Back to Journals » Eye and Brain » Volume 12
Neuro-Ophthalmological Manifestations of Obstructive Sleep Apnea: Current Perspectives
Authors Farahvash A , Micieli JA
Received 30 April 2020
Accepted for publication 18 June 2020
Published 7 July 2020 Volume 2020:12 Pages 61—71
DOI https://doi.org/10.2147/EB.S247121
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Armin Farahvash,1 Jonathan A Micieli2– 4
1Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; 2Department of Ophthalmology and Vision Sciences, University of Toronto, Toronto, Ontario, Canada; 3Division of Neurology, Department of Medicine, University of Toronto, Toronto, Ontario, Canada; 4Kensington Vision and Research Centre, Toronto, Ontario, Canada
Correspondence: Jonathan A Micieli Tel +1 (416) 928-1335
Fax +1 (416) 928-5075
Email [email protected]
Abstract: Obstructive sleep apnea (OSA) is a disease of obstructed airways during sleep that significantly impacts the quality of life and increases the risk of various systemic diseases. OSA has been studied as a risk factor for a number of neuro-ophthalmic conditions and a strong relationship has been established with non-arteritic anterior ischemic optic neuropathy (NAION). The incidence of glaucoma and stroke have also been significantly associated with OSA and are conditions that may also be seen by neuro-ophthalmologists. Patients with NAION have a significantly higher incidence of OSA and OSA diagnosis significantly increases the risk for NAION development. Non-compliance with continuous positive airway pressure (CPAP) in OSA patients has also been found to be a risk factor for fellow-eye involvement and there is increasing evidence to suggest that every patient with NAION should be formally evaluated with polysomnography. The relationship between OSA and idiopathic intracranial hypertension (IIH) has also been studied, but the relationship between these two conditions is less clear. There is insufficient evidence to recommend routine eye examinations in OSA patients for papilledema and conducting a sleep study for a newly diagnosed IIH patient should be left to the discretion of the clinician based on other symptoms and risk factors of OSA.
Keywords: ischemic optic neuropathy, non-arteritic anterior ischemic optic neuropathy, idiopathic intracranial hypertension, optic nerve, glaucoma, stroke
Case Vignette
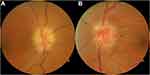
A 63-year-old man with no known medical conditions was seen in neuro-ophthalmology consultation for bilateral vision loss. He noticed blurred vision in his right eye 3 weeks prior to presentation and blurred vision in his left eye 5 days prior to presentation. He denied symptoms suggestive of giant cell arteritis and did not use any medications. He had a visual acuity of 20/400 (right) and 20/60 (left) with a right relative afferent pupillary defect. Humphrey 24–2 SITA-Fast visual fields showed severe generalized depression (right) and an inferior altitudinal defect (left). Dilated fundus examination demonstrated bilateral optic disc edema (Figure 1). Blood pressure was 128/80 mmHg and body mass index was 28.9kg/m2. Review of symptoms was significant for daytime sleepiness, frequent snoring, and witnessed episodes of choking and gasping for air at night by his wife. Informed written and verbal consent was obtained from the patient for inclusion of clinical details and images in this study.
Introduction
Obstructive sleep apnea (OSA) is defined as episodes of upper airway collapse that occur during sleep. These events lead to episodic reduction or cessation of ventilation, resulting in hypoxia, hypercapnia, or sleep arousal.1 OSA can significantly impact quality of life and increase the risk of cardiovascular disease, stroke, diabetes, hypertension, and hypercholesterolemia.2 The incidence of OSA has been estimated to be 3–50% in the general population depending on age, sex, and body mass index (BMI); often, individuals are unaware that they have this condition.3,4 The incidence of OSA globally does not follow a geographical trend. In men it has been the lowest in Hong Kong (8.8%) and highest in Switzerland (83.8%); while in women, New Zealand and Singapore have the lowest and highest reported rates at 3.4% and 62.3%, respectively.5
Conditions that influence the diameter of upper airways during sleep are risk factors of OSA. Obesity and high BMI have been associated with higher degrees of OSA since an increase in the amount of adipose tissue in the tongue or in the upper respiratory pathway can cause airway collapse.2 Male sex is another major risk factor for OSA with an unclear pathophysiology, although the risk appears to be similar in postmenopausal women.6,7 It has been hypothesized that progesterones help maintain an appropriate airway diameter and androgens increase pharyngeal muscle mass, increasing the likelihood of airway closure.2,8 Less established risk factors include smoking, family history of OSA and a variety of medical conditions such as hypertension, type 2 diabetes, congestive heart failure, hypothyroidism, acromegaly, and certain craniofacial anatomical abnormalities.2,9
OSA often manifests as daytime sleepiness, snoring, choking or gasping during sleep, and morning headaches. It may also present with complications including erectile dysfunction, neuropsychiatric symptoms, or nocturnal cardiovascular events. However, the correlation of symptoms to the severity of the disease is relatively poor.10 Currently, the gold standard diagnostic test for OSA is polysomnography to determine the apnea-hypopnea index (AHI).2,10,11 Apneas are defined as episodes of almost total obstructed airflow of more than 10 seconds during sleep, while hypopneas are decreased airflow and oxyhemoglobin saturation by 3% or arousal from sleep.10 The AHI score can be used to both diagnose and assess the severity of OSA. An AHI score of 5 or above is indicative of OSA diagnosis. AHI scores of 5–15 are considered mild, 16–30 are considered medium, and above 30 are considered severe OSA.11 In addition to polysomnography, multiple questionnaires have been developed to assess for OSA that are more accessible and less expensive, including the Berlin questionnaire (BQ), STOP-BANG questionnaire (SBQ), STOP questionnaire (STOP), and Epworth sleepiness scale (ESS). In a meta-analysis of OSA diagnostic questionnaires, SBQ was found to have the highest accuracy and sensitivity compared to the others.12
Positive airway pressure such as continuous positive airway pressure (CPAP), which keeps the airways open both during inspiration and expiration while the patient is sleeping, is an effective and commonly used treatment.2,10 However, patient adherence to CPAP therapy is low due to cost and inconvenience. Weight loss is another effective treatment that has been shown to improve outcomes in patients with OSA. In a meta-analysis of 4 randomized controlled trials, a weight loss of 14 kg was shown to reduce the AHI score by 16 points.13 Other treatment options include oral appliance to protract the mandible, avoiding supine sleeping, and surgical intervention to reduce the collapsibility of the pharynx and upper airways.10
Neuro-ophthalmology refers to a subspecialty of ophthalmology and neurology that concerns itself with central nervous system pathology affecting vision. An increasing amount of evidence has implicated OSA as a significant risk factor for optic nerve disorders (referred to as optic neuropathies). These include non-arteritic anterior ischemic optic neuropathy (NAION) and to a lesser extent idiopathic intracranial hypertension (IIH). This review will examine the evidence for OSA as a risk factor for neuro-ophthalmological conditions with a focus on NAION and IIH.
OSA and NAION
Non-arteritic anterior ischemic optic neuropathy (NAION) occurs due to infarction of the optic nerve head due to hypoperfusion of the short posterior ciliary arteries.14 This condition manifests as painless vision loss and the optic nerve becomes edematous after the initial insult and the optic disc edema persists for about 6–11 weeks after which optic disc atrophy develops.14 It is seen in patients with small optic nerves and a small or absent physiological cup (so-called “disc-at-risk”). NAION commonly affects individuals above the age of 50 and is associated with vascular risk factors such as hypertension and type 2 diabetes. Other associated risk factors include dyslipidemia, smoking, and systemic atherosclerosis, but few rigorous population studies exist to establish these factors.15 We used a systematic search strategy (Table 1) and identified 18 articles that have explored the association between OSA and NAION (Table 2).
 |
Table 1 Search Strategy Used for OVID MEDLINE (1946 to February 2020 Week 1) to Identify Studies That Investigated the Relationship Between OSA and NAION |
 |
Table 2 Studies Identified Using Our Search Strategy That Investigate the Relationship Between OSA and NAION |
The relationship between OSA and optic disc edema was first described by Bucci et al in a 46-year-old obese male with OSA.34 This patient had an extensive workup including a lumbar puncture that revealed a normal opening pressure and following the placement of a permanent tracheotomy for the treatment of his OSA symptoms, his optic disc edema resolved. This observation encouraged Mojon et al to systematically investigate the association between OSA and NAION for the first time.16 They examined the frequency of OSA by polysomnograms in patients with NAION and compared it to age- and sex-matched controls who had restless leg syndrome. They showed that NAION patients had significantly higher rates of OSA diagnosis at 71% compared to the controls at 18%.16
Another study conducted phone questionnaires of 73 NAION patients and 73 age- and gender-matched controls, and assessed the OSA status of the participants based on the Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ).19 Even though they used a different, indirect method of assessing for OSA, they found higher rates of OSA in NAION patients than the controls, 30.1% versus 17.8%, consistent with other studies.19
Unlike other prospective studies that matched the control group based only on age and sex, two studies matched them based on the diagnoses of diabetes and hypertension as well.21,24 The results were consistent in finding that the rate of OSA was 85% (17/20 patients) and 55.5% (15/27 patients) in NAION patients, which was significantly higher than the controls at 65% (13/20) and 22.2% (6/27), respectively.21,24 These studies demonstrated that even after controlling for all major risk factors of NAION including age, diabetes, and hypertension, OSA still appears to be strongly associated with NAION.
Conversely, other studies have investigated the frequency of NAION in patients with OSA. Comparative retrospective studies of OSA patients versus controls have shown higher incidences of NAION diagnosis in OSA patients at 0.92% and 0.36% compared to the controls at 0.42% and 0.20%, respectively.30,31 In addition, after adjustments for comorbidities, OSA patients had a significantly higher risk of developing NAION than patients without OSA over an 18-year period, with a reported hazard ratio of 1.66.26 Thus, OSA appears to be highly prevalent among NAION patients while NAION is much less common among OSA patients. This is likely a result of the multifactorial nature of NAION including the importance of a disc-at-risk. In addition, since OSA is consistently more prevalent in patients with NAION compared to controls, it suggests that OSA is a risk factor for NAION development.
A meta-analysis of 4 case-control studies,16,19,21,24 involving 137 subjects showed that OSA was significantly associated with the development of NAION with a pooled odds ratio of 3.126 (P<0.001) in the OSA group.35 Likewise, another meta-analysis of 5 studies,16,18,19,21,24,27 found a strong link between NAION and OSA with a pooled odds ratio of 6.18 (95% CI 2.00–19.11) versus non-OSA controls.36 In subgroup analysis, only matched-control studies showed a significant association between NAION and OSA with an odds ratio of 5.0 (95% CI 2.22–11.25), compared to studies that did not.36 This difference could be explained by the fact that NAION is a multifactorial disease.
A few studies have also reported the potential for CPAP therapy to minimize the risk of developing NAION in patients with OSA. Chang et al retrospectively studied 119 patients with unilateral NAION and OSA.32 Over a 5-year period, 29 of these patients developed NAION in the other eye as well. They found that poor adherence to CPAP was a major risk factor in developing bilateral NAION.32 This emphasizes the importance of treating OSA when present to prevent fellow-eye involvement, which often has a tremendous impact on their quality of life.
The exact mechanism of how OSA causes NAION is not fully understood but various hypotheses have been proposed. Many studies in the literature suggest that recurrent episodes of apnea lead to vascular dysregulation, compromising the ability to provide constant blood flow to the vessels supplying the optic nerve head.16,21,37 This dysregulation has been thought to be a result of prolonged hypoxia, production of reactive oxygen species, and an imbalance in the production of vasoactive substances.16,27 Vascular endothelial growth factor (VEGF) has been reported to stimulate the progression of cardiovascular disease and a meta-analyses of six studies found that levels of VEGF were significantly reduced in OSA after CPAP treatment.38
Given the strong association between OSA and NAION, there is increasing evidence that polysomnography should be performed in all patients with a new diagnosis of NAION.39 This is important since there is some evidence, as outlined above, that CPAP non-compliance is a risk factor for developing bilateral NAION.32 OSA should be of particular concern in patients that have a clear diagnosis of NAION, but no other risk factors for this condition. OSA is also a concern in younger patients below 50 years of age and NAION has been found to be the presenting condition of severe sleep apnea in younger individuals.33 Although there is no established treatment for NAION, efforts should be focused on identification and optimization of risk factors including OSA to reduce the likelihood of fellow-eye involvement, which can be devastating for these patients.
OSA and IIH
Idiopathic intracranial hypertension (IIH), also known as pseudotumor cerebri, refers to increased intracranial pressure (ICP) of unknown cause.40 It is most commonly seen in obese females of childbearing age and may present with headache, diplopia, or vision loss.41 IIH is diagnosed by the modified Dandy criteria and treatment is initiated to reduce the risk of permanent vision loss and help in reducing systemic symptoms. These treatments include weight loss, pharmacological treatments such as acetazolamide, or surgical intervention in severe cases.40 Studies have shown that in patients with OSA, ICP was relatively normal during wake times with morning ICP values significantly higher than the evening values (20.7 mmHg versus 17.7, p<0.02).60 However, this value was increased as much as 90 mmHg during apnea episodes. This correlation was highly significant and was associated with a decrease in PO2.60,61 Since IIH is a syndrome of raised ICP, this sparks the question of a potential relationship between IIH and OSA. Table 3 summarizes the 19 articles that have explored the association between OSA and IIH (Table 3). OSA has been reported to occur between 4% and 60% of IIH patients.47,49 Many cases have been described in the literature describing patients with OSA who were also diagnosed with IIH at presentation, proposing a possible link between the two conditions. However, unlike NAION, the evidence for an association between IIH and OSA is less conclusive.
 |
Table 3 Studies Identified Using Our Search Strategy That Investigate the Relationship Between OSA and IIH |
Thurtell et al conducted polysomnograms on 24 patients with a diagnosis of IIH and found that 8 of these patients had OSA.53 The mean AHI score of these individuals was compared to a model developed by sampling the AHI scores of 1741 random individual and corrected for age, sex, race, BMI, and menopausal status. The results showed that there was no difference between the AHI scores of the two groups, indicating that IIH by itself did not increase the risk of developing OSA.53 In another study, Bruce et al surveyed 721 patients with IIH and found that 5.7% of these patients also had OSA.47 In addition, when they looked at the rate of OSA between sexes they found that males had a higher rate at 24% compared to females at 4%.47 In a study focused on racial differences in IIH, it was found that black individuals had a higher proportion of OSA (9% vs 4%; p=0.01) and that OSA was more likely in patients that had severe visual loss (p=0.002).62
A few studies have found lower rates of OSA in patients with IIH compared to the controls. In a large retrospective study, Ardissino et al found that among 607 IIH patients, only 4 of them (0.7%) had OSA.56 Conversely, 2204 of the 230,792 controls (1%) had an OSA diagnosis.56 Chronic IIH and increased ICP have also been shown to contribute to the development of spontaneous cerebrospinal fluid leaks (sCSF-L). sCSF-L has been associated with thinning of the calvarium with no changes to extracranial bones and is linked with obesity and OSA.63,64 In particular, the incidence of OSA in sCSF-L patients was reported to be 83.3% and patients with OSA have been shown to have a thinner mean calvaria.63,64 This suggests that there might be an indirect link between OSA and chronic untreated IIH.
Overall, there is little evidence for increased ICP in patients with OSA and fundus examination screening for papilledema in OSA patients is not warranted at this time. A few studies demonstrated a potential link between IIH and OSA, but these were small and without comparative groups. There is therefore no strong evidence supporting routine testing for OSA in patients with IIH. However, weight loss has been shown to be an effective treatment for both IIH and is also an important treatment modality in patients with OSA.
OSA and Glaucoma
Glaucoma is a multifactorial collection of eye diseases that is characterized by degeneration of retinal ganglion cells leading to a progressive optic neuropathy and is associated with cupping of the optic disc and increased intraocular pressure. The incidence of glaucoma in patients with OSA has been reported to be anywhere between 2% and 27%.65,66 While some studies have found the rate of glaucoma to be the same between OSA patients and the general population,67 others have described a higher incidence in OSA patients.68
In a study of 69 OSA patients and 45 controls undergoing a sleep study, none of the patients in the control group had glaucoma while 7.2% of the OSA patients did, of which 40% had normal-tension glaucoma and 60% had primary open-angle glaucoma.68 Glaucoma incidence of 7.2% in the OSA patients was also significantly higher than the 2% rate in the general population (p<0.001).68
A meta-analysis of 12 studies has shown an overall increased risk of glaucoma development in patients with OSA with an odds ratio of 1.65 (CI, 1.44–1.88).69 Sub-group analysis revealed that this significant increase was only observed in patients with primary open-angle glaucoma but not in normal-tension glaucoma. In addition, patients with severe OSA had an even more significant increased odds (OR: 5.49) of developing glaucoma compared to patients with mild or moderate OSA.69
The risk of glaucoma development in 1012 OSA patients and 6072 healthy controls was investigated over 5 years.70 The incidence of glaucoma was 11.26% in patients with OSA compared to 6.76% in the controls. Even after adjustment for demographics and risk factors, OSA patients were 1.67 times more likely to develop glaucoma (95% CI, 1.30, 2.17; P<0.001).70
In addition to be correlated with a higher incidence of glaucoma development, OSA has been found to influence the anatomical progression of disease in glaucoma. In a cohort study of 32 patients, those with moderate and severe OSA had a statistically higher degree of retinal nerve fiber layer thinning than patients with no or mild OSA.71 In addition, severe OSA had an increased risk of retinal nerve fiber layer thinning by a factor of 8.448 (95% CI, 1.464–48.752; P<0.017).71
Overall, the evidence suggests that patients with OSA have an increased risk of developing glaucoma. This progressive ischemia of the optic nerve could be due to chronic hypoperfusion of the optic nerve head during the apnea episodes or dysregulation of vascular modulating cytokines as a result of OSA. Investigation with polysomnography can therefore be considered in patients with glaucoma, especially if there is progression despite the achievement of target intraocular pressure.
OSA and Stroke
Stroke affecting the retrochiasmal visual pathways is a leading cause of homonymous visual field defects and OSA has been established as a risk factor for stroke. The study by Dyken et al was one of the first studies that showed a higher incidence of OSA in patients with a recent episode of ischemic or hemorrhagic stroke.72 They conducted polysomnograms and found that 70.8% of the 24 recent stroke patients had OSA as opposed to 18.5% of the 27 age- and sex-matched controls. Furthermore, 54% of the patients experienced stroke during sleep, suggesting that the apnea episodes of OSA might be a cause.72
Artz et al conducted a study with a retrospective cross-sectional part of 1475 patients and a prospective 4-year longitudinal part that included 1189 patients who did not have a history of stroke.73 The cross-sectional study revealed that patients with an AHI score of 20 or higher have an increased odds of developing stroke with an OR of 4.33 (CI: 1.32–14.24) after adjustment for known confounding factors. Additionally, the longitudinal study showed that patients with an AHI score above 20 have a higher risk of first-time stroke occurrence over 4 years (OR 3.08, CI: 0.74–12.81).73
In another longitudinal study, a cohort of 394 non-hospitalized elderly (70 or older) subjects with no history of stroke were followed for 6 years.74 Twenty patients experienced stroke in the duration of the study, with an average AHI score of 28 as opposed to 20.1 in individuals who did not have a stroke. Moreover, after adjustment for confounding variables, subjects with severe OSA (AHI>30) were found to have an increased risk of stroke (HR 2.52, CI 1.04–6.01).74 Overall, this study demonstrated that even in older patients, OSA diagnosis is associated with a higher risk of stroke occurrence. Over a 20-year period, Marshall et al recorded 31 strokes in 393 patients who were assessed for OSA using a portable home-monitoring device.75 They found that moderate-severe OSA was associated with stroke with an HR of 3.7 (CI: 1.2–11.8).75
Overall, various observational and longitudinal studies have demonstrated a strong association between OSA and stroke, such that the more severe OSA leads to a higher risk of stroke occurrence. This may have an impact on vision since stroke is a leading cause of homonymous visual field defects.
Conclusion
The strongest association with obstructive sleep apnea in the area of neuro-ophthalmology is with NAION. Multiple prospective studies and meta-analyses have demonstrated an increased risk of this condition in patients with NAION. There is increasing evidence that polysomnography should be strongly considered in all patients with NAION and treatment with CPAP is recommended to reduce the risk of fellow-eye involvement. There is a much weaker relationship between IIH and OSA and there is insufficient evidence to recommend routine polysomnography in this patient population at this time. Patients with homonymous visual field defects are commonly diagnosed with stroke and it is important to recognize that there is an association between stroke and OSA, which may also be the first manifestation of undiagnosed OSA.
Return to Case Vignette
The patient was diagnosed with bilateral NAION after giant cell arteritis was excluded and magnetic resonance of the brain and orbits with contrast was normal. Given the concerning symptoms for OSA, he underwent polysomnography, which revealed severe OSA with an AHI of 48.4 per hour. He was treated with CPAP and worked on lifestyle modifications to help with weight loss. His visual function was mildly improved to 20/80 (right) and 20/40 (left) at the 6-month follow-up.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi:10.1152/physrev.00043.2008
2. Veasey SC, Rosen IM, Solomon CG. Obstructive sleep apnea in adults. N Engl J Med. 2019;380(15):1442–1449. doi:10.1056/NEJMcp1816152
3. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi:10.1093/aje/kws342
4. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi:10.1016/S2213-2600(15)00043-0
5. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the Global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
6. Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin sleep cohort study. Am J Respir Crit Care Med. 2003;167(9):1181. doi:10.1164/rccm.200209-1055OC
7. Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98(10):984–989. doi:10.1016/j.rmed.2004.03.002
8. Liu PY, Yee B, Wishart SM, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003;88(8):3605–3613. doi:10.1210/jc.2003-030236
9. Ozcan KM, Selcuk A, Ozcan I, et al. Incidence of hypothyroidism and its correlation with polysomnography findings in obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2014;271(11):2937–2941. doi:10.1007/s00405-014-2962-1
10. Laratta CR, Ayas NT, Povitz M, Pendharkar SR. Diagnosis and treatment of obstructive sleep apnea in adults. CMAJ. 2017;189(48):E1481–E1488. doi:10.1503/cmaj.170296
11. Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32(2):150–157. doi:10.1093/sleep/32.2.150
12. Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi:10.1016/j.smrv.2016.10.004
13. Mitchell LJ, Davidson ZE, Bonham M, O’Driscoll DM, Hamilton GS, Truby H. Weight loss from lifestyle interventions and severity of sleep apnoea: a systematic review and meta-analysis. Sleep Med. 2014;15(10):1173–1183. doi:10.1016/j.sleep.2014.05.012
14. Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015;373(24):2390.
15. Peeler C, Cestari DM. Non-arteritic anterior ischemic optic neuropathy (NAION): a review and update on animal models. Semin Ophthalmol. 2016;31(1–2):99–106. doi:10.3109/08820538.2015.1115248
16. Mojon DS, Hedges TR, Ehrenberg B, et al. Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. 2002;120(5):601–605. doi:10.1001/archopht.120.5.601
17. Behbehani R, Mathews MK, Sergott RC, Savino PJ. Nonarteritic anterior ischemic optic neuropathy in patients with sleep apnea while being treated with continuous positive airway pressure. Am J Ophthalmol. 2005;139(3):518–521. doi:10.1016/j.ajo.2004.11.004
18. Palombi K, Renard E, Levy P, et al. Non-arteritic anterior ischaemic optic neuropathy is nearly systematically associated with obstructive sleep apnoea. Br J Ophthalmol. 2006;90(7):879–882. doi:10.1136/bjo.2005.087452
19. Li J, McGwin G, Vaphiades MS, Owsley C. Non-arteritic anterior ischaemic optic neuropathy and presumed sleep apnoea syndrome screened by the sleep apnea scale of the sleep disorders questionnaire (SA-SDQ). Br J Ophthalmol. 2007;91(11):1524–1527. doi:10.1136/bjo.2006.113803
20. Stein JD, Kim DS, Mundy KM, et al. The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol. 2011;152(6):989–998.e3. doi:10.1016/j.ajo.2011.04.030
21. Arda H, Birer S, Aksu M, et al. Obstructive sleep apnoea prevalence in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2013;97(2):206–209. doi:10.1136/bjophthalmol-2012-302598
22. Blaivas AJ, Uddin F. Obstructive sleep apnea caused by substernal goiter presenting as nonarteritic ischemic optic neuropathy. Sleep Breath. 2013;17(2):469–471. doi:10.1007/s11325-012-0758-3
23. Kolb SD, Backhouse O. Obstructive sleep apnoea prevalence in non-arteritic anterior ischaemic optic neuropathy: a response. Br J Ophthalmol. 2013;97(6):794. doi:10.1136/bjophthalmol-2013-303179
24. Bilgin G, Koban Y, Arnold AC. Nonarteritic anterior ischemic optic neuropathy and obstructive sleep apnea. J Neuroophthalmol. 2013;33(3):232–234. doi:10.1097/WNO.0b013e31828eecbd
25. Mohamed EE, Massoud TH. Effect of sleep related breathing disorders on ocular function. Egypt J Chest Dis Tuberc. 2014;63(3):663–669. doi:10.1016/j.ejcdt.2014.02.010
26. Lacharme T, Almanjoumi A, Aptel F, et al. Twenty-four-hour rhythm of ocular perfusion pressure in non-arteritic anterior ischaemic optic neuropathy. Acta Ophthalmol. 2014;92(5):e346–52. doi:10.1111/aos.12352
27. Aptel F, Khayi H, Pépin JL, et al. Association of nonarteritic ischemic optic neuropathy with obstructive sleep apnea syndrome: consequences for obstructive sleep apnea screening and treatment. JAMA Ophthalmol. 2015;133(7):797–804. doi:10.1001/jamaophthalmol.2015.0893
28. Ghaleh Bandi MF, Naserbakht M, Tabasi A, Marghaiezadeh A, Riazee Esfahani M, Golzarian Z. Obstructive sleep apnea syndrome and non-arteritic anterior ischemic optic neuropathy: a case control study. Med J Islam Repub Iran. 2015;29:300.
29. Morsy NE, Amani BE, Magda AA, et al. Prevalence and predictors of ocular complications in obstructive sleep apnea patients: a cross-sectional case-control study. Open Respir Med J. 2019;13(1):19–30. doi:10.2174/1874306401913010019
30. Sun MH, Lee CY, Liao YJ, Sun CC. Nonarteritic anterior ischaemic optic neuropathy and its association with obstructive sleep apnoea: a health insurance database study. Acta Ophthalmol. 2019;97(1):e64–e70. doi:10.1111/aos.13832
31. Yang HK, Park SJ, Byun SJ, Park KH, Kim JW, Hwang JM. Obstructive sleep apnoea and increased risk of non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2019;103(8):1123–1128. doi:10.1136/bjophthalmol-2018-312910
32. Chang MY, Keltner JL. Risk factors for fellow eye involvement in nonarteritic anterior ischemic optic neuropathy. J Neuro Ophthalmol. 2019;39(2):147–152. doi:10.1097/WNO.0000000000000715
33. Lei S, Micieli JA. Severe obstructive sleep apnea diagnosed after non-arteritic anterior ischaemic optic neuropathy in a young man. BMJ Case Rep. 2019;12(11):
34. Bucci FA, Krohel GB. Optic nerve swelling secondary to the obstructive sleep apnea syndrome. Am J Ophthalmol. 1988;105(4):428–430. doi:10.1016/0002-9394(88)90318-2
35. Huon LK, Liu SY, Camacho M, Guilleminault C. The association between ophthalmologic diseases and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2016;20(4):1145–1154. doi:10.1007/s11325-016-1358-4
36. Wu Y, Zhou LM, Lou H, Cheng JW, Wei RL. The association between obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. Curr Eye Res. 2016;41(7):987–992. doi:10.3109/02713683.2015.1075221
37. Hayreh SS. The optic nerve head circulation in health and disease. Exp Eye Res. 1995;61(3):259–272. doi:10.1016/S0014-4835(05)80121-6
38. Qi JC, Zhang L, Li H, et al. Impact of continuous positive airway pressure on vascular endothelial growth factor in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2019;23(1):5–12. doi:10.1007/s11325-018-1660-4
39. Mentek M, Aptel F, Godin-Ribuot D, Tamisier R, Pepin JL, Chiquet C. Diseases of the retina and the optic nerve associated with obstructive sleep apnea. Sleep Med Rev. 2018;38:113–130. doi:10.1016/j.smrv.2017.05.003
40. Madriz Peralta G, Cestari DM. An update of idiopathic intracranial hypertension. Curr Opin Ophthalmol. 2018;29(6):495–502. doi:10.1097/ICU.0000000000000518
41. Wall M. Update on idiopathic intracranial hypertension. Neurol Clin. 2017;35(1):45–57. doi:10.1016/j.ncl.2016.08.004
42. Doyle KJ, Tami TA. Increased intracranial pressure and blindness associated with obstructive sleep apnea. Otolaryngol Head Neck Surg. 1991;105(4):613–616. doi:10.1177/019459989110500413
43. Purvin VA, Kawasaki A, Yee RD. Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol. 2000;118(12):1626–1630. doi:10.1001/archopht.118.12.1626
44. Marcus DM, Lynn J, Miller JJ, Chaudhary O, Thomas D, Chaudhary B. Sleep disorders: a risk factor for pseudotumor cerebri? J Neuroophthalmol. 2001;21(2):121–123. doi:10.1097/00041327-200106000-00014
45. Lee AG, Golnik K, Kardon R, Wall M, Eggenberger E, Yedavally S. Sleep apnea and intracranial hypertension in men. Ophthalmology. 2002;109(3):482–485. doi:10.1016/S0161-6420(01)00987-3
46. Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68(3):229–232. doi:10.1212/01.wnl.0000251312.19452.ec
47. Bruce BB, Kedar S, Van Stavern GP, et al. Idiopathic intracranial hypertension in men. Neurology. 2009;72(4):304–309. doi:10.1212/01.wnl.0000333254.84120.f5
48. Fraser JA, Bruce BB, Rucker J, et al. Risk factors for idiopathic intracranial hypertension in men: a case-control study. J Neurol Sci. 2010;290(1–2):86–89. doi:10.1016/j.jns.2009.11.001
49. Thurtell MJ, Bruce BB, Rye DB, Newman NJ, Biousse V. The Berlin questionnaire screens for obstructive sleep apnea in idiopathic intracranial hypertension. J Neuroophthalmol. 2011;31(4):316–319. doi:10.1097/WNO.0b013e31821a4d54
50. Javaheri S, Qureshi Z, Golnik K. Resolution of papilledema associated with OSA treatment. J Clin Sleep Med. 2011;7(4):399–400. doi:10.5664/JCSM.1202
51. Szewka AJ, Bruce BB, Newman NJ, Biousse V. Idiopathic intracranial hypertension: relation between obesity and visual outcomes. J Neuroophthalmol. 2013;33(1):4–8. doi:10.1097/WNO.0b013e31823f852d
52. Abraham A, Peled N, Khlebtovsky A, et al. Nocturnal carbon dioxide monitoring in patients with idiopathic intracranial hypertension. Clin Neurol Neurosurg. 2013;115(8):1379–1381. doi:10.1016/j.clineuro.2012.12.037
53. Thurtell MJ, Trotti LM, Bixler EO, et al. Obstructive sleep apnea in idiopathic intracranial hypertension: comparison with matched population data. J Neurol. 2013;260(7):1748–1751. doi:10.1007/s00415-013-6858-6
54. Wardly D, Wolford LM, Veerappan V. Idiopathic intracranial hypertension eliminated by counterclockwise maxillomandibular advancement: a case report. Cranio. 2017;35(4):259–267. doi:10.1080/08869634.2016.1201634
55. Cappuzzo JM, Hess RM, Morrison JF, et al. Transverse venous stenting for the treatment of idiopathic intracranial hypertension, or pseudotumor cerebri. Neurosurg Focus. 2018;45(1):E11. doi:10.3171/2018.5.FOCUS18102
56. Ardissino M, Moussa O, Tang A, Muttoni E, Ziprin P, Purkayastha S. Idiopathic intracranial hypertension in the British population with obesity. Acta Neurochir. 2019;161(2):239–246. doi:10.1007/s00701-018-3772-9
57. Radojicic A, Vukovic-Cvetkovic V, Pekmezovic T, Trajkovic G, Zidverc-Trajkovic J, Jensen RH. Predictive role of presenting symptoms and clinical findings in idiopathic intracranial hypertension. J Neurol Sci. 2019;399:89–93. doi:10.1016/j.jns.2019.02.006
58. Onder H, Ergun O, Kaygisiz M, Daltaban IS. Total improvement after surgery for obstructive sleep apnea syndrome in a patient with concurrent malignant idiopathic intracranial hypertension. J Neurosurg. 2018;131(2):582–586. doi:10.3171/2018.3.JNS171663
59. Onder H, Aksoy M. Resolution of idiopathic intracranial hypertension symptoms by surgery for obstructive sleep apnea in a pediatric patient. J Pediatr Neurosci. 2019;14(2):110–112. doi:10.4103/jpn.JPN_30_19
60. Jennum P, Børgesen SE. Intracranial pressure and obstructive sleep apnea. Chest. 1989;95(2):279–283. doi:10.1378/chest.95.2.279
61. Sugita Y, Iijima S, Teshima Y, et al. Marked episodic elevation of cerebrospinal fluid pressure during nocturnal sleep in patients with sleep apnea hypersomnia syndrome. Electroencephalogr Clin Neurophysiol. 1985;60(3):214–219. doi:10.1016/0013-4694(85)90033-1
62. Bruce BB, Preechawat P, Newman NJ, Lynn MJ, Biousse V. Racial differences in idiopathic intracranial hypertension. Neurology. 2008;70(11):861–867. doi:10.1212/01.wnl.0000304746.92913.dc
63. Rabbani C, Saltagi MZ, Ye MJ, et al. Association of obstructive sleep apnea with calvarial and skull base thinning. JAMA Otolaryngol Head Neck Surg. 2018;144(6):513–518. doi:10.1001/jamaoto.2018.0347
64. Rabbani CC, Saltagi MZ, Manchanda SK, et al. Prevalence of obstructive sleep apnea (OSA) in spontaneous cerebrospinal fluid (CSF) leaks: a prospective cohort study. Otol Neurotol. 2018;39(6):e475–e480. doi:10.1097/MAO.0000000000001805
65. Bendel RE, Kaplan J, Heckman M, Fredrickson PA, Lin SC. Prevalence of glaucoma in patients with obstructive sleep apnoea–a cross-sectional case-series. Eye. 2008;22(9):1105–1109. doi:10.1038/sj.eye.6702846
66. Geyer O, Cohen N, Segev E, et al. The prevalence of glaucoma in patients with sleep apnea syndrome: same as in the general population. Am J Ophthalmol. 2003;136(6):1093–1096. doi:10.1016/S0002-9394(03)00709-8
67. Aptel F, Chiquet C, Tamisier R, et al. Association between glaucoma and sleep apnea in a large French multicenter prospective cohort. Sleep Med. 2014;15(5):576–581. doi:10.1016/j.sleep.2013.11.790
68. Mojon DS, Hess CW, Goldblum D, et al. High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology. 1999;106(5):1009–1012. doi:10.1016/S0161-6420(99)00525-4
69. Wu X, Liu H. Obstructive sleep apnea/hypopnea syndrome increases glaucoma risk: evidence from a meta-analysis. Int J Clin Exp Med. 2015;8(1):297–303.
70. Lin CC, Hu CC, Ho JD, Chiu HW, Lin HC. Obstructive sleep apnea and increased risk of glaucoma: a population-based matched-cohort study. Ophthalmology. 2013;120(8):1559–1564. doi:10.1016/j.ophtha.2013.01.006
71. Fan YY, Su WW, Liu CH, et al. Correlation between structural progression in glaucoma and obstructive sleep apnea. Eye. 2019;33(9):1459–1465. doi:10.1038/s41433-019-0430-2
72. Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27(3):401–407. doi:10.1161/01.STR.27.3.401
73. Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi:10.1164/rccm.200505-702OC
74. Munoz R, Duran-Cantolla J, Martínez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37(9):2317–2321. doi:10.1161/01.STR.0000236560.15735.0f
75. Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton health study cohort. J Clin Sleep Med. 2014;10(4):355–362. doi:10.5664/jcsm.3600
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.