Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Neurocognitive Impairment and Associated Factors Among People Living with HIV: A Systematic Review and Meta-Analysis of African Studies
Authors Zenebe Y , Akele B, W/Selassie M , Necho M
Received 16 June 2022
Accepted for publication 23 March 2023
Published 28 March 2023 Volume 2023:19 Pages 673—687
DOI https://doi.org/10.2147/NDT.S377636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Yosef Zenebe,1 Baye Akele,2 Mulugeta W/Selassie,3 Mogesie Necho1
1Department of Psychiatry, Wollo University, Dessie, Ethiopia; 2Department of Pharmacy, Wollo University, Dessie, Ethiopia; 3Department of Pediatrics and Child Health Nursing, Wollo University, Dessie, Ethiopia
Correspondence: Yosef Zenebe, Email [email protected]; [email protected]
Background: Neurocognitive impairment (NCI) is one of the most common neurological complications in HIV-positive individuals, particularly in resource-limited countries. Neurocognitive impairments can occur at any stage of HIV infection, although the risk increases as the infection progresses. However, in Africa, there are few studies with highly variable and inconsistent results. Therefore, this study aimed to determine the prevalence and factors associated with NCI among HIV-positive people in Africa.
Methods: For this systematic review and meta-analysis, we used PubMed/Medline, Scopus, Web of Science, the Cochrane Library, Embase, and PsycINFO to comprehensively search a number of papers. Studies reporting the prevalence of NCI and its factors were included in the estimation of the pooled prevalence. A consistent data extraction format was created in Microsoft Excel to extract the data, which was then imported into STATA 11 statistical software for analysis. The heterogeneity was evaluated using the I2 test, and a random effect meta-analysis model was employed to calculate the pooled prevalence of NCI because the included studies showed significant heterogeneity.
Results: In all, Africa had a pooled prevalence of NCI of 45.15% (95% CI: 36.86, 53.43). According to the subgroup analysis of this study, West Africa had the lowest frequency, at 42.40% (95% CI: 22.03, 62.77), whereas Central and South Africa had the highest prevalence, at 49.33% (95% CI: 10.72– 87.95).
Conclusion: In Africa, the cumulative prevalence of NCI was high. Being a woman, not having a formal education, those with only an elementary education, being older, having late-stage HIV, and abusing drugs were all often associated with NCI. The average burden of NCI in Africa is high and that would be a significant figure for interventional actions in the area.
Keywords: Africa, meta-analysis, NCI, HIV
Introduction
Patients with HIV infection often experience HIV Associated Neurocognitive Disorders. The specific pattern of neuropsychological deficits is attributed to damage to the frontostriatal circuitry, which is commonly observed in HIV infection.1–3 Deficits in different areas of life such as cognitive, motor, emotional, and even personality changes can be caused by HIV especially as the disease progresses. This HIV Associated Neurocognitive Disorder occurs on a spectrum ranging from asymptomatic NCI to mild neurocognitive disorder (cognitive impairment with mild functional impairment) to frank HIV-associated Dementia (marked cognitive impairment and marked functional impairment).4–8 This classification is based on three parameters: performance during neuropsychological testing, functional impairment, and the absence of any other condition explaining the existing symptoms.4
The HIV-associated neurocognitive disorder can affect many aspects of patient’s lives, including medication adherence, daily activities such as cooking and cleaning, and the ability to hold a steady job. Therefore, it is important to identify and treat NCI as part of routine HIV care.9,10
One of the continents that is heavily impacted by HIV is Africa, particularly the sub-Saharan area. Numerous cross-sectional research have shown that HIV-associated NCI becomes common as a result of the high HIV prevalence and subpar healthcare environments. A study conducted in Zambia by Norma Kabuba et al revealed that 34.6% of Zambians with HIV had a global NCI and met the criteria for HIV-associated neurocognitive disorder (HAND).11 Another study by Nyamayaro et al demonstrated that 49.7% of NCI treatment-experienced adults living with HIV attend primary care clinics in Zimbabwe.12
The presence of other AIDS-defining infections or tumors, low haemoglobin concentration, advanced age, female sex, level of education, income level, social support, medical comorbidities, intravenous drug use, and HIV medication adherence, as well as self-reported alcohol use, Khat chewing, lifetime tobacco use, being married, and unmarried status are all associated with cognitive impairment in HIV.13–22
However, as noted above, facility-based research was carried out in different parts of the African continent about NCI associated with HIV infection, there are only two systematic reviews and meta-analyses done in Sub-Saharan countries and not for African countries in general. In addition, the determinant factors reported by different researchers were not consistent. This might be due to the demographic and clinical characteristics of study populations. So, regardless of sociocultural differences, the goal of this meta-analysis was to ascertain the incidence of NCI across the continent and identify the factors determining the problem’s burden there. Therefore, this study is crucial for giving detailed information to scholars, decision-makers, and medical professionals who tackle the problem at the continental level.
Methods
Search Strategy
Through December 16, 2020, PubMed/Medline, Scopus, Web of Science, Cochrane Library, Embase, PsycINFO, and reference lists of prior pertinent studies were used for literature searches.The following search terms were applied: “epidemiology” OR “prevalence” OR “magnitude” AND “associated factors” OR “determinants” AND “Cognitive functioning” OR “Cognition Disorder/s” OR “Neurocognitive Dysfunction” OR “Cognitive Dysfunction” OR “Cognitive Impairment” OR “Neurocognitive problems” OR “Cognitive problems” OR “NCI” OR “HIV associated neurocognitive disorder” OR “HIV associated dementia” OR “HIV” AND “Africa”. No restrictions were imposed. Additionally, reference lists of the included articles were screened by the first author to detect complementary articles that met the selection criteria.
Eligibility Criteria
An article was eligible for inclusion in the analysis if it fulfilled the following criteria: (1) The initial criteria were that the study must assess NCI in People living with HIV, (2) the study design should be either cross-sectional, cohort, or case-control design, (3) the investigated outcome should be NCI and/or studies should assess the associated factors for NCI, (4) the age of the participants should be 18 years old and more, (5) the sample size should be above 50 and (6) the study must be conducted in Africa. In our review, NCI includes HIV Associated Neurocognitive Disorder, HIV-associated neurocognitive deficit, cognitive dysfunction, HIV-associated neurocognitive impairment, HIV dementia, neurocognitive disorder, and cognitive impairment. Previous reviews, studies conducted on animals, editorials, and works written in languages other than English were all rejected. After carefully reviewing each publication, YZ, BA, and MW independently decided which ones would be incorporated into the final meta-analysis. Any differences between these authors on inclusion/exclusion criteria were resolved by discussion with a fourth author and consensus (MN).
Data Extraction
From eligible studies, four authors independently extracted relevant information, including the first author’s name, publication year, country of the study, sample size, and the number of participants with NCI and age of the population. Any disputed findings were resolved by discussions between investigators until a consensus was reached.
Quality Assessment
The modified Newcastle-Ottawa Scale (NOS)23 was employed for the assessment of the quality of studies. An ascertainment of NCI, statistical quality, sample representativeness, sample size, and comparability between participants were the domains NOS scale to assess the quality of individual studies.
Statistical Analysis
In this meta-analysis, the pooled prevalence of NCI and its associated factors with their 95% CIs was computed using a random-effects model.24 Meta-XL version 5.325 and the STATA-11 Meta-prop package26 were used for the analysis. Considering the Cochrane Handbook, heterogeneity among the studies was assessed using the I2 index. In this regard, there are three categories for the I2 index: (25–49% indicates moderate heterogeneity, 50–75% indicates substantial heterogeneity and over 75% indicates considerable heterogeneity).27 For a sensitivity analysis of the pooled estimate’s power, studies were removed one at a time. Based on area, study design, and study instrument, subgroup analysis was carried out to determine the cause of the heterogeneity among studies. To examine publication bias, funnel plots and the Egger test was utilized. Any statistical analysis performed throughout this investigation that had a P-value of 0.05 or lower was considered statistically significant. Tables and forest plots were used to present the results in the end.
Results
Search Result
The search strategy generated 579 citations of which 22 studies met inclusion criteria as presented according to the PRISMA guidelines in Figure 1.
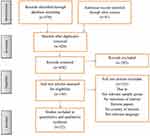 |
Figure 1 Flowchart of the study selection process. |
Characteristics of Studies Included
A total of 22 articles in Africa9–12,15,16,20–22,28–40 that examined NCI and associated factors in 6741 people living with HIV have been included in this study. Regarding the regional distribution, the three articles16,28,32 were done in Central and South Africa, fifteen9,11,12,15,20–22,29–31,33–35,38,40 were from East Africa and four10,36,37,39 from West Africa.
Based on the study design, 199,10,12,15,16,20–22,28–35,38–40 of them were cross-sectional, one cohort,37 and two case-control11,36 studies. More than half of these studies10,15,16,21,22,28,30,32–36,38,40 used IHDS to screen NCI among individuals living with HIV. Two31,39 used Frascati criteria, three9,11,12 used GDS, and the remaining three20,29,37 studies used others (MoCA, WAIS, NR). In addition, nine15,28,31,32,34–37,39 were published from 2010–2015 years, and the remaining thirteen9–12,16,20–22,29,30,33,38,40 studies were published from 2016–2020 years (Table 1). Thirteen of the included studies assessed the clinical characteristics of study participants.10,11,15,20–22,28,30,31,33,37,38,41
 |
Table 1 The Prevalence of NCI and the Tool Used in Each Study Among People Living with HIV in Africa |
Quality of Included Studies
According to the NOS, the 22 studies’ overall quality assessment scores range from 6 to 10. Twenty of the studies had good methodological quality, while the other two had moderate quality. However, it was determined that none of the research was of poor quality.
The Prevalence of NCI Among People Living with HIV
Twenty-two studies9–12,15,16,20–22,28–40 in Africa were pooled in the final meta-analysis to determine the average magnitude of NCI among HIV-positive people. The reported prevalence of NCI in People living with HIV among studies included in the analysis varies from as low as 15% in Malawi31 to as high as 88%% in Kenya.33 We used the standard error, prevalence, and author year of each study to compute the overall prevalence of NCI. Using a random effect model, the pooled prevalence of NCI among HIV-positive people in Africa was 45.15% (95% CI: 36.86, 53.43). Significant heterogeneity (I2=100%, P-value 0.001) had an impact on this average estimate. Below is a forest plot with detailed information about the combined prevalence of NCI among people living with HIV in Africa (Figure 2).
 |
Figure 2 The pooled estimated prevalence of neurocognitive impairment among people living with HIV in Africa. |
Subgroup Analysis of the Prevalence of NCI Among People Living with HIV
In addition, we performed subgroup analysis based on the regional location of the study, the study design, the study instrument used, and the year of publication. Among the studies included, three16,28,32 were done in Central and South Africa, fifteen9,11,12,15,20–22,29–31,33–35,38,40 were in East Africa, and four10,36,37,39 of them were in West Africa.
Accordingly, the pooled prevalence of NCI was 49.33% (95% CI; 10.72, 87.95) (I2=100%; P-value<0.001) in Central and South Africa, 45.04% (95% CI; 35.55, 54.53) (I2=100%; P-value<0.001) in East Africa and, 42.40% (95% CI; 22.03, 62.77) (I2=100%; P-value<0.001) in West Africa. Case-control and cohort studies showed a higher prevalence of NCI (50.90% (95% CI; 34.41, 67.39)) than cross-sectional studies (44.24% (95% CI; 34.98, 53.49) (I2=100%; P-value 0.001). As determined by MoCA, WAIS, and NR, the prevalence of NCI was greater at 50.70% (95% CI: 16.21, 85.19) (I2=100%; P-value: 0.001). In addition, the pooled prevalence of NCI among studies that utilized the IHDS screening tool was 48.13% (95% CI; 37.43, 58.83) (I2=100%; p-value<0.001). However; the lower prevalence of NCI was reported among studies that utilized GDS and Frascati criteria, 33.46% (95% CI; 24.32, 42.60) (I2=100%; p-value<0.001), screening tool. The prevalence of NCI was lower among studies published from 2016–2020 Years; 42.55% (95% CI; 32.42, 52.69) (I2=100%; p-value<0.001) than studies published from 2010–2015 Years; 48.89% (95% CI; 34.41, 63.36) (I2=100%; p-value<0.001) (Table 2).
 |
Table 2 A Subgroup Analysis of the Prevalence of NCI Among People Living with HIV |
Meta-Regression
To identify independent determinants of the observed differences in the prevalence of NCI in People living with HIV, we did a meta-regression analysis. The analysis process was a univariate regression analysis followed by a final meta-regression analysis. In the univariate regression analysis, variables with P-value < 0.8 were selected and fitted to the final meta-regression model as recommended by Ferrari et.al. In the final meta-regression analysis model, we computed the impacts of study design (Case-control/cohort studies and cross-sectional studies), studies location (studies conducted in Central and South Africa, East Africa, and West Africa), the instrument used to screen NCI (MoCA, WAIS, and NR, IHDS, GDS and Frascati criteria) and year of publication of the study (from 2010–2015 and 2016–2020). The overall fraction of difference elucidated by the above covariates in the regression model was 9% (R2 = 0.09; P value=0.870). However, all four of the above independent variables were not significant determinants for the observed difference in the prevalence of NCI in People living with HIV.
Sensitivity Analysis on the Prevalence of NCI Among People Living with HIV
The sensitivity analysis was done to see if any of the twenty-two studies had over-weighted the average prevalence of NCI among HIV-positive individuals. However, as the 95% CI interval was derived by excluding each of the twenty-two studies individually during the 95% CI interval of the overall result, the outcome demonstrated that there was no single influential study (Figure 3).
 |
Figure 3 A sensitivity analysis of the prevalence of neurocognitive impairment among people living with HIV in Africa. |
Funnel Plot on the Risk of Publication Bias for the Prevalence of NCI Among People Living with HIV
To identify publication bias, Egger’s publication bias plot test was used. However, as the P-value of the Eggers publication bias plot test is negligible (P-value=0.630), there was no publication bias in this meta-analysis. A funnel plot for a Logit event rate of prevalence of NCI among HIV-positive individuals against its standard error might be used to visually reinforce this (Figure 4).
 |
Figure 4 Funnel plot of the risk of publication bias for the prevalence of neurocognitive impairment among people living with HIV in Africa. |
Associated Factors for NCI Among People Living with HIV
From the total included studies, nineteen10–12,15,16,20–22,28,30–35,37–40 reported data regarding the associated factors of NCI in People living with HIV. female gender,31 no formal education,15,21,38 having a primary-level education,21 older age,38 age group of 41−64 years,40 older age of 50 years or above,15 unemployment status,22 impairment in the activity of daily living,30 body mass index 16 kg/m2,22 late clinical-stage of the illness,30 being in WHO clinical stage T3 category/advancing stages of the disease,22,40 ≥ 3log10 copies/mL,40 having a co-morbid opportunistic infection,15 increasing stress scores,35 stopping medication,10 khat chewing,40 and substance use15 were strongly and positively associated with NCI (Table 3). The factors most frequently associated with NCI in People living with HIV were being female,31,33,34 having no formal education,15,21,38 having a primary level education,21,28 older age,16,38,40 late clinical stage of the illness22,30,40 and substance use.15,40
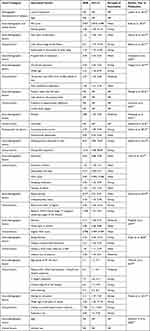 |
Table 3 Associated Factors for NCI Among People Living with HIV |
The pooled odds ratio of being female was 3.09. In addition, the pooled odds ratio for people with no formal education is 4.20. The pooled odds ratio in people with primary-level education was 5.80. Moreover, The pooled odds ratio of older people in the above-mentioned studies was 3.57. Besides, the late clinical stage of the illness was also found to have a significant association with the development of NCI with a pooled odds ratio of 4.45. Substance use was also an associated factor for the development of NCI with a pooled estimate odds ratio of 4.54 (Table 4).
 |
Table 4 Pooled Odds Ratio of NCI Among People Living with HIV |
Discussion
This study used systematic review and meta-analysis to best estimate the prevalence and associated factors for NCI among people living with HIV in Africa. In this review, a small number of studies with a high level of heterogeneity found that 45.15% of People living with HIV were likely to have NCI in the continent. According to experts, this is the first study of its kind to quantitatively and qualitatively evaluate the prevalence and contributing variables of NCI in HIV-positive patients in an African environment. Therefore, the study’s goal was to add to epidemiological knowledge about NCI prevalence and related determinants in HIV-positive individuals. Therefore, the information obtained from the pooled prevalence and associated factors for NCI could serve as important evidence for stakeholders working in the area.
In this study, the overall pooled prevalence of NCI was 45.15% (Total number of study participants=6741). This was higher than the pooled estimated prevalence of NCI among HIV-1 infected pre-ART adults in sub-Saharan Africa which was 42.37%, and those on ART for 6 months was 30.39%,42 a systematic review and meta-analysis study among HIV/AIDS patients on anti-retroviral therapy in Sub-Saharan Africa was 44.46% (Total number of study participants=5820),41 and a meta-analysis study on a global prevalence and burden of HIV-associated neurocognitive disorder which was 42.6% (Total number of study participants=35,513),43 and an Ethiopian systematic review and meta-analysis which was 39.15% (Total number of study participants=3529).44 The current pooled prevalence was lower than the global pooled prevalence of HIV-associated neurocognitive disorders 50.41% (Total number of study participants=14,107).45 The discrepancy could be due to the study period, sample size, and the ART status of the study participants. It reaffirmed that ART confers substantial benefits against NCI, lowering it by over 60%.46
In the present analysis, the average pooled estimated prevalence of NCI in Central and South Africa (49.33%) was larger than the pooled estimated prevalence in East Africa (45.04%) and West Africa (42.40%). Region-specific HIV distribution, cultural and socio-economic factors, access to ART service, quality of ART service, retention of patients to ART care, and skilled human power related to ART services might be responsible for such variation.47–50
The average pooled prevalence of NCI in case-control and cohort studies was higher than in cross-sectional studies. This might be due to the methodology employed in case-control and cohort studies than cross-sectional studies.
There was also a significant variety of pooled estimated NCI across the study instrument used. The average estimated prevalence of NCI in People living with HIV as measured with IHDS, GDS, and Frascati criteria, and others (MoCA and WAIS) was 48.13%, 33.46%, and 50.70% respectively. The disparity may be caused by the fewer studies that were included in some of the evaluation tool categories, which would reduce the accuracy of the estimate.
Those published between 2016 and 2020 indicated a lower pooled estimated prevalence of NCI (42.55%) than studies published between 2010 and 2015 (488.9%). This reason could be that African countries launched recently an intensive screening program for HIV to meet the 90–90-90 target set by WHO.51 Furthermore, diagnosed patients received quality ART service which could improve the treatment outcome, leading to a decreased prevalence of NCI. Between 2012 and 2015, international guidelines went the last step further and recommended treating all HIV-infected adults regardless of their CD4 count. Since 2016 African countries initiated ART for all HIV-positive patients irrespective of their CD4 count. This early initiation of ARV drugs has a hallmark that the CD4 count remains high which could account for a difference in the prevalence of NCI across the study period.52–56
In this study, females were three times more likely to develop NCI than males. This could be due to the high vulnerability of females to contracting HIV. Several factors can increase the risk of HIV in females. For example, during vaginal or anal sex, a female has a greater risk of getting HIV because, in general, receptive sex is riskier than insertive sex.57 The more vulnerability to HIV, females might develop the late stages of the disease and NCI.
People living with HIV who had no formal education were four times to have NCI as compared with those who had Secondary & above grades. This might be due to the low awareness of the treatment of HIV and late presentation to health facilities.
In our review, older people were almost four times at higher risk of developing NCI than people with 18–25 years. This finding was inconsistent with a meta-analysis study conducted in two Sub-Saharan Africa studies.41,42 The strong association observed in our study might reflect an increased vulnerability to NCI which may be intensified by lifestyle changes and physical changes during old age.
Besides, the current study revealed that the late clinical stage of the illness was 4.45 times more likely to have NCI than clinical stage I HIV. This might be because the late clinical stage of HIV may affect the central nervous system and may expose the individual to develop different kinds of neurocognitive impairment.
People with HIV who used substances were nearly five times more likely to have NCI as compared to people with HIV who did not use the substance. The current study was consistent with another study.58 HIV-associated neurocognitive impairment was associated with problematic methamphetamine use and higher plasma HIV RNA levels.58
Meta-Analysis on the Difference Among Included Studies
Due to the differences across the included studies, there may be significant variability in the prevalence of NCI and related variables among HIV-positive individuals. Therefore, more investigation into the cause of such significant variability was necessary. An analysis of subgroups was conducted as a result. The results of the subgroup analysis showed that the location of the study, the type of study design used, the type of study instrument used to screen for NCI, and the year of publication were responsible for the variation in NCI prevalence between the included studies. Additionally, a temporal sensitivity analysis had also been done, although none of the research had been found to have an impact on the final estimate.
Limitations
The following restrictions apply to this comprehensive review and meta-analysis. The inclusion of only English-language evaluations raises the possibility of publication bias. It might be challenging to generalize to all African countries because studies from only 10 African nations were considered. Furthermore, there was a significant amount of heterogeneity across the included studies, which can be related to variations in the technique, research scope, and screening tool types. The overall pooled prevalence of NCI may be high due to the false positive estimate of IHDS, Frascati criteria, and GDS scale.59–61
Conclusion
This comprehensive review and meta-analysis study found significant heterogeneity and a high average burden of NCI among HIV-positive individuals. Being a woman, not having a formal education, those with only an elementary education, being older, having late-stage HIV, and abusing drugs were all often associated with NCI. The authors make a lot of their findings of different rates of NCI between African regions, however, this is only a few percentage points, compared to the large difference between methods (about double) and between studies (15–88%). The average burden of NCI in Africa is high and that would be a significant figure for interventional actions in the area.
Our narrative synthesis showed that NCI was highly and positively associated with female gender, low education, advanced age, unemployment, impairment in daily living activities, having a co-morbid opportunistic infection, elevated stress scores, medication non-adherence, and substance use. In light of the above-mentioned factors and the systematic review and meta-analysis study, healthcare treatments for NCI among HIV-positive individuals would be significant.
Abbreviations
AIDS, Acquired Immune Deficiency Syndrome; AOR, Adjusted odds ratio; ART, Antiretroviral therapy; CI, Confidence Interval; GDS, Global Deficit Score; HIV, Human immunodeficiency virus; IDHS, International HIV dementia scale; MoCA, Montreal Cognitive Assessment; NCI, neurocognitive impairment; NOS, Newcastle-Ottawa Scale; NR, Not reported; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; WHO, World Health Organization.
Data Sharing Statement
All the data are included in this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No specific fund was secured for this review.
Disclosure
The authors declare that they have no known competing interests that could have appeared to influence the work reported in this paper.
References
1. Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev. 2002;26(3):353–359. doi:10.1016/S0149-7634(02)00006-4
2. Ances B, Roc A, Wang J, et al. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66(6):862–866. doi:10.1212/01.wnl.0000203524.57993.e2
3. Cherner M, Cysique L, Heaton RK, et al. Neuropathologic confirmation of definitional criteria for human immunodeficiency virus-associated neurocognitive disorders. J Neurovirol. 2007;13(1):23–28. doi:10.1080/13550280601089175
4. Saylor D, Dickens AM, Sacktor N, et al. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(4):234–248. doi:10.1038/nrneurol.2016.27
5. Carroll A, Brew B. HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Research. 2017;6:312. doi:10.12688/f1000research.10651.1
6. Focà E, Magro P, Motta D, et al. Screening for neurocognitive impairment in HIV-infected individuals at first contact after HIV diagnosis: the experience of a large clinical center in Northern Italy. Int J Mol Sci. 2016;17(4):434. doi:10.3390/ijms17040434
7. Adams C, Zacharia S, Masters L, Coffey C, Catalan P. Mental health problems in people living with HIV: changes in the last two decades: the London experience 1990–2014. AIDS Care. 2016;28(sup1):56–59. doi:10.1080/09540121.2016.1146211
8. Achappa B, Priyadarshni S, Madi D, et al. Neurocognitive dysfunction among HIV positive patients using International HIV dementia scale. Asian J Med Sci. 2014;5(4):61–64. doi:10.3126/ajms.v5i4.8724
9. Yechoor N, Towe SL, Robertson KR, Westreich D, Nakasujja N, Meade CS. Utility of a brief computerized battery to assess HIV-associated neurocognitive impairment in a resource-limited setting. J Neurovirol. 2016;22(6):808–815. doi:10.1007/s13365-016-0456-1
10. Yusuf AJ, Hassan A, Mamman AI, Muktar HM, Suleiman AM, Baiyewu O. Prevalence of HIV-associated neurocognitive disorder (HAND) among patients attending a tertiary health facility in Northern Nigeria. J Int Assoc Provid AIDS Care. 2017;16(1):48–55. doi:10.1177/2325957414553839
11. Kabuba N, Menon JA, Franklin DR, Heaton RK, Hestad KA. Use of western neuropsychological test battery in detecting HIV-associated neurocognitive disorders (HAND) in Zambia. AIDS Behav. 2017;21(6):1717–1727. doi:10.1007/s10461-016-1443-5
12. Nyamayaro P, Gouse H, Hakim J, Robbins RN, Chibanda D. Neurocognitive impairment in treatment-experienced adults living with HIV attending primary care clinics in Zimbabwe. BMC Infect Dis. 2020;20:1–10. doi:10.1186/s12879-020-05090-8
13. Attonito JM, Dévieux JG, Lerner BD, Rosenberg R, Rosenberg R. Exploring substance use and HIV treatment factors associated with neurocognitive impairment among people living with HIV/AIDS. Public Health Front. 2014;2:105. doi:10.3389/fpubh.2014.00105
14. Vance DE, Fazeli PL, Dodson JE, Ackerman M, Talley M, Appel SJ. The synergistic effects of HIV, diabetes, and aging on cognition: implications for practice and research. J Neurosci Nurs. 2014;46(5):292. doi:10.1097/JNN.0000000000000074
15. Mossie TB, Tegegne MT. HIV dementia among HIV positive people at Debre markos hospital, Northwest Ethiopia. Am J Psychol Neurosci. 2014;2:18–24. doi:10.11648/j.ajpn.20140202.11
16. Pascal M, Gaspard T, Philomène NK, Emmanuel Y, Avilah AWP, Hortense H. Determinants of neurocognitive impairment in HIV in a cohort of patients on antiretroviral therapy followed in Bangui (Central African Republic). Neurosci Med. 2016;7(01):1. doi:10.4236/nm.2016.71001
17. Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology. 2014;82(20):1776–1783. doi:10.1212/WNL.0000000000000433
18. Watkins CC, Treisman GJ. Cognitive impairment in patients with AIDS–prevalence and severity. Hiv Aids. 2015;7:35.
19. Wabel NT. Psychopharmacological aspects of catha edulis (khat) and consequences of long term use: a review. Psychiatry Behav Sci. 2011;1(4):187.
20. Sanmartí M, Meyer A, Jaen A, et al. HIV‐associated neurocognitive impairment in stable people living with HIV on ART in rural Tanzania. HIV Med. 2021;22(2):102–112. doi:10.1111/hiv.12979
21. Araya T, Abebaw D, Belete A, Derajew H, Umer H. Prevalence and factors associated with neuro cognitive disorders among HIV-positive patients in Ethiopia: a hospital-based cross-sectional study. Ethiop J Health Dev. 2020;34:1.
22. Debalkie Animut M, Sorrie MB, Birhanu YW, Teshale MY. High prevalence of neurocognitive disorders observed among adult people living with HIV/AIDS in Southern Ethiopia: a cross-sectional study. PLoS One. 2019;14(3):e0204636. doi:10.1371/journal.pone.0204636
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi:10.1007/s10654-010-9491-z
24. Doi SA, Thalib L. A quality-effects model for meta-analysis. Epidemiology. 2008;19:94–100. doi:10.1097/EDE.0b013e31815c24e7
25. Barendregt J, Doi S. MetaXL User Guide Version 5.3. EpiGear International Pty Ltd; 2016.
26. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):1–10. doi:10.1186/2049-3258-72-39
27. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
28. Atashili J, Gaynes BN, Pence BW, et al. Prevalence, characteristics and correlates of a positive-dementia screen in patients on antiretroviral therapy in Bamenda, Cameroon: a cross-sectional study. BMC Neurol. 2013;13(1):1–7. doi:10.1186/1471-2377-13-86
29. Awori V, Mativo P, Yonga G, Shah R. The association between asymptomatic and mild neurocognitive impairment and adherence to antiretroviral therapy among people living with human immunodeficiency virus. South Afr J HIV Med. 2018;19:1. doi:10.4102/sajhivmed.v19i1.674
30. Belete T, Medfu G, Yemiyamrew E. Prevalence of HIV associated neurocognitive deficit among HIV positive people in Ethiopia: a cross sectional study at Ayder Referral Hospital. Ethiop J Health Sci. 2017;27(1):67–76. doi:10.4314/ejhs.v27i1.9
31. Kelly CM, van Oosterhout JJ, Ngwalo C, et al. HIV associated neurocognitive disorders (HAND) in Malawian adults and effect on adherence to combination anti-retroviral therapy: a cross sectional study. PLoS One. 2014;9(6):e98962. doi:10.1371/journal.pone.0098962
32. Lawler K, Mosepele M, Ratcliffe S, et al. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. J Int AIDS Soc. 2010;13(1):1–9. doi:10.1186/1758-2652-13-15
33. Mugendi A, Kubo M, Nyamu D, Mwaniki L, Wahome S, Haberer J. Prevalence and correlates of neurocognitive disorders among HIV patients on antiretroviral therapy at a Kenyan hospital. Neurol Res Int. 2019;2019:1–10. doi:10.1155/2019/5173289
34. Nakasujja N, Allebeck P, Agren H, Musisi S, Katabira E. Cognitive dysfunction among HIV positive and HIV negative patients with psychosis in Uganda. PLoS One. 2012;7(9):e44415. doi:10.1371/journal.pone.0044415
35. Nakku J, Kinyanda E, Hoskins S. Prevalence and factors associated with probable HIV dementia in an African population: a cross-sectional study of an HIV/AIDS clinic population. BMC Psychiatry. 2013;13(1):1–7. doi:10.1186/1471-244X-13-126
36. Oshinaike OO, Akinbami AA, Ojo OO, Ojini IF, Okubadejo UN, Danesi AM. Comparison of the minimental state examination scale and the international HIV dementia scale in assessing cognitive function in Nigerian HIV patients on antiretroviral therapy. AIDS Res Treat. 2012;2012:1–6. doi:10.1155/2012/581531
37. Sunmonu TA, Sellner J, Ogunrin OA, et al. Intellectual impairment in patients with newly diagnosed HIV infection in southwestern Nigeria. Biomed Res Int. 2015;2015:1–6. doi:10.1155/2015/185891
38. Tsegaw M, Andargie G, Alem G, Tareke M. Screening HIV-associated neurocognitive disorders (HAND) among HIV positive patients attending antiretroviral therapy in South Wollo, Ethiopia. J Psychiatr Res. 2017;85:37–41. doi:10.1016/j.jpsychires.2016.10.016
39. Yakasai AM, Gudaji MI, Muhammad H, et al. Prevalence and correlates of HIV-associated neurocognitive disorders (HAND) in Northwestern Nigeria. Neurol Res Int. 2015;2015:1–9. doi:10.1155/2015/486960
40. Yideg Yitbarek G, Mossie Ayana A, Bariso Gare M, Garedew Woldeamanuel G. Prevalence of cognitive impairment and its predictors among HIV/AIDS patients on antiretroviral therapy in Jimma University medical center, Southwest Ethiopia. Psychiatry J. 2019;2019:1.
41. Yitbarek GY, Ayehu GW, Ayele BA, Bayih WA, Gebremariam AD, Tiruneh SA. Cognitive impairment and its associated factors among HIV/AIDS patients on anti retro-viral therapy in Sub-Saharan Africa: systematic review and meta-analysis. Neurol Psychiatry Brain Res. 2020;38:83–91. doi:10.1016/j.npbr.2020.11.002
42. Habib AG, Yakasai AM, Owolabi LF, et al. Neurocognitive impairment in HIV-1-infected adults in Sub-Saharan Africa: a systematic review and meta-analysis. Int J Infect Dis. 2013;17(10):e820–e31. doi:10.1016/j.ijid.2013.06.011
43. Wang Y, Liu M, Lu Q, et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology. 2020;95(19):e2610–e21. doi:10.1212/WNL.0000000000010752
44. Zenebe Y, Akele B, Necho M, Necho M. A systematic review and meta-analysis of HIV associated neurocognitive disorders (HAND) among people with HIV in Ethiopia. AIDS Res Ther. 2021;18(1):1–9. doi:10.1186/s12981-021-00424-1
45. Zenebe Y, Necho M, Yimam W, Akele B. Worldwide occurrence of HIV-associated neurocognitive disorders and its associated factors: a systematic review and meta-analysis. Front Psychiatry. 2022;13:814362. doi:10.3389/fpsyt.2022.814362
46. Joska JA, Westgarth-Taylor J, Hoare J, et al. Validity of the international HIV dementia scale in South Africa. AIDS Patient Care STDS. 2011;25(2):95–101. doi:10.1089/apc.2010.0292
47. Prudden HJ, Beattie TS, Bobrova N, et al. Factors associated with variations in population HIV prevalence across West Africa: findings from an ecological analysis. PLoS One. 2015;10(12):e0142601. doi:10.1371/journal.pone.0142601
48. Mark D, Armstrong A, Andrade C, et al. HIV treatment and care services for adolescents: a situational analysis of 218 facilities in 23 sub‐Saharan African countries. J Int AIDS Soc. 2017;20:21591. doi:10.7448/IAS.20.4.21591
49. Callaghan M, Ford N, Schneider H. A systematic review of task-shifting for HIV treatment and care in Africa. Hum Resour Health. 2010;8(1):1–9. doi:10.1186/1478-4491-8-8
50. Dwyer-Lindgren L, Cork MA, Sligar A, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570(7760):189–193. doi:10.1038/s41586-019-1200-9
51. Granich R, Williams B, Gupta S, Zuniga JM. 90-90-90, epidemic control and Ending AIDS: global situation and recommendations. bioRxiv. 2017;2017:196972.
52. Geffen N, Low MO. When to start antiretroviral treatment? A history and analysis of a scientific controversy. South Afr J HIV Med. 2017;18(1):8. doi:10.4102/sajhivmed.v18i1.734
53. World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. World Health Organization; 2015.
54. Yoshimura K. Current status of HIV/AIDS in the ART era. J Infect Chemother. 2017;23(1):12–16. doi:10.1016/j.jiac.2016.10.002
55. Bartlett J. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. The Department of Health and Human Services Panel on Antiretroviral Guidelines for Adult and Adolescents; 2008:42–43.
56. Günthard HF, Aberg JA, Eron JJ, et al. International Antiviral Society-USA Panel. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312(4):410–425. doi:10.1001/jama.2014.8722
57. Beer L, McCree DH, Jeffries WL, Lemons A, Sionean C. Recent US Centers for Disease Control and Prevention activities to reduce HIV stigma. J Int Assoc Provid AIDS Care. 2019;18:2325958218823541. doi:10.1177/2325958218823541
58. Weber E, Morgan EE, Iudicello JE, et al. Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. J Neurovirol. 2013;19(1):65–74. doi:10.1007/s13365-012-0141-y
59. Nightingale S, Dreyer AJ, Saylor D, Gisslén M, Winston A, Joska JA. Moving on from HAND: why we need new criteria for cognitive impairment in persons living with human immunodeficiency virus and a proposed way forward. Clin Infect Dis. 2021;73(6):1113–1118. doi:10.1093/cid/ciab366
60. Underwood J, De Francesco D, Leech R, et al. Medicalising normality? Using a simulated dataset to assess the performance of different diagnostic criteria of HIV-associated cognitive impairment. PLoS One. 2018;13(4):e0194760. doi:10.1371/journal.pone.0194760
61. Dreyer AJ, Nightingale S, Heaps-Woodruff JM, et al. Rates of cognitive impairment in a South African cohort of people with HIV: variation by definitional criteria and lack of association with neuroimaging biomarkers. J Neurovirol. 2021;27(4):579–594. doi:10.1007/s13365-021-00993-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
