which may be perceived as a limitation. Findings from population-based studies suggest that elation is generally very rare in patients with dementia.21 By recruiting patients with mild to moderate dementia who were manageable as outpatients, ie, those who are most likely to be treated with EGb 761® in daily practice, the rates of psychotic symptoms were probably kept particularly low.22
Conclusion
In summary, we found that G. biloba extract EGb 761® at a daily dose of 240 mg alleviates a variety of NPS in patients with mild to moderate dementia and reduces the NPS-related distress perceived by the patients’ caregivers.
Disclosure
A Nacu participated in the clinical trial as principal investigator for the Republic of Moldova; R Hoerr is a full-time employee of Dr Willmar Schwabe GmbH & Co. KG, receiving a fixed salary. The authors have no other conflicts of interest to disclose in this work.
References
Cummings JL, McPherson S. Neuropsychiatric assessment of Alzheimer’s disease and related dementias. Aging Clinical and Experimental Research. 2001;13(3):240–246. | |
Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. | |
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. | |
Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. | |
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. | |
Ihl R, Frölich L, Winblad B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease. World J Biol Psychiatry. 2011;12(1):2–32. | |
Gauthier S, Schlaefke S. Efficacy and tolerability of Ginkgo biloba extract EGb 761® in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin Interv Aging. 2014;9:2065–2077. | |
Herrschaft H, Nacu A, Likhachev S, Sholomov I, Hoerr R, Schlaefke S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: a randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J Psychiatr Res. 2012;46(6):716–723. | |
ICH International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Good Clinical Practice: Consolidated Guideline. ICH Topic E6-Step 5. Geneva: ICH; 1996. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed December 18, 2015. | |
Mahoney R, Johnston K, Katona C, Maxmin K, Livingston G. The TE4D-Cog: a new test for detecting early dementia in English-speaking populations. Int J Geriatr Psychiatry. 2005;20(12):1172–1179. | |
Kim YS, Nibbelink DW, Overall JE. Factor structure and scoring of the SKT test battery. J Clin Psychol. 1993;49(1):61–71. | |
Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):S10–S16. | |
Scripnikov A, Khomenko A, Napryeyenko O; GINDEM-NP Study Group. Effects of Ginkgo biloba extract EGb 761® on neuropsychiatric symptoms of dementia: findings from a randomised controlled trial. Wien Med Wochenschr. 2007;157(13–14):295–300. | |
Bachinskaya N, Hoerr R, Ihl R. Alleviating neuropsychiatric symptoms in dementia: the effects of Ginkgo biloba extract EGb 761®. Findings from a randomized controlled trial. Neuropsychiatr Dis Treat. 2011;7:209–215. | |
Hoerr R. Behavioural and psychological symptoms of dementia (BPSD): Effects of EGb 761®. Pharmacopsychiatry. 2003;36 Suppl 1:S56–S61. | |
Halama P, Bartsch G, Meng G. Hirnleistungsstörungen vaskulärer Genese. Randomisierte Doppelblindstudie zur Wirksamkeit von Ginkgo-biloba-Extrakt [Cerebrovascular insufficiency – Placebo-controlled, randomized, doubleblind trial on the effect of Ginkgo biloba extract]. Fortschr Med. 1988;106(19):408–412. German. | |
Mjørud M, Røsvik J, Rokstad, Kirkevold M, Engedal K. Variables associated with change in quality of life among persons with dementia in nursing homes: a 10 months follow-up study. PLoS One. 2014;9(12):e115248. | |
Santos RL, Sousa MF, Simões-Neto JP, et al. Caregivers’ quality of life in mild and moderate dementia. Arq Neuropsiquiatr. 2014;72(12):931–937. | |
Okura T, Langa KM. Caregiver burden and neuropsychiatric symptoms in older adults with cognitive impairment: the Aging, Demographics, and Memory Study (ADAMS). Alzheimer Dis Assoc Disord. 2011;25(2):116–121. | |
Rabins PV, Schwartz S, Black BS, et al. Predictors of progression to severe Alzheimer’s disease in an incidence sample. Alzheimers Dement. 2013;9(2):204–207. | |
Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170–177. | |
Tatsch MF, Bottino CM, Azevedo D, et al. Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: prevalence and relationship with dementia severity. Am J Geriatr Psychiatry. 2006;14(5):438–445. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.