Back to Journals » Neuropsychiatric Disease and Treatment » Volume 10
Neuropsychiatric symptoms and celiac disease
Authors Urban-Kowalczyk M , Śmigielski J, Gmitrowicz A
Received 8 June 2014
Accepted for publication 15 July 2014
Published 14 October 2014 Volume 2014:10 Pages 1961—1964
DOI https://doi.org/10.2147/NDT.S69039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Małgorzata Urban-Kowalczyk,1 Janusz Śmigielski,2 Agnieszka Gmitrowicz3
1Affective and Psychotic Disorders Department, Medical University of Łódź, Łódź, Poland; 2Department of Geriatric Medicine Medical University of Łódź, Łódź, Poland; 3Department of Adolescent Psychiatry, Medical University of Łódź, Łódź, Poland
Background: Neuropsychiatric symptoms may represent an atypical manifestation of celiac disease that occur before a gastroenterological diagnosis is made. Some studies suggest that a gluten-free diet is effective in treating the depression, anxiety, and neurological complications associated with celiac disease.
Method: The article describes the case of a patient suffering from chronic, treatment-resistant symptoms of depression and anxiety. The diagnosis of celiac disease and introduction of an elimination diet caused a significant improvement in mental state and everyday functioning in the presenting patient.
Conclusion: The presence of persistent anxiety and depressive symptoms, with a poor reaction to pharmacological treatment, indicates a need to identify somatic reasons for the underlying condition. It is important to remember that celiac disease can occur at any age, not only in childhood. The presence of this somatic cause of persistent depressive and anxiety symptoms should be considered in the diagnostic process in adults.
Keywords: gluten, depression, anxiety, anemia, neurological complications
Introduction
Celiac disease (CD) is an inflammatory disease characterized by small intestinal mucosal injury and nutrient malabsorption in genetically susceptible individuals following the dietary ingestion of gluten. Proteins in the dietary cereal grains, wheat, rye, and barley are the major known environmental factors that are required for disease activation.1 The disease occurs in about 1% of the general population; it may be diagnosed at any age and affects many organ systems.2 There are two main models of CD presentation in adults: classic (with predominant diarrhea, dyspepsia, weight loss, and anemia) and non-classic.3,4 The nonclassic population includes those with atypical presentations, those presenting with complications of CD, and those who are truly asymptomatic. Diagnosis of CD is based on serology and duodenal biopsy combined with clinical presentation.4 The therapy for the disease is a gluten-free diet – it is the only treatment that protects against the severe complications of CD.2 CD has been associated with such neurological and psychiatric disorders as cerebellar ataxia, brain atrophy, peripheral neuropathy, epilepsy, cognitive-function deterioration, depression, and anxiety.5,6 This paper presents the case of a patient with psychiatric symptoms and neurological changes in course of long-term undiagnosed CD.
Case report
The patient was a 39-year-old woman with a secondary education. She was an only child who had never worked and was living with her family. Since childhood, she had been painfully shy, withdrawn, and easily embarrassed. In school, she avoided contact with her peers due to emotional problems. Her mother played a dominant role in her life, and her parents chose to perform all her duties for her. The patient described herself as completely helpless, incompetent, and subordinate to others. She claimed she was not able to perform the simplest household tasks. The patient was in an informal relationship for several years, but decided not to live with her partner or to formalize the relationship as she did not feel mature enough.
The patient had been treated for a few years as a psychiatric outpatient with depressive and anxiety disorders. She reported treatment with citalopram and sulpiride in the prior 6 months. The patient reported sadness, anxiety, apathy, fatigue, and poor functioning, and, due to lack of response to treatment, she was referred to the psychiatric ward. On admission, she was conscious, with a depressive mood and psychomotor slowness. She complained of apathy, lack of motivation, chronic fatigue, weakness, poor physical-exercise tolerance, and deterioration of cognitive functions. In addition, she reported repeatedly occurring palpitations, dizziness, and fainting. In the patient’s opinion, her functioning had been significantly deteriorating for several years. Her first Hamilton Depression Rating Scale (HDRS-17) assessment was 17 points. The severity of depressive symptoms was moderate according to the 10th revision of the International Classification of Diseases criteria. She had broken up with her partner, had become idle at home, could not make simple decisions without consulting her mother and did not leave the house alone. Moreover, she had broken off contact with a few friends and spent her time mostly in the company of her mother.
Although she admitted having had “problems with blood” in the past and was referred to a hematologist, she decided not to undergo the diagnostic process. In the physical examination, skin and conjunctiva pallor were observed, as was tachycardia of 110–120 beats/minute. The results of the neurological examination were normal. A blood cell count showed microcytic anemia: red blood cell count (RBC) 4.2 million cells/mcL, hemoglobin 5.9 g/dL, hematocrit 23.6%, mean corpuscular volume 53 μm3, and iron deficiency 3.4 μmol/L. Thyroid function, vitamin B12, and glucose were normal.
The patient was transferred to the internal diseases department, where CD was diagnosed: anti-tissue transglutaminase antibodies were positive (immunoglobulin A =4,04, immunoglobulin G =0,37) and a duodenal biopsy showed increased intraepithelial lymphocytosis, crypt hyperplasia, and villous atrophy (intraepithelial lymphocyte count >40; Marsh 3b). A gluten-free diet and intramuscular iron supplementation were administered, and the patient continued treatment in the psychiatric ward. Magnetic resonance imaging (MRI) of the head showed bilateral, hemispheric white matter, mainly subcortical single uncharacteristic small focus with high signal in T2 and FLAIR sequences without intensification after contrast administration – vascular changes against atherosclerosis, coagulation disorders, or inflammation of blood are possible reasons for this. In addition, symmetric, supratentorial cortical atrophy was seen (Figure 1).
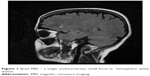  | Figure 1 Brain MRI – a single uncharacteristic small focus in hemispheric white matter. |
Sertraline treatment (50 mg/day) was started and blood counts were observed to normalize, as did the mental state and activity of the patient. She began to take care of her appearance and participate in therapeutic activities in hospital. Her interpersonal relationships and functioning also improved.
Two neuropsychological assessments were performed – the first just after CD diagnosis and second after 3 months, with the patient in a stable mental state and without anemia. The following tests were used: Wechsler Adult Intelligence Scale – revised, Benton Visual Retention Test, Trail Making Test, Clock Drawing Test, d2 Test, The Rey Complex Figure, Ruff Figural Fluency Test, and the Minnesota Multiphasic Personality Inventory. In the first assessment, impairment of perceptual organization was found. The patient’s global level of intellectual functioning was found to be average (IQ =100). Personality disorders with dependent and avoidant features were described. In the second examination, significant improvements in cognitive functioning (attention and psychomotor speed) were observed. Depressive and anxiety symptoms were persistent but not severe enough to meet the diagnostic criteria for depressive or anxiety disorders. Finally, organic personality disorder was diagnosed.
Significant improvements were observed in mental state (HDRS-17 =5 points) and RBC count normalization (RBC 4.42 million cells/mcL, hemoglobin 11.9 g/dL, hematocrit 37.6%, mean corpuscular volume 83 μm3). After discharge from the psychiatric ward, she was referred to a rehabilitation center for individuals with psychiatric disorders, where the patient quickly acclimatized, established relationships with other residents, and met a partner. Progressive improvement in everyday functioning was observed, and the patient was discharged home after a few months’ stay. She still lives with her parents but has taken a job as a cleaner. On her own initiative, she has started a training course to get better and more-interesting employment.
Discussion
The patient had complained of depressive mood, apathy, fatigue, and anxiety before CD diagnosis, and although she did not report any gastrointestinal symptoms, features of untreated anemia had been observed for some time before hospitalization. Anemia may be treated for years with iron supplements without identification of the underlying disease, and it is thought that this condition coexists in about 8% of patients with CD.3
It is difficult to determine in this case whether the psychiatric symptoms were caused by the chronic anemia or the CD itself. Psychiatric symptoms can be common complications and manifestations of undiagnosed CD. Some authors report a significantly higher prevalence of depressive symptoms in adult CD patients compared to patients without CD.7 In most cases, depressive disorders developed before intestinal biopsy. Apathy, anxiety, and irritability are commonly described symptoms.8 The mechanism and pathogenesis of mental disorders related to CD are unknown, but the possibility of impaired tryptophan availability and disturbances in central serotoninergic function have been suggested to play a role. Decreased plasma levels of tryptophan and monoamine precursors have been found in untreated CD patients, as have decreased cerebrospinal fluid levels of serotonin, dopamine, and norepinephrine metabolites.9,10 It is well known that many inflammatory and autoimmune disorders may trigger clinical depression. Depression is probably associated with a chronic low-grade inflammatory response, activation of cell-mediated immunity, and activation of the compensatory anti-inflammatory reflex system, characterized by negative immunoregulatory processes. New evidence shows that clinical depression is accompanied by increased oxidative and nitrosative stress.11
Some reports indicate that a gluten-free diet can significantly improve depressive symptoms in CD patients.12,13 Van Hees et al14 found that adherence to the gluten-free diet for longer than 5 years may reduce the risk of depressive symptoms. Nevertheless, other studies have found no correlation between depression or CD duration and dietary compliance.8 It may also be the case that personality features predisposed the patient to the development of psychiatric symptoms. Moreover, uncharacteristic, most likely ischemic, foci were observed by brain MRI. No pathology was found upon neurological examination, but neuropsychological assessment showed some cognitive impairment. It is still not known whether antigliadin antibodies directly interact with the nervous system.13 However, about 73% of patients with untreated CD, but only 7% adhering to a gluten-free diet, have been reported to demonstrate cerebral blood-flow abnormalities similar to those observed in patients with depressive disorders.15
In the case of our patient, introducing a gluten-free diet and iron supplementation were related to cognitive improvement and the resolution of anxiety and depressive symptoms. Moreover, in a follow-up after a 3 months, significant improvement in functioning was observed. Nevertheless, sertraline treatment can contribute to clinical improvement because of its serotonergic and low dopaminergic properties. It is known that deficiencies in these neurotransmitters may play role in depression related to CD.8,9 It is important to remember that CD can occur in any age, not only in childhood. The presence of this somatic cause of persistent depressive and anxiety symptoms should be considered in the diagnostic process in adults. Despite long-lasting depressive-anxiety features and personality disorders, the period of gradual neurocognitive deterioration and daily routine impairment may be indicated in our patient. Persistent, treatment-resistant depressive and anxiety symptoms were at least partially associated with growing untreated anemia. Malabsorption and secondary severe anemia probably contributed to ischemic lesion of brain. Reported change of clinical manifestation, symptom severity and persistence with poor treatment effects may indicate their organic basis. The variety in clinical manifestations of CD should encourage clinicians to consider it as a differential diagnosis in selected cases, not only those that are accompanied by gastrointestinal symptoms or deviations in neurological examination.
Disclosure
The authors report no conflicts of interest in this work.
References
Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21(2):405–425. | ||
Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357(17): 1731–1743. | ||
Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128(4 Suppl 1): S74–S78. | ||
Ludvigsson JF, Bai JC, Biagi F, et al; BSG Coeliac Disease Guidelines Development Group. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014; 63(8):1210–1228. | ||
Luostarinen L, Pirttilä T, Collin P. Coeliac disease presenting with neurological disorders. Eur Neurol. 1999;42(3):132–135. | ||
Mäki M, Collin P. Coeliac disease. Lancet. 1997;349(9067): 1755–1759. | ||
Smith DF, Gerdes LU. Meta-analysis on anxiety and depression in adult celiac disease. Acta Psychiatr Scand. 2012;125(3):189–193. | ||
Ciacci C, Iavarone A, Mazzacca G, De Rosa A. Depressive symptoms in adult coeliac disease. Scand J Gastroenterol. 1998;33(3):247–250. | ||
Hernanz A, Polanco I. Plasma precursor amino acids of central nervous system monoamines in children with coeliac disease. Gut. 1991;32(12): 1478–1481. | ||
Hallert C, Aström J, Sedvall G. Psychic disturbances in adult coeliac disease. III. Reduced central monoamine metabolism and signs of depression. Scand J Gastroenterol. 1982;17(1):25–28. | ||
Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. | ||
Pynnönen PA, Isometsä ET, Verkasalo MA, Savilahti E, Aalberg VA. Untreated celiac disease and development of mental disorders in children and adolescents. Psychosomatics. 2002;43(4):331–334. | ||
Pynnönen PA, Isometsä ET, Verkasalo MA, et al. Gluten-free diet may alleviate depressive and behavioural symptoms in adolescents with coeliac disease: a prospective follow-up case-series study. BMC Psychiatry. 2005;5:14. | ||
van Hees NJ, Van der Does W, Giltay EJ. Coeliac disease, diet adherence and depressive symptoms. J Psychosom Res. 2013;74(2):155–160. | ||
Addolorato G, Di Giuda D, De Rossi G, et al. Regional cerebral hypoperfusion in patients with celiac disease. Am J Med. 2004;116(5): 312–317. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
