Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Neuroprotection by 2,3,5,4ʹ-tetrahydroxystilbene-2-O-β-D-glucoside extracts from Polygonum multiflorum against cerebral ischemia/reperfusion injury through the 5-hydroxytryptamine/5-hydroxytryptamine receptor pathway
Authors Yi CA, Wang J, Wang Y , Wu XY
Received 11 July 2018
Accepted for publication 18 March 2019
Published 27 May 2019 Volume 2019:15 Pages 1429—1438
DOI https://doi.org/10.2147/NDT.S179845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Chuan-An Yi,1–3 Jun Wang,4 Ye Wang,2 Xiao-Ying Wu1,3
1Department of Pathology, School of Basic Medicine, Central South University, Changsha, Hunan Province, 410008, People’s Republic of China; 2Medical Morphology Experiment Center, Hunan University of Medicine, Huaihua, Hunan Province, People’s Republic of China; 3Department of Pathology, Xiangya Hospital, Central South University, Changsha, Hunan Province, 410078, People’s Republic of China; 4Ningbo No. 7 Hospital, Ningbo, Zhejiang Province 315000, People’s Republic of China
Objective: To investigate the therapeutic effect of 2,3,5,4ʹ-tetrahydroxystilbene-2-O-β-D-glucoside (TSG) on the expression of 5-hydroxytryptamine (5-HT)/5-HT receptor 2A (5-HT2A), 5-HT transporter (5-HTT), and uncoupling protein 4 (UCP4) after cerebral ischemia/reperfusion (I/R) injury.
Methods: Sprague–Dawley rats were randomly divided into control, model and 125 (low-dose), 250 (middle-dose), and 500 (high-dose) mg/mL TSG groups. Rat cerebral I/R injury model was established by middle cerebral artery occlusion (MCAO). After successful establishment of rat MCAO model, rats in control and model groups were decapitated immediately. Rats in TSG group were orally administered 125, 250, and 500 mg/mL TSG in corresponding groups at a dose of 1 mL/100 g per day for 7 continuous days, and then the rats were decapitated. The infarct size was determined using triphenyl tetrazolium chloride staining and the expression of UCP4 and 5-HT2A in the hippocampus and thalamic nucleus was detected using immunohistochemistry and western blot assay. The expression of 5-HTT in brain tissue was detected using western blot assay. Serum 5-HT levels were detected using ELISA.
Results: After treatment, the infarct size due to cerebral I/R injury decreased with increased concentrations of TSG. Synchronous reduction of 5-HT in the blood and 5-HTT in the brain was observed, and 5-HT2A was expressed in normal brain tissue but its level was increased in rats after cerebral I/R injury. A high level of UCP4 was found in normal brain tissue, which rose by 6 hrs after cerebral I/R injury but reduced to minimal levels 24 hrs after injury. With increasing TSG concentration, the levels of 5-HT, 5HTT, and UCP4 were increased, while the level of 5-HT2A was decreased.
Conclusion: TSG is effective in treating cerebral I/R injury in rats, and its mechanism may be implemented through the 5-HT/5-HTR pathway, by increasing 5-HT release, enhancing the activity of 5-HTT, increasing expression of UCP4, and inhibiting 5-HT2A activity.
Keywords: cerebral ischemia/reperfusion injury, 5-HT/5-HT2A, UCP4, Polygonum multiflorum extract, 2354ʹ-tetrahydroxystilbene-2-O-β-D-glucoside (TSG)
Introduction
Ischemic cerebrovascular diseases are characterized by high incidence and high mortality. Although the causes of the diseases are clear, the pathogenesis is not detailed and treatment effects are not ideal. Increasing evidence has indicated that chronic cerebral ischemia may cause the changes in the level and functions of 5-hydroxytryptamine (5-HT) and other neurotransmitters in the brain.1 5-HT transporter (5-HTT) is a transporter with the highest affinity for 5-HT and is widely present in the limbic system of the brain.2 For ischemic cerebrovascular diseases, 5-HT reuptake and metabolism and changes in 5-HTT in the brain may directly vary 5-HT neuronal activity in the brain. Previous studies have shown that in rats with vascular dementia, Polygonum multiflorum extract inhibited the abnormal increase in the level of the 5-HT receptor 2A (5-HT2A) in the hippocampus and gradually restored their cognitive function.3 A previous study has shown that neurotransmitter alterations were observed after ischemic brain injury, and injury to the hippocampus, anterior thalamic nuclei, and frontal lobe may lead to reduction in 5-HT synthesis and cause metabolic dysfunction.4 Thus, 5-HT2A is of general interest in the treatment of cerebrovascular diseases. Modern pharmacological studies have shown that Polygonum multiflorum Thunb mainly contains anthraquinone compounds, stilbene glucoside compounds, a large amount of lecithin, and a variety of trace elements, and 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside (TSG) extracted from Polygonum multiflorum have antiaging, lipid-lowering, and neuroprotective effects.5,6 However, there have been no reports indicating that the neuroprotective effect of TSG acts through the 5-HT/5-HT receptor (5-HTR) signaling pathway. Uncoupling protein 4 (UCP4) is a proton transporter located on the mitochondrial inner membrane that is directly involved in neurotransmission, synaptic plasticity, and neurodegeneration by regulating mitochondrial membrane potential, calcium flow, oxygen free radical generation, and local body temperature.7,8 Existing data suggest that cerebral ischemia/reperfusion (I/R) injury can reduce cell membrane fluidity, and there is a tight correlation between membrane proteins and mitochondrial function.9 Increased intracellular ROS lead to reduced mitochondrial transmembrane potential and induce increased UCPs.10 Herein, we hypothesized that UCP4 plays an important regulatory role in cerebral I/R injury. We investigated the pathogenesis of cerebral I/R injury in Sprague–Dawley rats in correlation with 5-HT/5-HT2A, 5-HTT, and UCP4 and explored the effects of TSG on the 5-HT/5-HT2A pathway, thereby providing new strategies for diagnosis, treatment, and new drug development for cerebrovascular diseases.
Materials and methods
Animals
272 healthy, male Sprague–Dawley rats weighing 220–250 g were provided by the Experimental Animal Center, Anhui Medical University, China (license No. SCXK (Anhui) 2011-002). The study protocol received approval from the Ethics Committee of Xiangya Hospital, Central South University and complied with the Animal Management Rules of the Chinese Ministry of Health and existing current animal welfare guidelines.
Drugs and reagents
UCP-4 polyclonal antibody (Santa Cruz Biotechnology Inc., Dallas, TX, USA); SABC immunohistochemistry kit, 5-HT rabbit monoclonal antibody, 5-HT2A rabbit monoclonal antibody, 5-HTT rabbit monoclonal antibody, DAB color kit (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China); polyvinylidene fluoride (PVDF) membrane (Millipore Corp., Billerica, MA, USA); hydrated trichloroacetaldehyde (Shanghai Chemical Reagent Research Institute Co., Ltd., Shanghai, China); RIPA (Beyotime Biotechnology, Jiangsu Province, China); and Bioshine ChemiQ 4600mini chemiluminescence imaging system (Bioshine, Shanghai, China)were used. Cooked Polygonum multiflorum Thumb was purchased from Longyuan Pharmaceutical Co., Ltd. (Hunan Province, China) with batch No. 20150901, and TSG extracts from Polygonum multiflorum were made in the Chinese Medicine Research Center, Hunan University of Medicine (Huaihua, Hunan Province, China). Polygonum multiflorum was extracted twice by 70% ethanol via reflux extraction, then dried in a basic environment, and concentrated in a vacuum. The extracted TSG (6.25, 12.50, and 25.00 g) was dissolved in 50 mL of water to prepare 125, 250, and 500 mg/mL solutions.
Establishment and evaluation of a rat model of middle cerebral artery occlusion (MCAO)
The rats were intraperitoneally anesthetized by 10% chloral hydrate (0.3 mL/100 g) and fixed in a supine position. An incision was made at the center of the neck to expose and separate the right common carotid artery, right internal carotid artery, and external carotid artery, around which a 4-0 ligation line was placed. The common carotid artery and external carotid artery were ligated, an incision was made at the bifurcation of the carotid artery, and paraffin-treated fishing line was inserted into the internal carotid artery to a depth of 18–20 mm. Then, the internal carotid artery was ligated and sutured with a short line. Twenty-four hours later, the fishing line was pulled out and left at the bifurcation of the carotid artery to restore blood circulation. The neurological deficits of the rats were scored using Bederson’s method11 as follows: 0 point, no apparent neurological deficits; 1 point, left forelimb flexion when lifting the tail; 2 points, walking to the right; 3 points, circling to the left when holding the tail; 4 points, no spontaneous walking, with a loss of consciousness. A score of 1–3 points indicated successful model induction in the rats.
Experimental animal groups
200 rats were taken and randomly divided into five groups: control, model, 125 (low-dose), 250 (middle-dose), and 500 mg/mL (high-dose) TSG groups (n=40 per group), and 8 rats in each group were decapitated. The brain tissue was obtained and used to detect the infarct size using triphenyl tetrazolium chloride (TTC) staining, the expression of UCP4, 5-HTT, and 5-HT2A using western blot assay, and the expression of 5-HTT using ELISA, respectively.
Another 48 rats were taken and divided into control, model, and 500 mg/mL (high-dose) TSG groups, and 8 rats in each group were decapitated. The brain tissue was obtained and used to detect the expression of UCP4 using immunohistochemistry and western blot assay, respectively.
The remaining 24 rats were taken and divided into control, model, and 500 mg/mL (high-dose) TSG groups, and 8 rats in each group were decapitated. The brain tissue was obtained and used to detect the expression of 5-HT2A using immunohistochemistry.
Administration methods
After successful establishment of rat MCAO model, rats in control and model groups were decapitated immediately. Rats in the TSG group were orally administered 125, 250, and 500 mg/mL TSG in corresponding groups at a dose of 1 mL/100 g per day for 7 continuous days, and then the rats were decapitated. The infarct size was determined using TTC staining, the expression of UCP4 and 5-HT2A in the hippocampus and thalamic nucleus was detected using immunohistochemistry and western blot assay, respectively. The expression of 5-HTT in brain tissue was detected using western blot assay. Serum 5-HT levels were detected using ELISA.
Determination of infarct size in rat brain after cerebral I/R injury
After removal of those without neurological dysfunction or with coma or that died, rats with successful I/R injury were rapidly decapitated, and their brain tissues were removed onto an ice tray and placed into a −20°C freezer for 10 mins before removal and discarding of the olfactory bulb, cerebellum, and lower brain stem. Frozen brain tissues from the first group of rats were cut into five coronal slices that were then quickly placed in 5 mL of PBS containing 1% TTC and incubated in the dark at 37°C for 15 mins, after which the slices were turned over. Stained normal brain tissue was rose-red and the infarct tissue was pale. There was a distinct boundary between the two kinds of brain tissue. Image J (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, MD, USA,
Immunohistochemical analyses in brain tissues
Brain tissue paraffin slices of 5 µm thickness were dewaxed with xylene, hydrated in an ethanol gradient, stained with hematoxylin for 5 mins, and then washed with distilled water. Then, the slices were immersed in PBS for 5 mins and incubated with 3% hydrogen peroxide at room temperature for 5 mins, followed by 3 rinses in PBS (2 mins each). After addition of 10% normal goat serum in PBS, the slices were placed in a humid box and incubated at room temperature for 10–15 mins to block the free binding sites and reduce nonspecific staining. Excess goat serum was removed, and the primary antibody was added overnight at 4°C. Afterward, the slices were rinsed in PBS (3×2 mins). Biotin-labeled secondary antibody (goat anti-rabbit lgG) was added and the slices were incubated at 37°C for 30 mins followed by 3 rinses in PBS (2 mins each). Horseradish peroxidase-labeled streptavidin working solution was added, and the slices were incubated at 37°C for 20 mins followed by 3 rinses in PBS (2 mins each). Subsequently, the slices were developed with 3,3’-diaminobenzidine and counterstained with hematoxylin. The brain slices were observed under a light microscope to control the time of color development. Finally, the slices were dehydrated, cleared, and mounted.
Western blot analysis of brain tissues
For total protein extraction, the brain tissue samples were ground on ice, placed into a 1–2 mL homogenizer and homogenized with RIPA lysis buffer at a ratio of 0.1 g per 1 mL. Protein samples were transferred into a 1.5 mL centrifuge tube and centrifuged at 4°C at 10,000 rpm/min for 15 mins. The supernatant was transferred into an Eppendorf tube and stored at −80°C. The extracted protein samples were mixed with loading buffer in a volume ratio of 1:4 and denatured in a heat block at 100°C for 10 mins. Samples were then loaded onto a polyacrylamide gel (10%), electrophoresed, transferred onto PVDF membranes, blocked with 5% blocking buffer on a shaker at room temperature for 1 hr, and rinsed three times in Tris-buffered saline with Tween-20 (TBST) (5 mins each). The PVDF membranes with target proteins were incubated with the primary antibody overnight at 4°C, rinsed three times in TBST (5 mins each), and subsequently incubated with the secondary antibody at room temperature for 1 hr. After rinsing with TBST (3×5 mins), the electrochemiluminescence (ECL) detection solution (a mixture of solution A and solution B at a ratio of 1:1) was smeared on the PVDF membrane with a filter paper to absorb excess liquid. The protein bands were captured using a Bioshine ChemiQ 4600mini chemiluminescence imaging system and analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA).
ELISA assay
The amount of 5-HT was detected in strict accordance with the instructions of the ELISA test kit as follows: 1) blood samples from the abdominal aorta were centrifuged and the supernatant was obtained; 2) required microtiter plates were taken out of an aluminum foil bag that had been equilibrated at room temperature for 20 mins, and the remaining plates were sealed in a self-sealing bag and stored at 4°C; 3) After setting standard and sample wells, 50 μL of standard samples at different concentrations were added per standard well; 4) Samples (10 μL) to be tested for 5-HT were added into sampling wells and diluted to 50 μL. Blank wells had nothing added; 5) horseradish peroxidase-labeled detection antibody (100 μL) was added into each well and the reaction wells were sealed with sealing film, followed by incubation in a 37°C water bath or an incubator for 60 mins; 6) The remaining liquid was removed by patting the plate on absorbent paper. Washing solution was added to each well until full and left for 1 min. After removal of the washing solution, the plates were patted on absorbent paper. The rinsing step was repeated five times; 7) substrate A (50 μL) and substrate B (50 μL) were added to each well, and the plates were incubated at 37°C in the dark for 15 mins; 8) stop solution (50 μL) was added to each well and within the next 15 mins, the optical density at 450 nm was measured for each well.
Statistical analysis
Data were expressed as the mean ± SD and analyzed with GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA, USA) and with SPSS 22.0 (IBM Corp, NY, USA). The differences between two groups were analyzed with Student’s t-test, and the differences among groups were analyzed with ANOVA. p<0.05 was considered statistically significant.
Results
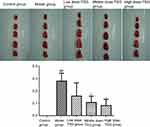
Effects of TSG on infarct size in the rat brain after cerebral I/R injury
After 7-day oral administration of TSG at a dose of 1 mL/100 g/day, TTC staining showed that the infarct size due to cerebral I/R injury was ranked largest to smallest as follows: model group, >125 mg/mL group, >250 mg/mL group, and >500 mg/mL, indicating TSG markedly reduced the infarct size in rat brains (P<0.05 or P<0.01; Figure 1).
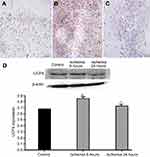
Effects of TSG on UCP4 expression at different stages after cerebral I/R injury
As shown in Figures 2 and 3, there was a high expression of UCP4 with granular appearance or brown staining of cytoplasm and processes in normal rat brain tissue. After cerebral ischemia, the expression of UCP4 increased within the first 6 hrs, then reduced, and was minimized at 24 hrs. Findings from western blot assay showed that TSG upregulated the decreased expression of UCP4 in a concentration-dependent manner after cerebral I/R injury (P<0.05 or P<0.01). These findings indicate that TSG can restore the activity of UCP4 in the ischemic brain.
Effects of TSG on 5-HT content in cerebral I/R injury rats
As shown by ELISA test in Figure 4, there was a basal level of 5-HT the normal rat serum, while the content of 5-HT was decreased in the model group. After treatment with TSG, the downregulation of 5-HT was reversed with increasing TSG concentrations (P<0.05 or P<0.01), indicating TSG can increase the release of 5-HT.
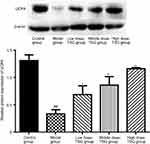
Effects of TSG on 5-HTT expression in cerebral I/R injury rats
As shown in Figure 5, there was high expression of 5-HTT in the normal rat brain and the expression level of 5-HTT was decreased after cerebral I/R injury. After treatment with TSG, the expression level of 5-HTT was markedly upregulated with increasing TSG concentrations (P<0.05 or P<0.01). These findings indicate that TSG can strengthen the activity of 5-HTT in rats with cerebral I/R injury.
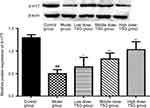
Effects of TSG on 5-HT2A expression in cerebral I/R injury rats
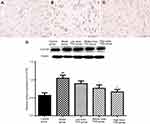
As shown by immunohistochemical findings in Figure 6, 5-HT2A-positive cells in the thalamic nucleus were rounded or elliptical in shape and brown in color, with a colorless and transparent cytoplasm, and a dark brown band was visible in the cell membrane. There was a significant difference in the integrated optical density value between the model group and the TSG groups, as well as between the model group and control group (P<0.05). Western blot results showed that there was a lower expression of 5-HT2A in the normal rat brain, and its expression level was increased in response to cerebral I/R injury. After treatment with TSG, the downregulation of 5-HT2A was markedly blocked with increasing TSG concentrations (P<0.05 or P<0.01). These findings indicate that TSG can downregulate the level of 5-HT2A and inhibit its activity in rats with cerebral I/R injury.
Discussion
In this study, we investigated the role of the 5-HT/5-HTR pathway in the rats with cerebral I/R injury and found variations in expression of 5-HTT, 5-HT2A, and UCP4 in response to TSG treatment at different concentrations. After treatment with TSG, the infarct size in the rats with cerebral I/R injury was reduced in a concentration-dependent manner and was lowest in the 500 mg/mL TSG group. After treatment with TSG, the downregulation of 5-HT, 5-HTT, and UCP4 and the upregulation of 5-HT2A in response to cerebral I/R injury was abolished in a concentration-dependent manner. Therefore, we suggest that TSG has neuroprotective effects against cerebral I/R injury and its mechanism may be related to the 5-HT/5-HTR pathway.
The pathogenesis of ischemic cerebrovascular diseases is still unclear, despite there being a variety of theories, such as excitatory amino acid poisoning, oxidative stress, and mitochondrial dysfunction. Moreover, there are no ideal therapeutic treatments. TSG extracted from Polygonum multiflorum Thunb has antiaging and neuroprotective effects, and its mechanism of action may be to increase the level of superoxide dismutase, reduce the content of malondialdehyde, increase Bcl-2 expression, protect cholinergic nerve fibers in the brain, and release brain-derived neurotrophic factor.12,13 As a classic monoamine neurotransmitter, 5-HT is located in the raphe nuclei of the brain stem, with projections to almost the entire central nervous system. After cerebral I/R injury, the extent of fiber projections in the infarct site decreased, resulting in a reduction in the content of 5-HT in the cortex, hippocampus, and thalamic nucleus. Reuptake of 5-HT depends on 5-HTT in the presynaptic axon terminals and the cell body. A portion of the 5-HT that is ingested into nerve endings is inserted into the vesicles for storage and reuse, and the remainder reacts with monoamine oxidase on the mitochondrial surface. As 5-HTT is a transmembrane transporter protein that transports serotonin, its expression changes directly affect the involvement of 5-HT neurons in brain activities;14,15 5-HTT is regulated by a variety of proteins, and activation of protein kinases can downregulate 5-HTT-mediated 5-HT uptake. Experimental changes in 5-HTT levels can alter the function of 5-HT2A in the brain, and an increase in the 5-HTT level strengthens the function of the 5-HT2A receptor.16 In rats and humans, blocking 5-HTT can eliminate 5-HT2A-mediated neuroendocrine responses. In this study, the content of 5-HT was decreased and the activity of 5-HTT was inhibited in cerebral I/R injury rats compared with the normal rats (Figures 3 and 4). However, the downregulation of 5-HT and 5-HTT was partly blocked by TSG treatment, indicating TSG can effectively regulate 5-HT and 5-HTT. The 5-HT2A receptor, with a low level of expression in normal brain tissue, is important for neurochemical and behavioral responses. In some parts of the brain, the 5-HT2A receptor can promote neuronal depolarization and implement nerve signal transmission.17 In peripheral tissues, the 5-HT2A receptor is mainly located in blood platelets and vascular smooth muscle functioning to mediate platelet aggregation and vasoconstriction. Excessive 5-HT2A receptor expression in vascular smooth muscle results in cerebral vasoconstriction, brain tissue necrosis, and an enlarged apoptotic area. After cerebral I/R injury, TSG treatment reduced the infarct size in a concentration-dependent manner (Figure 1); the increased 5-HT2A expression after injury demonstrated neuroprotection of 5-HT2A against I/R injury and TSG treatment reduced the increased level (Figure 6). Studies have shown that the upregulation of 5-HT2A in depressive rats in response to cerebral ischemia was inhibited by Kaixin Jieyu Fang, a Chinese medicine prescription.18
Mitochondria, as the main site for intracellular energy production, are extremely sensitive to cerebral ischemia and hypoxia. Under ischemic conditions, mitochondria are swollen and exhibit internal structural damage, cytoplasmic concentration, chromatin condensation, and mitochondrial permeability conversion. Moreover, mitochondria can release oxygen free radicals under I/R conditions.19 Under conditions of oxidative stress and inflammation, ROS production can induce the increases in UCP expression, and the expression of UCPs was gradually increased with the aggravation of inflammation and oxidative stress, and the uncoupling activity of UCPs was increased. The expression of UCPs can be reduced by attenuating oxidative stress and inflammatory response.10 Mitochondrial dysfunction and a secondary increase in ROS can cause neuronal cell death and apoptosis.20 UCP4 is a mitochondrial component that is mainly expressed in brain tissue. In this study, we found that the expression of UCP4 increased at 6 hrs after cerebral I/R injury but decreased at 24 hrs. The decreased expression of UCP4 was elevated after treatment with TSG, and we also found that 5-HT2A and UCP4 synchronously increased in the early stage of cerebral I/R injury in rats, and then the expression of UCP4 was reduced. In this study, we used TSG to restore the levels of 5-HT2A and UCP4. Therefore, 5-HT2A and UCP4 are key proteins for cerebral I/R injury, and further investigation on the regulation of UCP4 by 5-HT2A is warranted.
At present, the pathogenesis of cerebral I/R injury has not yet been clarified, and the effects of cholinesterase inhibitors, neurotrophic and neuroprotective drugs, N-methyl-D-aspartate receptor antagonists, antioxidants, drugs for improvement of microcirculation and anti-inflammatory drugs in the treatment of cerebral I/R injury are not ideal. TGS extracted from natural products Polygonum multiflorum is a monomer of stilbene, which has antiaging, lipid-lowering, and neuroprotective effects, and has no side effects. TSG extracts from Polygonum multiflorum can alter the expression of 5-HTT, and UCP4, increase 5-HT release, inhibit 5-HT2A activity, and maintain the function of serotonergic neurons in rats with cerebral I/R injury. TSG at different concentrations exhibits varying therapeutic effects on cerebral I/R injury, and a higher concentration promoted better effects.
This study shows the effectiveness of TSG, but its design has some limitations, and further in-depth in vivo studies are needed to investigate the role of 5-HT2A receptor and UCP4 protein played on cerebral I/R injury and the underlying regulatory mechanisms.
Acknowledgments
This study was supported by the Science and Technology Plan of Huaihua City (No. 2015S2202); the Science and Technology Plan of Hunan Provincial Health and Family Planning Committee (No. B2015-67) and A Project Supported by Scientific Research Fund of Hunan Provincial Education Department (No. 15C0992).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Han JS. Neuroscience.
2. Fan DF, Xi GS, Liang KD, Zhang WH. Serotonin transporter rogress. Shengming Kexue. 2011;23(4):385–389.
3. Yi CA, Wang Y, Hu XS, Yu PH, Wang B. Effect of polygonum multiflorum extracts on 5-HT receptor and biochemical indices in vascular dementia rats. Zhongguo Laonianxue Zazhi. 2014;34(4):945–947.
4. Duan XJ. Effect of Acupuncture on Monoamine Neurotransmitters and Receptorsof Rats with Post-Stroke Depression. Zhengzhou: Henan University of Chinese Medicine; 2013.
5. Hua HM, Cao XS, Gu SL, Chi TY, Ji XF, Zou LB. Effect of 5,6-dihydroxyethyl baicalin on brain tissue energy metabolism in rats with cerebral ischemia. Zhongguo Yaolixue Tongbao. 2014;30(7):1035–1036.
6. Huang XP, Deng CQ, Qiu YY, Wang B, Tang YH, Zeng R. Effects of composition of astragaloside IV and three active ingredients of Panax notoginseng on oxidative stress and Nrf2/HO-1 pathway after cerebral ischemia/reperfusion in mice. Zhongguo Yaolixue Tongbao. 2013;29(11):1596–1601.
7. Wang LR, Liu RZ. The relationship between the expression of UCP4 and ROS in primary cultured neuron after ischemia/reperfusion. Shanxi Yike Daxue Xuebao. 2008;39(5):399–401.
8. Wei Z, Chigurupati S, Bagsiyao P, Henriquez A, Chan SL. The brain uncoupling protein UCP4 attenuates mitochondrial toxin-induced cell death: role of extracellular signal-regulated kinases in bioenergetics adaptation and cell survival. Neurotox Res. 2009;16(1):14–29. doi:10.1007/s12640-009-9039-8
9. Chu AC, Ho PW, Kwok KH, et al. Mitochondrial UCP4 attenuates MPP+ - and dopamine-induced oxidative stress, mitochondrial depolarization, and ATP deficiency in neurons and is interlinked with UCP2 expression. Free Radic Biol Med. 2009;46(6):810–820. doi:10.1016/j.freeradbiomed.2008.12.015
10. He XL, Bi MG, Du GH. Brain mitochondria proteome and energy metabolism in rats after chronic cerebral hypoperfusion. Zhongguo Yaolixue Tongbao. 2012;28(9):1200–1205.
11. Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476.
12. Qiu G, Wu XQ, Luo XG. Effect of radix polygoni multiflori on expression of BDNF in hippocampal neurons induced by Aβ1-40 in rats. Zhongnan Daxue Xuebao (Yixueban). 2006;31(2):194–199.
13. Zhang L, Li L, Li YL. Protective mechanism of stilbene glucoside, an active ingredient of polygonum multiflorum thunb, on nerve cells. Zhongguo Linchuang Kangfu. 2004;8(18):118–120.
14. Zheng LM, Shi YF, Wu HM, Xu ZW. The role of 5-HT energy system in the central system in the early-onset depression. Zhongguo Yaolixue Tongbao. 2015;31(1):19–23.
15. Qian QJ, Wang YF, Li J. Interaction of 5-hydroxytryptamine transporter gene and 5-hydroxytryptamine 2A receptor gene in children with attention deficit hyperactivity disorder. Shiyong Erke Linchuang Zazhi. 2010;25(2):133–136.
16. Song Q, Zhang XW, Zhang R. Status quo of serotonin receptors and subtypes. Gansu Yiyao. 2010;29(1):1–4.
17. Filip M, Bader M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep. 2009;61(5):761–777.
18. Zhang YC. Therapeutic Mechanism of Kaixi Jieyu Fang for Vascular Depression Based on 5-HTR, VEGF and Their Receptors. Beijing: Beijing University of Chinese Medicine; 2014.
19. Li LS, Wang QS, Yan GH, Cai CQ. Changes of brain mitochondrial function in rats after cerebral ischemia. Zhonghua Shenjingke Zazhi. 1996;29(6):329–331.
20. Jin J, Zhang F, Yang LL, Zhang JF, Tang J, Yang F. Advances in mitochondrial uncoupling agents. Shengming Kexue. 2013;25(7):707–713.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.