Back to Journals » Journal of Pain Research » Volume 10
Neuropathic pain responds better to increased doses of pregabalin: an in-depth analysis of flexible-dose clinical trials
Authors Serpell M, Latymer M, Almas M, Ortiz M , Parsons B , Prieto R
Received 9 December 2016
Accepted for publication 17 May 2017
Published 26 July 2017 Volume 2017:10 Pages 1769—1776
DOI https://doi.org/10.2147/JPR.S129832
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Michael Serpell,1 Mark Latymer,2 Mary Almas,3 Marie Ortiz,4 Bruce Parsons,4 Rita Prieto5
1University Department of Anaesthesia, Stobhill Ambulatory Care Hospital, Glasgow, 2Pfizer Ltd, Tadworth, UK; 3Pfizer, Groton, CT, 4Pfizer, New York, NY, USA; 5Pfizer GEP SLU, Madrid, Spain
Background: Pregabalin is an effective treatment option for many patients with neuropathic pain. Higher doses of pregabalin have been shown to be more effective in improving pain outcomes but, in practice, failing to appropriately increase the dose can leave patients under-treated.
Methods: This was a pooled analysis of 6 flexible-dose clinical trials of pregabalin in patients with neuropathic pain (diabetic peripheral neuropathy, peripheral herpetic neuralgia, posttraumatic pain, or postsurgical pain). Patients were divided into “dose pathway” groups based on their weekly pregabalin dose from the start of their trial to the first week of their maintenance phase. These were: 150 mg/day only; 150 to 300 mg/day; 150 to 300 to 450 mg/day; 150 to 300 to 450 to 600 mg/day; 150 to 300 to 600 mg/day; 300 to 600 mg/day. Pain outcomes assessed for each group at each new dose were proportion of 30% and 50% responders (≥30% or ≥50% reduction in mean pain score from baseline) and mean change in pain score. Percent change in mean pain score from baseline was assessed using a marginal structural model.
Results: Seven hundred and sixty-one patients treated with flexible-dose pregabalin were included in the analysis. For each dose pathway group, there was a notably greater proportion of 30% and 50% responders and change in pain score, at each escalating dose. As assessed by the marginal structural model, higher doses of pregabalin were estimated to result in a significantly greater change in mean pain score at each week. This dose response with flexible-dose pregabalin was consistent with that previously observed with fixed-dose pregabalin.
Conclusion: Many patients who do not respond to lower doses of pregabalin will respond with notable improvements in pain outcomes when the dose is escalated. These data should encourage physicians treating patients with neuropathic pain to escalate pregabalin to the dose that delivers optimal analgesia and tolerable side effects.
Keywords: neuropathic pain, pregabalin, dosing
Introduction
Neuropathic pain, defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system”1 is a common chronic pain condition. The condition represents a significant burden on patients and healthcare systems, and can be challenging to diagnose and effectively treat.2,3 Despite the availability of a number of effective pharmacological treatment options,4 many patients with neuropathic pain, particularly those with more severe conditions, may be untreated or undertreated.5
Pregabalin (Pfizer, New York, USA), an α2δ ligand, is indicated in the USA for the treatment of diabetic peripheral neuropathy (DPN), peripheral herpetic neuralgia (PHN), and spinal cord injury,6 and in Europe for the treatment of peripheral and central neuropathic pain.7 It is recommended as a first-line treatment for diverse neuropathic pain conditions (including DPN, PHN, and central pain) by the European Federation of Neurological Societies,4 the International Association for the Study of Pain,8 and the American Academy of Neurology.9,10 While pregabalin is not effective in every patient with neuropathic pain, its efficacy has been demonstrated in clinical trials and meta-analyses.11 Cumulatively, it is estimated that there have now been over 34 million patient-years of exposure to pregabalin.12
A meta-analysis of clinical trials of different treatments for the neuropathic pain condition DPN concluded that pregabalin at doses ≥300 mg/day was more effective in improving pain than pregabalin at doses ≤150 mg/day;13 although many patients do respond to treatment with pregabalin at lower doses (≤300 mg/day).13,14 As a consequence, it is recommended that in clinical practice, pregabalin should be carefully escalated to the dose that delivers optimal analgesia and tolerable side effects.4,6,15 Despite this, some physicians may not attempt a higher dose of pregabalin in patients who do not respond at an initial low dose and many patients receive doses ≤150 mg/day.16
New evidence showing that there are patients who do not respond to a low dose of pregabalin but who do subsequently respond when the dose is increased may help to provide clearer guidance to physicians and more effective treatment to patients. This analysis pooled individual patient-level data from 6 randomized, placebo-controlled studies of flexible-dose pregabalin in neuropathic pain with the aim of assessing how patients who do not initially respond to pregabalin at lower doses react to increasing doses.
Methods
Source data
This analysis included all Pfizer-sponsored studies of pregabalin completed at the time the analysis started that met the following criteria: randomized, parallel, placebo-controlled; conducted in patients with neuropathic pain; and included a treatment arm of flexible-dose pregabalin (150 to 600 mg/day). Only Pfizer-sponsored studies were included as patient-level data were required for the analysis. A total of 6 trials met these criteria. A 12-week trial conducted in 406 patients with DPN, A0081030 (ClinicalTrials.gov: NCT00156078).17 A 4-week trial conducted in 269 patients with PHN, A0081004 (NCT00159666).18 A 12-week trial conducted in 338 patients with either DPN or PHN, 1008–1155.19 An 8-week trial conducted in 308 patients with either DPN or PHN, A0081081 (NCT00301223).20 An 8-week trial conducted in 254 patients with posttraumatic or postsurgical (PT/PS) pain, A0081064 (NCT00292188).21 An 8-week trial conducted in 240 patients with DPN, PHN, or PT/PS, A0081037 (NCT00141219).22 Some historical trials are not recorded at ClinicalTrials.gov.
The trials were conducted between July 2001 and May 2008 and included patients from Asia, Europe, the Middle East, and North and South America. The primary efficacy outcome in each study was the change in mean pain score at endpoint compared with placebo. Mean pain score was the mean score over the past 7 days as recorded by patients in a daily pain diary and measured using an 11-point numeric rating scale scored from 0 (no pain) to 10 (worst possible pain).
Data on the pain response to fixed-dose pregabalin were taken from all Pfizer-sponsored, randomized, placebo-controlled trials of pregabalin in patients with neuropathic pain. This was a total of 27 trials: 1008–1014,23 1008–1029,24 1008–1030,25 1008–1040,25 1008–1045,26 1008–1125,27 1008–1127,28 1008–1131,29 1008–1149,30 1008–1155,19 1008–1196,31 A0081004 (ClinicalTrials.gov: NCT00159666),18 A0081030 (NCT00156078),17 A0081037 (NCT00141219),22 A0081060 (NCT00159679),32 A0081064 (NCT00292188),21 A0081066,33 A0081071 (NCT00143156),17 A0081081 (NCT00301223),20 A0081107 (NCT00407745),34 A0081120 (NCT00394901),35 A0081163 (NCT00553475),36 A0081244 (NCT01049217),37 A0081265 (NCT01332149), A0081268 (NCT01455415),38 A0081269 (NCT01474772),39 and A0081276 (NCT01455428).40 Some historical trials are not recorded at ClinicalTrials.gov.
The protocol for each trial adhered to the International Ethical Guidelines for Biomedical Research Involving Human Subjects, the International Conference on Harmonisation Good Clinical Practice guidelines, and the Helsinki Declaration. All trials were approved by the appropriate independent ethics committee and all patients provided written informed consent.
Dose pathway groups
To be included in this analysis, patients had to have ≥80% compliance on the study drug (calculated by total number of days on a valid dose/duration of treatment), to have remained in their study for at least 28 days, and to have been receiving a stable dose of pregabalin for at least 4 days. Patients were grouped into dose pathways based on the pattern of their pregabalin dose at each week during titration phase and up to 1 week of their maintenance phase. The dose pathway groups were: 150 mg/day only; 150 to 300 mg/day; 150 to 300 to 450 mg/day; 150 to 300 to 450 to 600 mg/day; 150 to 300 to 600 mg/day; and 300 to 600 mg/day. Each patient was included in a single-dose pathway group. Patients who decreased their dose at any visit or who did not remain at the particular dose level for at least 4 days were not included in a dose pathway group.
Statistical analysis
Descriptive statistics were used to describe outcomes in treatment-compliant and non-compliant patients for each dose pathway. Assessed outcomes included: proportion of 30% responders (patients with ≥30% reduction in mean pain score from baseline); proportion of 50% responders (patients with ≥50% reduction in mean pain score from baseline); and mean change in pain score at each new dose. A ≥30% reduction in mean pain score from baseline, or a 2-point change in pain score, was accepted to represent a clinically important difference.41,42 For the descriptive analysis, patients were also required to have had ≥80% compliance on pregabalin over the entire study.
In addition, the percent change in mean pain score from baseline was assessed using a marginal structural model (MSM).43,44 A MSM was selected for this analysis as it can accommodate both multiple time-independent covariates, such as baseline clinical and demographic variables and time-dependent covariates, such as treatment and treatment outcomes, even in the presence of missing data and time-varying confounders, as is the case in flexible-dose studies. Specifically, the MSM was a weighted repeated-measures approach using treatment as a time-varying covariate in which weights, based on inverse probability of treatment weighting, control for time-dependent confounders. This produced a pseudo-population with balance in both time-invariant and time-varying covariates that allowed for causal treatment comparisons using standard repeated-measure models. The weighting could also be adjusted to incorporate adjustments for missing data that provides validity under missing at random. Patients were included in the MSM analysis regardless of their level of treatment compliance but a separate analysis limited to patients with ≥80% compliance on pregabalin was conducted for comparison. As a sensitivity analysis, an unweighted estimate of the change in mean pain score from baseline was also conducted. The MSM-estimated change with flexible-dose pregabalin conducted in this analysis was also compared directly with the estimated change in mean pain score from baseline with fixed-dose pregabalin. The study conducted in PHN patients alone, A0081004 (NCT00159666),18 was included in the descriptive analysis but not in the MSM-estimated models as the length of the study was only 4 weeks (compared with 8 or 12 weeks for all other studies).
Results
Patient population
A total of 889 patients were treated with flexible-dose pregabalin; 761 of which met the inclusion criteria (≥80% compliance on pregabalin, had remained in their study for ≥28 days, and receiving a stable dose of pregabalin for ≥4 days) and were included in the descriptive analysis. Of these, 717 could be grouped into one of the six established dose response pathways. The demographic characteristics and baseline clinical characteristics of patients were broadly similar regardless of treatment compliance (Table 1).
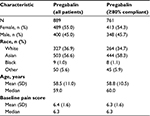  | Table 1 Baseline demographic and clinical characteristics |
Improvement in pain outcomes in each dose pathway
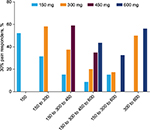
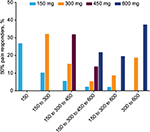
Cumulative assessment of the proportion of 30% (Figure 1) and 50% (Figure 2) pain responders for each dose pathway showed that across every dose pathway, there was recruitment of new pain responders (30% or 50%) at each new and higher dose of pregabalin. Table 2 shows the number (and proportion) of 30% responders at each dose limited to only those patients who were non-responders at the previous dose in their dose pathway (i.e., non-cumulative data). For example, in the 150 to 300 mg/day dose pathway, 100 (of 146) patients were non-responders at 150 mg/day. When these patients were escalated to 300 mg/day, a further 43 (of the 100 non-responding) patients were responders (Table 2). An equivalent pattern was observed with 50% responders (Table 3). These data demonstrate that for every dose pathway, previously non-responsive patients would become 30% or 50% responders with every increase in pregabalin dose.
The change in pain score from baseline for each dose pathway indicated that patients in the shorter dose pathways (i.e., those who remained on 150 mg/day or only escalated to 300 mg/day) had a notable larger change in pain score with pregabalin 150 mg/day (and 300 mg/day) than did those patients who subsequently went on to be escalated to higher doses (Table 4). This finding reflects the fact that patients with a greater response at lower doses were less likely to be escalated to a higher dose. At the same time, for those patients who were escalated to a higher dose, there was a notably greater change in pain score at each escalating dose (Table 4).
These data exclude non-compliant patients (those with <80% compliance on pregabalin over the entire study). Considering only patients who were non-compliant for all dose pathways combined, there was a notably lower proportion of 30% (32.3%) and 50% (15.2%) responders than for treatment-compliant patients (Figures 1 and 2).
Weighted estimation of change in pain score for each dose
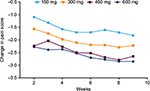
As assessed by MSM, higher doses of pregabalin were estimated to result in a significantly greater change in mean pain score at each week of assessment (Figure 3). Restricting the analysis to only those patients with ≥80% compliance with pregabalin resulted in similar results (not shown). This was also supported by a comparison of the weighted MSM analysis with an unweighted estimate of the change in mean pain score, with each analysis showing similar results (not shown).
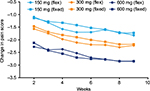
Direct comparison of the MSM-estimated change with flexible-dose pregabalin with the estimated change in mean pain score from baseline with data from fixed-dose clinical trials of pregabalin (a total of 3128 patients; 516 receiving pregabalin 150 mg/day, 1679 receiving pregabalin 300 mg/day, and 933 receiving pregabalin 600 mg/day) demonstrated a similar dose-response pattern for each dose of pregabalin (Figure 4).
Discussion
Pregabalin is recommended as a treatment for diverse neuropathic pain conditions4,8–10 where it is advised that it be carefully escalated to the optimal dose.4,6,15 In the USA, the maximum approved dose of pregabalin is 300 mg/day for DPN and 600 mg/day for PHN,6 while in Europe it is 600 mg/day for all neuropathic pain.45 Despite this, many patients may not receive the most effective dose of pregabalin, with a recent drug utilization study in the UK indicating that the approximate median prescribed dose of pregabalin for neuropathic pain was only 150 mg/day.16 This analysis provides evidence that new patients will tend to respond with every increase in pregabalin dose.
Neuropathic pain can be challenging to treat effectively.2 While there are a number of treatment options, not all are effective in all patients. For instance, data on the efficacy of pregabalin in patients with lower back pain are inconsistent.46,47 In a randomized withdrawal trial, pregabalin was not shown to be effective in patients with chronic lumbosacral radiculopathy.48 Even for those conditions where its efficacy is well established, many patients will not respond to treatment with pregabalin.11 As physicians are advised to ensure that effective and tolerable treatment for neuropathic pain is initiated as soon as possible,2 it is important to ascertain quickly if a treatment is ineffective so that other options can be tested. In these circumstances, it may be understandable why the time is not taken to escalate some patients to a higher, efficacious dose of pregabalin. However, of the 701 patients in this analysis who took the 150 mg/day dosing, 136 (19.4%) responded at that dose. Of those who continued to higher doses, 231 (33.0%) responded at a higher dose. These data do not correct for the tendency for higher doses to be used when patients have inadequate response at a lower dose. The MSM analyses make some correction for this tendency in dose escalation and indicate that it can be worthwhile to persist with pregabalin until the dose that delivers optimal analgesia and tolerable side effects is utilized.
Physicians may be reluctant to escalate the dose of pregabalin due to concerns about adverse events. A previous analysis described the incidence of common adverse events with each dose of pregabalin.49 In that analysis, the incidence of most adverse events was higher with higher fixed doses (450 or 600 mg/day) of pregabalin.49 However, the incidence of adverse events with flexible-dose pregabalin was lower than with any fixed dose >150 mg/day.49 In addition, most adverse events emerged soon after the start of treatment and resolved within 1–2 weeks.49 It was advised that potential adverse events with pregabalin be discussed with patients, before and during treatment, as greater awareness of what to expect could help manage expectations.49 Together with the data reported here, this suggests that communication and careful dose titration could result in improved pain outcomes for patients.
Fixed dose studies have previously demonstrated a clear dose response with pregabalin.11,13 In these studies, patients were assigned to a specific fixed dose prior to treatment with the dose response being shown for a population of patients. Here, for the first time, this analysis shows that this dose response also exists for flexible-dose pregabalin, with individual patients being shown to respond to increasing doses. This dose response was shown to be broadly equivalent in the direct comparison between flexible- and fixed-dose pregabalin shown in Figure 4. This may suggest that in the future, some initial fixed-dose clinical trials could theoretically be replaced with flexible-dosing trials that would allow for fewer treatment arms. However, this would require additional data, and confirmation with other treatments and in other patient groups before it could be considered. While a previous analysis also used a MSM to evaluate dose-response in inflexible-dose trials of an antipsychotic, the analysis did not directly compare this with data from fixed-dose trials.50 We are not aware of any comparable analysis for any treatment for neurological pain.
Limitation
The descriptive part of this analysis was limited to those patients who were ≥80% compliant with treatment. As would be expected, there was a notably lower rate of efficacy among those patients who were <80% compliant. As these patients could not be considered representative of patients treated with the designated dose, they were excluded from the analysis. As a result, the proportion of patients responding at each dose in this analysis may be overstated relative to the full population. This could be considered a limitation of this analysis as in routine clinical practice it is likely that many patients would be <80% compliant with treatment, emphasizing the importance of promoting treatment compliance in clinical practice. Nevertheless, the MSM analysis was conducted in both those patients who were ≥80% compliant and in all patients together. The data for all patients are presented here (Figures 3 and 4) but the results when considering compliant patients only were equivalent.
Conclusion
The analysis demonstrates that many patients who do not respond to pregabalin at lower doses will subsequently respond when the dose is increased. Physicians are advised to follow treatment guidelines and escalate pregabalin to the dose that delivers optimal analgesia and tolerable side effects.
Acknowledgments
This study was sponsored by Pfizer. Michael Serpell has received honoraria from Astellas, Grünenthal, NAPP, and Pfizer for speaking at meetings. His institution has received research support in the past 5 years from commercial studies sponsored by Astellas, Grünenthal, and NAPP. Medical writing support was provided by Joshua Fink, PhD, of Engage Scientific Solutions, and funded by Pfizer.
Disclosure
Mark Latymer, Mary Almas, Marie Ortiz, Bruce Parsons, and Rita Prieto are employees of Pfizer and hold stock options with Pfizer. The authors report no other conflicts of interest in this work.
References
Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. | ||
Smith BH, Lee J, Price C, Baranowski AP. Neuropathic pain: a pathway for care developed by the British Pain Society. Br J Anaesth. 2013;111(1):73–79. | ||
Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain. 2004;5(3):143–149. | ||
Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):e1113–e1188. | ||
Torrance N, Ferguson JA, Afolabi E, et al. Neuropathic pain in the community: more under-treated than refractory? Pain. 2013;154(5):690–699. | ||
Pfizer Inc. LYRICA prescribing information. Availablefrom: http://www.pfizer.com/files/products/uspi_lyrica.pdf. Accessed July 23, 2016. | ||
Lyrica® Summary of Product Characteristics. Sandwich, UK: Pfizer Ltd. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000546/WC500046602.pdf. Accessed July 23, 2016. | ||
O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 Suppl):S22–S32. | ||
Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758–1765. | ||
Dubinsky RM, Kabbani H, El-Chami Z, Boutwell C, Ali H; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;63(6):959–965. | ||
Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009(3):CD007076. | ||
IMS MIDAS data from quarter ending June 2004 till quarter ending September 2016. Average daily dose based on IMS MIDAS data Q3 2016. Data on file. | ||
Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract. 2014;14(2):167–184. | ||
Juhn MS, Parsons B, Varvara R, Sadosky A. Pregabalin for painful diabetic peripheral neuropathy: strategies for dosing, monotherapy versus combination therapy, treatment-refractory patients, and adverse events. Curr Med Res Opin. 2015;31(5):1017–1026. | ||
Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31(7):1448–1454. | ||
Asomaning K, Abramsky S, Liu Q, Zhou X, Sobel RE, Watt S. Pregabalin prescriptions in the United Kingdom: a drug utilisation study of The Health Improvement Network (THIN) primary care database. Int J Clin Pract. 2016;70(5):380–388. | ||
Freeman R, Emir B, Parsons B. Predictors of placebo response in peripheral neuropathic pain: insights from pregabalin clinical trials. J Pain Res. 2015;8:257–268. | ||
Stacey BR, Barrett JA, Whalen E, Phillips KF, Rowbotham MC. Pregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain relief. J Pain. 2008;9(11):1006–1017. | ||
Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115(3):254–263. | ||
Guan Y, Ding X, Cheng Y, et al. Efficacy of pregabalin for peripheral neuropathic pain: results of an 8-week, flexible-dose, double-blind, placebo-controlled study conducted in China. Clin Ther. 2011;33(2):159–166. | ||
van Seventer R, Bach FW, Toth CC, et al. Pregabalin in the treatment of post-traumatic peripheral neuropathic pain: a randomized double-blind trial. Eur J Neurol. 2010;17(8):1082–1089. | ||
Moon DE, Lee DI, Lee SC, et al. Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter study. Clin Ther. 2010;32(14):2370–2385. | ||
Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6(4):253–260. | ||
Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104–2110. | ||
Sharma U, Griesing T, Emir B, Young JP Jr. Time to onset of neuropathic pain reduction: a retrospective analysis of data from nine controlled trials of pregabalin for painful diabetic peripheral neuropathy and postherpetic neuralgia. Am J Ther. 2010;17(6):577–585. | ||
Sabatowski R, Galvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109(1–2):26–35. | ||
Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67(10):1792–1800. | ||
Dworkin RH, Corbin AE, Young JP Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60(8):1274–1283. | ||
Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628–638. | ||
Tolle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008;12(2):203–213. | ||
van Seventer R, Feister HA, Young JP Jr, Stoker M, Versavel M, Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin. 2006;22(2):375–384. | ||
Arezzo JC, Rosenstock J, Lamoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008;8:33. | ||
Simpson DM, Schifitto G, Clifford DB, et al; 1066 HIV Neuropathy Study Group. Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology. 2010;74(5):413–420. | ||
Cardenas DD, Nieshoff EC, Suda K, et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology. 2013;80(6):533–539. | ||
Ogawa S, Suzuki M, Arakawa A, Araki S, Yoshiyama T. Efficacy and tolerability of pregabalin for postherpetic neuralgia: a multicenter, randomized, double-blind, placebo-controlled clinical trial. J Japan Soc Pain Clin. 2010;17(2):141–152. | ||
Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2011;28(1):109–116. | ||
Simpson DM, Rice AS, Emir B, et al. A randomized, double-blind, placebo-controlled trial and open-label extension study to evaluate the efficacy and safety of pregabalin in the treatment of neuropathic pain associated with human immunodeficiency virus neuropathy. Pain. 2014;155(10):1943–1954. | ||
Raskin P, Huffman C, Yurkewicz L, et al. Pregabalin in Subjects With Painful Diabetic Peripheral Neuropathy Using an NSAID for Other Pain Conditions: A Double-Blind Crossover Study. Clin J Pain. 2016;32(3):203–210. | ||
Huffman C, Stacey BR, Tuchman M, et al. Efficacy and Safety of Pregabalin in the Treatment of Patients With Painful Diabetic Peripheral Neuropathy and Pain on Walking. Clin J Pain. 2015;31(11):946–958. | ||
Liu Q, Chen H, Xi L, et al. A randomized, double-blind placebo-controlled trial to evaluate the efficacy and safety of pregabalin for postherpetic neuralgia in a population of Chinese patients. Pain Pract. 2017;17(1):62–69. | ||
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. | ||
Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. | ||
Faries DE, Kadziola ZA. Analysis of longitudinal observational data using marginal structural models. In: Faries DE, Leon AC, Haro JM, et al, editors. Analysis of Observational Health Care Data Using SAS. Cary, NC: SAS Institute Inc. 2010: 211–230. | ||
Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. | ||
Pfizer Ltd. Lyrica® Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000546/WC500046602.pdf. Accessed July 23, 2016. | ||
Baron R, Binder A, Attal N, Casale R, Dickenson AH, Treede RD. Neuropathic low back pain in clinical practice. Eur J Pain. 2016;20(6):861–873. | ||
Taguchi T, Igarashi A, Watt S, et al. Effectiveness of pregabalin for the treatment of chronic low back pain with accompanying lower limb pain (neuropathic component): a non-interventional study in Japan. J Pain Res. 2015;8:487–497. | ||
Baron R, Freynhagen R, Tolle TR, et al. The efficacy and safety of pregabalin in the treatment of neuropathic pain associated with chronic lumbosacral radiculopathy. Pain. 2010;150(3):420–427. | ||
Freynhagen R, Serpell M, Emir B, et al. A comprehensive drug safety evaluation of pregabalin in peripheral neuropathic pain. Pain Pract. 2015;15(1):47–57. | ||
Lipkovich I, Adams DH, Mallinckrodt C, Faries D, Baron D, Houston JP. Evaluating dose response from flexible dose clinical trials. BMC Psychiatry. 2008;8:3. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.