Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Neuroprotective effect and mechanism of baicalin on Parkinson’s disease model induced by 6-OHDA
Authors Tu L, Wu ZY, Yang XL, Zhang Q, Gu R, Wang Q, Tian T, Yao H, Qu X, Tian JY
Received 20 February 2018
Accepted for publication 28 June 2018
Published 3 January 2020 Volume 2019:15 Pages 3615—3625
DOI https://doi.org/10.2147/NDT.S165931
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Li Tu,1 Zhuo-Yu Wu,2 Xiu-Lin Yang,3 Qian Zhang,3 Ran Gu,3 Qian Wang,2 Tian Tian,2 Huan Yao,3 Xiang Qu,3 Jin-Yong Tian2,3
1Department of General Medical, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China; 2Department of Neurology, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China; 3Department of Emergency, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China
Correspondence: Jin-Yong Tian
Department of Emergency, Guizhou Provincial People’s Hospital, 83 Zhongshan East Road, Guiyang 550002, Guizhou, China
Tel +86 851 8593 7194
Email [email protected]
Objective: This research was aimed to investigate the effects of baicalin on 6-hydroxydopamine (6-OHDA)-induced rat model of Parkinson’s disease (PD) and the main mechanism of baicalin based on metabolomics.
Methods: The rat model of PD was induced by 6-OHDA. The protective effects of baicalin on rat model of PD were evaluated by open field test and rotarod test. The anti-PD efficacy of baicalin was evaluated by examining the morphologic changes of neurons and the level of monoamine neurotransmitters in the striatum, the number and morphology of tyrosine hydroxylase (TH)-positive neurons, and oxidative stress. Combined with metabolomics methods, the pharmacodynamic mechanism of baicalin on PD pathogenesis was also explored.
Results: Baicalin treatment improved the rod time and voluntary movement in rat model of PD (P<0.05) by the open field test and rotarod test. In addition, baicalin also protected from oxidative stress injury (P<0.05), and regulated the content of monoamine neurotransmitters dopamine, 3,4-dihydroxyphenylacetic acid, 5-hydroxytryptamine, and 5-hydroxyindoleacetic acid (P<0.05) and the number and morphology of TH-positive cells in 6-OHDA-induced PD model rats. By metabolomics, multivariate statistical analysis, and receiver operating characteristic curve analysis, we found that two metabolites N-acetyl aspartic acid and glutamic acid had a good diagnostic value. Quantitative analysis of metabolites showed a regulatory function of baicalin.
Conclusion: Baicalin has significant protective effect on 6-OHDA-induced PD rats, which may play a protective role through an antioxidant, promoting the release of neurotransmitters and regulating the metabolism of N-acetyl aspartate and glutamate.
Keywords: Parkinson’s disease, neurotransmitter, baicalin, metabolomics
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease in the elderly. The main pathologic mechanism is the gradual degeneration and loss of dopamine (DA) neurons in the substantia nigra compacta, resulting in the lack of neurotransmitter DA in the brain. However, due to the complex etiology of PD, pathogenesis has not yet been fully elucidated. It is widely believed that PD is a progressive and deteriorating polycentric neurodegenerative disease associated with neurotransmitter systems. The main clinical symptoms are abnormal voluntary movement such as rest tremor, bradykinesia, rigidity and posture balance, and cognitive dysfunction.1–4 At present, the existing anti-Parkinson drugs can only improve the effect of symptoms, however not delay the process of disease, and not prevent the degeneration of DA neurons. In recent years, the treatment of PD is mainly concentrated on neuroprotective factors, neurotrophic factors, and growth factors such as dopamine. A large number of studies showed increased oxidative stress and iron in substantia nigra (SN) in PD patients.
Elevated activity of iron can promote oxidative stress, leading to a large number of oxygen free radicals. Excessive active iron can promote the occurrence of oxidative stress, resulting in a large number of oxygen free radicals, resulting in cell death.5–8
Baicalin is isolated from Labiatae Scutellaria Linn Scutellaria baicalensis Georgi dry roots and extracted from flavonoids. Baicalin has antibacterial, antiviral, anti-inflammatory, antitumor, cardiovascular, and neuroprotective activities. Studies show that baicalin is protective on rotenone-induced and MPTP-induced dopaminergic neuron damage in PD model rats.9,10 Baicalin downregulated iron concentration, which positively regulated divalent metal transporter 1 expression and negatively regulated ferroportin 1 expression, and decreased iron accumulation in the SN.11 Baicalin and deferoxamine alleviate iron accumulation in different brain regions of PD rats.9 Preventive medication of baicalin shows a protective effect on C57 BL mouse with PD.10 However, MPTP-induced motor dysfunction in model mouse was not significantly improved by a short-time medication.
Metabolomics is a new method that can identify all metabolic components quickly. Potential biomarkers can be identified to evaluate subtle pathophysiologic stress by metabolomics. It is mainly for the qualitative analysis of all endogenous small molecular metabolites in the body under a specific physiologic cycle or physiologic condition, and quantitative study of multiple dynamic responses of living body to external stimuli, pathophysiologic changes, and its own gene mutation caused by its metabolite level in vivo.12–14 So far, nuclear magnetic resonance (NMR) is the most commonly used, and the main advantage of NMR is that sample can be detected without bias and with good reproducibility.15–17
In the current study, the PD rat model was established by 6-hydroxydopamine (6-OHDA), which is a kind of nerve agent. The selective destruction of 6-OHDA caused the destruction of DA synthesis and the normal transport to the striatum, and the DA content in the striatum of the lesioned rats decreased, which results in similar symptoms to human PD.18 We report the pharmacodynamic role of baicalin, which can improve the behavior and neurotransmitter changes, apoptosis and morphology of dopaminergic neurons, and oxidative stress injury. We also explore the potential pathogenesis of PD and the pharmacodynamic mechanism of baicalin by metabolomics. This provides a new direction for the development of PD treatment. The purpose of this study is to systematically evaluate the neuroprotective mechanism of baicalin on 6-OHDA-induced PD rats, and to explore the neuroprotective mechanism of baicalin and the pathogenesis of PD by metabonomics.
Materials and methods
Reagents and instruments
6-OHDA, 3,4-dihydroxyphenylacetic acid (DOPAC), and DA were purchased from Sigma (St. Louis, MO, USA). High-performance liquid chromatography (HPLC)-grade methanol was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Baicalin was purchased from Institute of Biochemistry (Shanghai, China); malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) were all purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Protein concentration determination kit was purchased from Beyotime Biotechnology (Haimen, Jiangsu). Rat tyrosine hydroxylase (TH) monoclonal antibody was purchased from Dako (Denmark).
Stereotaxic instrument (DW-200; Chengdu Thaimeng Technology Company) microdialysis system includes CMA402 microdialysis pump, CMA 12 microdialysis probe, cannula, and CMA120 (CMA/Microdialysis, Stockholm, Sweden).
Animal grouping and administration
Ninety male specific pathogen free Sprague Dawley (SD) rats, weighing 250–280 g, were from SLAK Laboratory Animal Shanghai, China. All animals were housed in a ventilated, dry, and quiet environment with 12-hour light–dark cycle at room temperature (25°C) and 45%–55% relative humidity. Rats were acclimated for a week. Routine behavioral tests were performed to ensure that all rats had no abnormal rotation behavior. Seventy-five SD rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate and then a microsyringe was used to aspirate a solution containing a 1.5 μg/μL 6-OHDA and 2.0 g/L of vitamin C solution in 10 μL saline. Injection was done at a flow rate of 10 nL/s by a micropump into the right striatum of the brain (the anterior fontanel 0.7 mm, the right midline 3.0 mm, and the subdural 5.5 mm). To fully disperse the drug, 5 μL injection was finished and retained for 5 minutes. After the last injection, the needle was retained for 10 minutes. Finally, the needle was slowly withdrawn and the wound was stitched. Seven rats died during the model building. Another 16 sham-operated rats were selected and operated according to the above method and the same volume of physiologic saline containing 2.0 g/L vitamin C was injected. One rat died during the sham operation. All operations were sterile with intramuscular injection of penicillin 200,000 units only before rats awakened. One week after surgery, behavioral testing was conducted.19 The success of the model was evaluated by the open field test and the rotarod test. The results showed that the PD model was established successfully, except that it was unsuccessful in seven rats. A total of 60 rats had PD model successfully established in our experiment. The model rats were randomly divided into four groups: PD group, PD + baicalin low-dose group (50 mg/kg), PD + baicalin middle-dose group (100 mg/kg), PD + baicalin high-dose group (150 mg/kg). Rats were continuously administered with baicalin for 8 weeks.20,21 The study was performed in strict accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. All experiments were performed following Guizhou Provincial People’s Hospital and national guidelines and regulations, and the experiment was approved by the ethics committee of Guizhou Provincial People’s Hospital.
Rotarod test and open field test
Rotarod test is often used to test fatigue, motor coordination, and degree of damage recovery. The rotarod test was commonly used to judge the success of the model, the degree of damage, and the treatment effect in the study of PD. Therefore, in our experiment, the rotarod test was used to evaluate the success of the model and the anti-PD efficacy of baicalin. A weekly rotarod test was carried out to evaluate the efficacy of baicalin.22
Open field test is an experiment used to detect the voluntary movement, anxiety, and exploratory behavior in new environment. The number of crossing the grid and upright are indicators of voluntary movement and limb activity in rats. In the experiment, the rats were placed in the central grid of the mine to adapt for 2 minutes and then number of crossing the grid and erect times are recorded to evaluate the degree of damage in the later 4 minutes.23
Determination of the activity of SOD, GSH-Px, and CAT and the content of MDAin striatum
After the behavioral experiment, the left striata of the experimental rats were removed. The cell lysate was added into the tissue in the ratio of 9:1. Then they were centrifuged at 3,000 rpm/min for 10 minutes. The supernatant was collected and the activity of SOD, CAT, and GSH-Px and the content of MDA were measured. All experiments were done according to the manufacturer’s instructions.
Determination of monoamine neurotransmitters DA, DOPAC, 5-hydroxytryptamine (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) in striatum
After drug administration for 8 weeks, the rats were anesthetized and the probe was slowly inserted into the striatum and the catheter was embedded at a depth of 5 mm using a stereotaxic instrument and fixed with three screws around the catheter followed by dental cement fixation. Microdialysis probes for brain tissue microdialysis sampling were inserted into guide cannulas. Samples were collected in awake rats at a rate of 1 μL/min. To remove the effect of surgery on rats, the sample was discarded for the first 90 minutes, then immediately analyzed by HPLC electrochemical detection.
Morphologic observation of brain
After behavioral tests, the rats in each group were anesthetized with 10% chloral hydrate and were killed. Then the brain tissue was quickly taken out and fixed in paraformaldehyde solution. The brain tissues were embedded by paraffin, and the consecutive coronal sections in the mesencephalic SN (thickness: 5 μm) were sliced up. Immunohistochemistry was used to examine the expression level of TH in SN. To further observe the ultrastructure of neurons, isolated SN was cut into pieces of 1 mm3 in size. They were double fixed with 2.5% glutaraldehyde and 1% osmium tetroxide and were subjected to a graded series of acetone-ethanol to dehydrate. The embedded brain tissues were sliced up with the thickness of 70–90 nm. Then the sections were double stained by uranyl acetate and lead citrate followed by transmission electron microscopy and imaged at 120 kV.24
Sample preparation and determination for metabolomics
After behavioral tests, the rats in each group were anesthetized with 10% chloral hydrate and were killed. Then the brain tissue was quickly taken out. The right striatum was isolated, quenched in liquid nitrogen rapidly, and then saved at -80°C. Before the preparation of nuclear magnetic samples, the striatum was unfrozen at room temperature, accurately weighed, placed in centrifuge tube, added 4 mL/g cold methanol and 0.85 mL/g distilled water, and homogenized with a glass homogenizer followed by vortex. Fifteen seconds later, 2 mL/g of chloroform was added, vortexed for 15 seconds, and then centrifuged at 3,000 rpm for 10 minutes at 4°C. The supernatant was lyophilized and then dissolved in 600 μL of H2O. Five hundred fifty microliters of supernatant was added to magnetic tube with a diameter of 5 mm for 1H-NMR analysis.
NMRdata processing and analysis
The samples were analyzed by 600 MHz 1 H-NMR spectroscopy measured by the Carr–Purcell–Meiboom–Gill pulse sequence with a scan time of 64 seconds. The sampling time was 5 seconds, while the other data are as follows: the sampling interval 40.5 seconds, the spectral width 12,345.679 Hz, the pulse interval 1 second, the delay time 1.0 second, the spectral width 12,345.7 Hz, the pulse time 14 seconds, and the sampling data point 65,536.
Statistics and data analysis
All statistical analyses were conducted using SPSS 19.0. Data were mean ± standard error of the mean (SEM). One-way ANOVA and Student's t-test were used for multiple-group comparison. The difference was considered statistically significant when a P-value was <0.05.
Results
Effect of baicalin on riding time in 6-OHDA-induced PD rat model
The rats were placed on the rod. Each rat was placed in a separate chamber. The latency to fall was also recorded. It was tested three times for 60 seconds and the average was taken. Rats had a 30-minute rest time to restore their physical strength. The rats in the sham group were able to move at a normal speed on the rotor, while in the PD group, there were obvious characteristics of abnormal behavior such as weakness of limbs and dislike activities on the rotating rod, and the movement time was shortened obviously on the rotating rod (P<0.05) significantly (Figure 1). After continuous administration of baicalin for 8 weeks, the limb weakness and other characteristics in each group were significantly improved with varying degrees of increase in the time on the rods. There were significant differences between before and after administration of medium dose and high dose (P<0.05).
Effects of baicalin on voluntary movement in 6-OHDA-induced PD rat model
Open field test is used to observe the voluntary movement of rats in the enclosed environment. As shown in Figure 2, the sham-operated rats had no significant symptoms of stiffness, physical tremor, and other abnormal behaviors. Compared to the sham-operated group, the voluntary movement, the number of rearing, and squares traversed were decreased (Figure 2A and B, P<0.05) with obvious timid and other behavioral traits in the model rats. After administration of baicalin, the voluntary movement of each group was partially reversed and the number of squares traversed and upright were significantly reversed in the middle-dose and high-dose groups (Figure 2A and B, P<0.05), indicating that baicalin can improve voluntary movement dysfunction in 6-OHDA-induced PD rat model.
Effects of baicalin on the activities of SOD, CAT, GSH-Px, and MDA content in striatum of 6-OHDA-induced PD rat model
The loss of DA cells in the brain of PD patients is mainly caused by oxidative stress. SOD, CAT, GSH-Px activity, and MDA content in the body are the indicators of oxidative stress. The results showed that the activities of SOD (Figure 3A), CAT (Figure 3B), and GSH-Px (Figure 3C) in the model group were significantly decreased and MDA content (Figure 3D) increased compared to the sham-operated group. These results indicated that the model rats were in oxidative peroxidative stress. After administration of baicalin, the activities of SOD, CAT, GSH-Px, and the content of MDA in the model rats were all rescued, especially in the middle-dose and the high-dose groups. Together, these results show that baicalin protects rats from 6-OHDA-induced oxidative damage.
Effects of baicalin on monoamine neurotransmitter in the striatum of 6-OHDA-induced PD rat model
DA is a key neurotransmitter controlling movements. When 80% dopaminergic neurons are lost in the nigrostriatal nucleus and DA levels are reduced in the striatum, symptoms of PD appear. Previous studies showed that the occurrence of PD is associated with decrease in neurotransmitter levels. When striatum is damaged in patients and DA levels reduced, syndrome of tremor and paralysis gets aggravated. Decreases in the DA metabolite DOPAC and the content of 5-HT and 5-HIAA were accompanied with that. The results showed that the contents of DA, DOPAC, 5-HT, and 5-HIAA in the model group were lower than those in the sham-operated group (Table 1). After administration of baicalin, the levels of monoamine neurotransmitters were reversed, which indicate that baicalin can promote the release of monoamine neurotransmitters to improve movements.
 |
Table 1 Levels of four monoamine neurotransmitters in the striatum of 6-hydroxydopamine-induced PD rat model |
Effect of baicalin on neurons in 6-OHDA-induced PD rat model
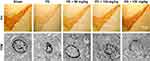
TH immunohistochemistry (Figure 4) showed that TH-positive neurons had a clear shape, high TH expression, and strong immunocompetence in the SN of rats in the sham-operated group. However, in PD model group, the number of TH-positive neurons in the brain was significantly decreased, TH levels were poor, the cytoplasm color was light, and the cell morphology blurred compared to the sham-operated group. After administration of baicalin, the number of neurons was increased. The effect of medium- and high-dose groups was obviously better than that in the low-dose group. Number of TH-positive cells increased with darker cytoplasm and clear cell morphology.
Transmission electron microscopy revealed that neurons in the sham-operated group (Figure 4) were structurally intact with normal cell morphology, which suggests that surgery did not induce damage. In the PD group, neurons shrunk, and chromatin coagulated and showed apoptosis-like morphology, which was typical of late-stage apoptosis. After administration of baicalin, the morphology of neurons in all groups was improved with the nuclear membrane integrity, especially normal cell morphology in medium- and high-dose groups.
NMR identification and multivariate statistical analysis
The compounds were assigned according to the literature and combined with the databases HMDB (http://www.hmdb.ca) and BMRB (http://www.bmrb.wisc.edu/). The identified metabolites mainly include amino acids, organic acids, and sugars (Table S1).
The data were analyzed by OPLS-DA. As shown in Figure 5A, the model group could separate from the blank group, indicating that the metabolic state changed in PD rats. The high-dose group was close to the sham-operated group, while the low-dose group was close to the model group, which indicates that baicalin could regulate the metabolism in rats. As shown in Figure 5B, the sham-operated group could separate from the blank group in the PCA score chart, indicating that the metabolic profile of the two groups underwent significant changes. The model was valid and could be used for subsequent analysis (Figure 5C). To further find potential biomarkers of the development of PD, load analysis was performed using OPLS-DA and S-plot (Figure 5D) in combination with VIP values (VIP>1) and found a total of nine differential metabolites that contributed much to the group: N-aspartic acid, aspartic acid, glutamic acid, gamma-aminobutyric acid, glycine, taurine, succinic acid, creatine, and lactic acid. They may correlate with the occurrence of PD. Statistical analysis of the peak areas of the nine metabolites showed that baicalin regulated six metabolites: N-acetyl-aspartate (NAA), aspartate, glutamate (Glu), γ-aminobutyric acid, glycine, and taurine. The results shown above suggest that the effect of baicalin on PD rat model may be related to the metabolic pathways.
Receiver operating characteristic (ROC) curve analysis
The ROC curve was used to evaluate and predict the diagnostic capabilities of the six differential metabolites. The area under the ROC curve (AUC) can reflect the diagnostic ability and accuracy of potential biomarkers. Generally when AUC value is higher than 0.9, the biomarker is considered to have better diagnostic ability and higher accuracy. When AUC value is between 0.7 and 0.9, it indicates that the potential diagnostic biomarker is accurate to some extent. AUC value between 0.5 and 0.7 indicates that accuracy is low with no diagnostic value. ROC curve analysis of the above six potential biomarkers was performed. A total of two potential diagnostic biomarkers (Figure 6), NAA and Glu, was found with a threshold of 0.9. AUC values are 0.937 and 0.941.
 |
Figure 6 Receiver operating characteristic curve analysis of NAAand Glu. Abbreviations: AUC, area under the receiver operator characteristic curve; Glu, glutamate; NAA, N-acetyl aspartate. |
Determination of NAA and Glu levels
To further evaluate the role of NAA and Glu in the pathogenesis of PD, quantitative analysis by MRS showed that NAA was decreased and Glu was increased. After administration of baicalin, the levels of NAA and Glu significantly reversed (Table 2).
 |
Table 2 NAAand Glu concentration (μmol/L, x±s) in rat striatum |
Discussion
PD is a degenerative disorder in the central nervous system caused by the loss of SN dopaminergic neurons gradually. The major pathologic change in PD is degeneration of neurons and loss of dopaminergic neurons in the SN and striatum, which leads to decreased DA levels. In addition, degenerative neurons are also vulnerable to oxidative stress injury in the brain of PD patients, which led to the severe pathology. Therefore, oxidative stress is an important indicator of PD. 6-OHDA unilateral nigrostriatal injection is a well-established model for PD. Therefore, 6-OHDA-induced PD model rats were used to explore the therapeutic effect and main mechanism of baicalin.25–27
Our study found that when DA neurons were impaired in the SN and striatum of PD patients, the movement of PD patients was impaired and the abnormal rotation occurred in PD model rats.28 Therefore, rotarod test and open field test were used for examination of the efficacy of baicalin on PD rat model. After continuous administration of baicalin for 8 weeks, rotarod test and open field test results in PD-treated groups were significantly improved, indicating that baicalin could improve spontaneous activity dysfunction in 6-OHDA-induced PD rat model.
DA synthesized by DA neurons is mainly transported via axons to the striatum. DA is an important monoamine neurotransmitter in the brain and PD dyskinesia correlated with DA levels. In addition, studies found that 5-HT neurons in degenerative diseases might be associated with sleep and breathing disorders in PD patients.29 Therefore, our study detected the DA, and 5-HT and its metabolites, and results showed that the levels of monoamine neurotransmitters significantly reduced in the striatum of 6-OHDA-induced rat model. After continuous administration of baicalin, the levels of monoamine neurotransmitters in striatum were significantly reversed, indicating that baicalin could promote the release of monoamine neurotransmitters and improve movement. This was consistent with the behavioral results.30,31 In addition, apoptosis of the SN neurons also led to a decrease in the TH content in the striatum. Therefore, studies have shown that neurons in SN and apoptosis of dopaminergic neurons led to reduced dopaminergic neurotransmission. Our study shows that baicalin can inhibit the apoptosis.
Oxidative stress is the main factor leading to the apoptosis of DA neurons in the brain of PD patients. A large number of studies show that PD patients are in a state of oxidative stress. Excessive free radicals can induce protein, lipid peroxidation, and DNA strand breaks, which induce neuronal apoptosis in turn. 6-OHDA can be absorbed by the SN and striatum and produce oxygen free radicals after metabolism, which leads to apoptosis of DA neurons. SOD, CAT, GPx activity, and MDA content are important indicators of oxidative stress.32 The results showed that the activities of SOD, CAT, and GPx in the striatum of 6-OHDA-induced PD model rats decreased while the content of MDA increased, which indicated that the striatum was in a state of oxidative stress. After continuous administration of baicalin, the activities of SOD, CAT, and GPx and the content of MDA were significantly reversed, demonstrating that baicalin can protect 6-OHDA-induced oxidative stress injury in PD model rats.
Studies in recent years showed that amino acid neurotransmitter in the brain is also of great significance for the occurrence of PD. Therefore, we explored the changes of metabolites and the therapeutic mechanism of baicalin in the brain of 6-OHDA-induced PD rats by metabonomics. A total of six differential metabolites were found by 1 H-NMR metabolomics combined with far-reaching statistical analysis and t-test. To assess the diagnostic capabilities of the six differential metabolites, the six metabolites were analyzed by ROC-binding AUC. The diagnostic ability of NAA and Glu is better. NAA is a hallmark of neuronal changes in the brain, and a decreased level suggests a loss or dysfunction of neurons.33 Excitatory amino acid Glu is mainly involved in synaptic excitability transmission, learning and memory formation, and neurodegenerative diseases. When Glu is abnormally elevated, DA receptors on DA neurons are activated. These result in an increase in intracellular Ca2+ levels, and lead to the destruction of cytoskeleton and degenerate neurons, which is also an important pathologic feature of PD.34,35 In our study, quantitative analysis of the content of NAA and Glu in the striatum by MRS showed that the content of NAA in the model group decreased and the content of Glu increased. After continuous administration of baicalin for 8 weeks, the content of baicalin significantly changed, indicating that baicalin may play a role by targeting to NAA and Glu.
Conclusion
In conclusion, our results showed that baicalin can prevent neurodegeneration in 6-OHDA-induced PD rats through antioxidative stress, inhibition of apoptosis of dopaminergic neurons in SN, and regulation of monoamine neurotransmitter release. The main mechanism of baicalin may be by the regulation of NAA and Glu metabolism. This study explored the pathogenesis of PD and the mechanism of baicalin against PD by metabonomics technology, which provided a basis for subsequent researchers to understand the pathogenesis of PD and explore the mechanism of action of drugs.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81560201) and Doctor Foundation of Guizhou Provincial People’s Hospital (GZSYBS[2015]03).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Riedel O, Bitters D, Amann U, Garbe E, Langner I. Estimating the prevalence of Parkinson’s disease (PD) and proportions of patients with associated dementia and depression among the older adults based on secondary claims data. Int J Geriatr Psychiatry. 2016;31(8):938–943.
2. Aarsland D. Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies. Parkinsonism Relat Disord. 2016;22(Suppl 1):S144–S148.
3. Sarkar S, Raymick J, Imam S. Neuroprotective and therapeutic strategies against Parkinson’s disease: recent perspectives. Int J Mol Sci. 2016;17(6):904.
4. Hindle JV, Hurt CS, Burn DJ, et al. The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson’s disease – a longitudinal cohort study. Int J Geriatr Psychiatry. 2016;31(1):13–23.
5. Chen Y, Lian Y, Ma Y, et al. The expression and significance of tyrosine hydroxylase in the brain tissue of Parkinson’s disease rats. Exp Ther Med. 2017;14(5):4813–4816.
6. Zhang X, Li Y, Liu C. Alteration of enteric monoamines with monoamine receptors and colonic dysmotility in 6-hydroxydopamine-induced Parkinson's disease rats. Transl Res. 2015;166(2):152-162.
7. Lindenbach D, Palumbo N, Ostock C. Side-effect profile of serotoninergic treatments for Parkinson’s disease and L-DOPA-induced dyskinesia in rats. Br J Pharmacol. 2014;172(1):119-130.
8. Kucinski A, Albin RL, Lustig C, Sarter M. Modeling falls in Parkinson’s disease: slow gait, freezing episodes and falls in rats with extensive striatal dopamine loss. Behav Brain Res. 2015;282:155–164.
9. Xiong P, Chen X, Guo C, Zhang N, Ma B. Baicalin and deferoxamine alleviate iron accumulation in different brain regions of Parkinson’s disease rats. Neural Regen Res. 2012;7(27):2092–2098.
10. Chen X, Zhang N, Zou HY. Protective effect of baicalin on mouse with Parkinson’s disease induced by MPTP. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(11):1010–1012.
11. Guo C, Chen X, Xiong P. Baicalin suppresses iron accumulation after substantia nigra injury: relationship between iron concentration and transferrin expression. Neural Regen Res. 2014;9(6):630.
12. Dong G, Wei D, Wang J, et al. Study of the cardiotoxicity of Venenum Bufonis in rats using an 1H NMR-based metabolomics approach. PLoS One. 2015;10(3):e0119515.
13. Deng M, Zhang M, Sun F, et al. A gas chromatography-mass spectrometry based study on urine metabolomics in rats chronically poisoned with hydrogen sulfide. Biomed Res Int. 2015;2015:295241–295246.
14. Long Y, Dong X, Yuan Y, et al. Metabolomics changes in a rat model of obstructive jaundice: mapping to metabolism of amino acids, carbohydrates and lipids as well as oxidative stress. J Clin Biochem Nutr. 2015;57(1):50–59.
15. Xu J, Jiang H, Li J, et al. 1H NMR-based metabolomics investigation of copper-laden rat: a model of Wilson’s disease. PLoS One. 2015;10(4):e0119654.
16. Zhao H, Si Z-H, Li M-H, et al. Pyrazinamide-induced hepatotoxicity and gender differences in rats as revealed by a 1H NMR based metabolomics approach. Toxicol Res. 2017;6(1):17–29.
17. Kim KS, Bang E. Metabolomics profiling of the effects of taurine supplementation on dyslipidemia in a high-fat-diet-induced rat model by 1H NMR spectroscopy. Adv Exp Med Biol. 2017;975:329–336.
18. Zheng X, Chen X, Guo M, et al. Changes in salsolinol production and salsolinol synthase activity in Parkinson’s disease model. Neurosci Lett. 2018;673:39–43.
19. Zhang W, Zhang L, Liu L, Wang X. Time course study of fractional anisotropy in the substantia nigra of a parkinsonian rat model induced by 6-OHDA. Behav Brain Res. 2017;328:130–137.
20. Antipova VA, Holzmann C, Schmitt O, Wree A, Hawlitschka A. Botulinum neurotoxin a injected ipsilaterally or contralaterally into the striatum in the rat 6-OHDA model of unilateral Parkinson’s disease differently affects behavior. Front Behav Neurosci. 2017;11:119.
21. Hansen HH, Fabricius K, Barkholt P, et al. Characterization of liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, in rat partial and full nigral 6-hydroxydopamine lesion models of Parkinson’s disease. Brain Res. 2016;1646:354–365.
22. Ru X, Yi S, Rui H, et al. Neuroprotective effects of SCM198 on 6-hydroxydopamine-induced behavioral deficit in rats and cytotoxicity in neuronal SH-SY5Y cells. Neurochem Int. 2011; 58(8): 851-860.
23. Yang J, Song S, Li J, Liang T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson’s disease rat. Pathol Res Pract. 2014;210(6):357–362.
24. Xie L, Xin Tiong C, Bian J. Therapeutic effect of ACS84 on Parkinson’s disease in 6-OHDA-induced rat model. PLoS One. 2013; 8(4): e60200.
25. Sánchez-Iglesias S, Méndez-Alvarez E, Iglesias-González J, et al. Brain oxidative stress and selective behaviour of aluminium in specific areas of rat brain: potential effects in a 6-OHDA-induced model of Parkinson’s disease. J Neurochem. 2009;109(3):879–888.
26. Ozsoy O, Yildirim FB, Ogut E, et al. Melatonin is protective against 6-hydroxydopamine-induced oxidative stress in a hemiparkinsonian rat model. Free Radic Res. 2015;49(8):1004–1014.
27. Noor NA, Mohammed HS, Mourad IM, Khadrawy YA, Aboul Ezz HS. A promising therapeutic potential of cerebrolysin in 6-OHDA rat model of Parkinson’s disease. Life Sci. 2016;155:174–179.
28. Zhao C, Li H, Zhao X-J, et al. Heat shock protein 60 affects behavioral improvement in a rat model of Parkinson’s disease grafted with human umbilical cord mesenchymal stem cell-derived dopaminergic-like neurons. Neurochem Res. 2016;41(6):1238–1249.
29. Pang Y, Lin S, Wright C, et al. Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-OHDA in rats. Neuroscience. 2016;318:157–165.
30. Chen L, Zhang L, Wang X, Lin H, du L. Determination of dopamine and its relativity of baicalin in rat nuclei after intravenous administration of flavonoids from Scutellariae radix. Biomed Chromatogr. 2007;21(1):84–88.
31. Zhu W, Ma S, Qu R, Kang D, Liu Y. Antidepressant effect of baicalin extracted from the root of Scutellaria baicalensis in mice and rats. Pharm Biol. 2006;44(7):503–510.
32. Wang WW, Jc H, Ding HJ. Effect of tianma gouteng drink on the behavioural and oxidation stress response of Parkinson’s disease rat. Zhongguo Laonian Xue Zazhi. 2010;30: 1657-1659.
33. Coune P, Craveiro M, Gaugler M, et al. An in vivo ultrahigh field 14.1 T 1 H-MRS study on 6-OHDA and α-synuclein-based rat models of Parkinson’s disease: GABA as an early disease marker. NMR Biomed. 2013;26(1):43–50.
34. Meng T, Yuan S, Zheng Z, Liu T, Lin L. Effects of endogenous melatonin on glutamate and GABA rhythms in the striatum of unilateral 6-hydroxydopamine-lesioned rats. Neuroscience. 2015;286:308–315.
35. El Arfani A, Albertini G, Bentea E, et al. Alterations in the motor cortical and striatal glutamatergic system and D-serine levels in the bilateral 6-hydroxydopamine rat model for Parkinson’s disease. Neurochem Int. 2015;88:88–96.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.