Back to Journals » Cancer Management and Research » Volume 11
Neuraxis Metastases Of Primary Central Nervous System Tumors: A Review Of Clinicopathological And Radiographic Characters Of 198 Cases In A Single Center
Authors Liu H , Zhang J, Liu Y , Sun Y, Li C, Gu C, Wang H, Zhang H, Yu C, Zhang M
Received 29 May 2019
Accepted for publication 26 October 2019
Published 20 November 2019 Volume 2019:11 Pages 9829—9841
DOI https://doi.org/10.2147/CMAR.S217672
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Hailong Liu,1,2 Junping Zhang,1 Yongqiang Liu,3 Youliang Sun,4 Cheng Li,1 Chunyu Gu,1 Haoran Wang,1 Hongwei Zhang,1 Chunjiang Yu,1 Mingshan Zhang1
1Department of Neurosurgery, Sanbo Brain Hospital Capital Medical University, Beijing 100093, People’s Republic of China; 2Department of Neurosurgery, The First Medical Center, Chinese People’s Liberation Army General Hospital, Beijing 100853, People’s Republic of China; 3Institute of Clinical Pharmacology, Guangzhou University of Chinese Medicine, Guangzhou 510405, People’s Republic of China; 4School of Basic Medical Science, Capital Medical University, Beijing 100069, People’s Republic of China
Correspondence: Mingshan Zhang
Department of Neurosurgery, Sanbo Brain Hospital Capital Medical University, No. 50 Yi-Ke-Song Road, Xiangshan Ave, Beijing 100093, People’s Republic of China
Tel +86 10 6285 6704
Fax +86 10 6285 6902
Email [email protected]
Background: Neuraxis metastases (NM) from systemic and central nervous system (CNS) tumors have become increasingly common. However, a lack of systematic information restricts the development of the accurate diagnosis and treatment. The aim of this study is to facilitate the understanding of NM arising from CNS tumors in the largest cohort.
Methods: Based on the clinicopathological and neuroimaging findings, we retrospectively analyze the epidemiological characters, radiographic classification, therapeutic strategies and prognostic factors.
Results: A total of 198 cases are enrolled and the most common primary tumor is medulloblastoma (34.34%). The median age is 15.0 years and the majority of NM (79.29%) occur in the children and young adult groups. One hundred and forty-nine (75.25%) cases suffer from intracranial metastases, and 169 (85.35%) have intraspinal NM. The whole leptomeninges and cauda equine are the most preferential disseminated sites. Upon MRI parameters, the massive and miliary subgroup occurs most frequently in the intracranial and intraspinal NM, respectively. Treatment includes surgery (21.71%), chemotherapy alone (19.19%), radiation alone (10.10%) and combined therapy (55.56%). Operations are performed in order to identify pathology and relive masses, as well as the triple chemotherapeutic scheme consisting of ifosfamide, carboplatin and etoposide is recommended for most of NM. The median overall survival is 11.6 months. Younger age, coexistence of NM with primary tumors, shorter interval from primaries to metastases, glioma, leptomeningeal seeding and nodal subtype on MRI significantly correlate with poor prognosis.
Conclusion: In spite of controversial therapies and poor outcomes, the neuroimaging classification and comprehensive treatment contribute to the efficient administration of NM.
Keywords: neuraxis metastases, survival, MRI, classification, operation
Introduction
Because survival of patients with CNS tumors has been improved and sensitivity of examinations including MRI, PET/CT or CSF test has been enhanced, metastases have become an increasingly common complication of cancers.1–3 Over the past decades, leptomeningeal metastases (LM) from systematic cancers have been widely reported, which only reflects the appearances of tumor cells in the leptomeninges or CSF cisterns.4,5 Actually, metastases from CNS tumors frequently occur at the ventricles or cerebrospinal parenchyma. Thus, it is suggested that neuraxis metastases (NM) should be defined as the disseminated lesions along the neuraxis including leptomeninges, ventricular system and even cerebrospinal parenchyma.6 NM derived from CNS tumors exist as a distinct entity due to the multiple pathological subgroups, the complexity of metastatic regional anatomical structures and the interdisciplinary management. Given the lack of studies systematically demonstrating this devastating stage of brain tumors and the available literature only reporting some individual series, a deeper understanding of the clinicopathological features across the whole spectrum of NM is necessary for the accurate diagnosis and optimal treatment.
MRI has been abundantly utilized as the effective tool for implementing the diagnosis and designing the operation plans.7 Especially, the sensitivity and specificity of MRI with gadolinium (Gd) enhancement have been elevated, contributing to detecting the inaccessible NM in the past years.8 However, in recent studies, only a fraction of NM originating from glioma limits developing the neuroimaging classification in a comprehensive view and estimating the survival in a statistical manner.9–11 Therefore, with the goal of updating the radiographic classification of NM, an illustrative review of MRI features will provide more meaningful refers to facilitate the precise diagnosis.
Studies have reported that patients with cancer metastases usually suffer from poor prognosis and the median survival is about half a year.12,13 Interdisciplinary treatments including resection, chemotherapy and radiation have been well-established. Moreover, the molecular reagents play critical roles in targeting the NM from lung, breast or prostate cancers.14–16 Nevertheless, consensus still lacks standard therapeutic indications of NM. Additionally, some existing data are partially restricted to the LM from systematic cancers and little is known concerning the prognostic factors of patients with NM from CNS tumors,17,18 which prompts us to demonstrate the management strategies by analyzing the neuraxis metastatic data.
Based on the clinical practice and informative materials in our single center, this study attempts to report the NM originating from CNS neoplasms through a retrospective analysis of 198 cases including 32 histopathological subgroups, which is the largest series documenting demography, diagnosis and treatment.
Materials And Methods
Study Participates
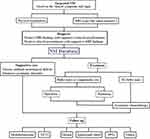
From April 2004 to June 2018, 198 Chinese patients bearing NM at the Department of Neurosurgery and Neuro-oncology in Sanbo Brain Hospital Capital Medical University were enrolled in this study. All patients receiving treatment for primary CNS tumors underwent routine follow-up in the outpatient at 2-month interval for the first 6 months and then at 6-month interval for the next 2 years and annually thereafter for 5 years by utilizing the whole brain and spine MRI monitors, and hormone and tumor marker tests were additionally performed in patients with GCTs. Patients suffering from possible progressions, such as the new or unexplainable symptoms and signs from primary tumors, would increase follow-up frequencies. When metastatic lesions were detected on MRI, patients would be submitted to the NM database and receive the following treatment and follow-up. In addition, cases receiving only supportive care for primary or metastatic tumors were excluded in the current study.
One hundred and ninety-four cases underwent operations for primary lesions, whose pathogeneses were independently confirmed by two neuropathologists and the other 4 cases with germ cell tumors (GCTs) were diagnosed according to symptoms, MRI presentations and laboratory examinations. Clinical information was obtained from medical records, which included gender, age, initial cancer-associated data (locations, surgery approaches and histopathology), interval between primary diagnosis and NM, NM-associated data (neurological symptoms and signs, sites, treatment and response) and date of death. All procedures for medical research about human subjects were approved by the Research Ethics Committee of Sanbo Brain Hospital Capital Medical University, and written informed consent was obtained from each patient (or the children's guardians).
MRI Interpretation
The entire neuraxis MRI scans were performed in all cases and the results were interpreted independently by two neuroradiologists, who focused on the following interests: location, size and shape, lesion boundary, T1/T2-weighted imaging signals, tumor enhancement, leptomeningeal or cisternal enhancement, cancerous homogeneity and presence/absence of hydrocephalus. The shape of metastases determined the neuroimaging classifications of NM. Neurological examinations were conducted at the time of diagnosis.
Statistical Analysis
Data statistical handling was performed using SPSS 19.0 and GraphPad Prism 8.0 software, and qualitative data were shown as the case (percentage), and quantitative data were presented as the median (range). The statistical analysis should be generated by the major groups, but not the subgroup data because of the rarity of some disorders. Comparison of mean values between multiple groups was evaluated by χ2 test. The Pearson's chi-square test was adopted in the groups with values being more than 5, and the Fisher exact test was used in the group with values being less than 5. Log rank test was performed to detect the differences in the overall survival (OS) and the survival curves were estimated by the Kaplan–Meier method. The Cox proportional hazards model was utilized to determine the prognostic factors, of which the item with a significant difference in univariate analysis was submitted to the multivariate evaluation. For all statistical methods, p < 0.05 was accepted as statistically significant.
Results
Epidemiological Characteristics Of NM
Over the past 10 years, we have constructed the work-up based on our clinical practice and NCCN guidelines,19 showing that patients who were suspected with NM would receive a neurological examination and MRI scans, and then the confirmed NM cases were included in the NM database and the patients received the corresponding treatment and follow-up (Figure 1). Accordingly, a total of 198 subjects were retrieved from the database accounting for about 1.20% of all homochronous CNS neoplasms. We summarized the general, clinicopathological and therapeutic parameters of the participants in Table 1. All initial lesions including 32 pathological subtypes were divided into 192 (96.97%) intracranial cases and 6 (3.03%) intraspinal cases. There were 78 (39.39%) patients with primary embryonal tumors occupying the most common incidence rate, followed by GCTs (19.19%), glioma (17.68%), ependymoma (7.58%) and pineal parenchymal tumors (PPTs, 5.05%). Among the embryonal tumors, the most commonly diagnosed was medulloblastoma (Table 1).
 |
Table 1 Demographic And Clinicopathological Characters Of NM Patients |
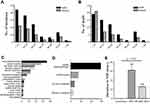
The obvious gender preponderance existed in the males because cases with male tendency (medulloblastoma, GCTs and PPTs) occupied 58.59% of the entire cohort (Table 1). Across all the cases, the median age was 15.0 years (range, 0.5 ~ 71.0 years). Furthermore, a majority of NM (79.29%) occurred in the younger age group including 96 cases in children group (< 14 years) and 63 cases in young adult group (14 ~ 35 years, Supplementary Table 1). In detail, the peak of incidence rate stayed in the < 10-year age group, of which the males were two times higher than the females (Figure 2A). This phenomenon showed a significant difference compared with the metastases from systematic cancers because they assembled in the relatively older-age population.4,20 The median follow-up period was 46.6 months (range, 0.5 ~ 153.0 months). Similar to the incidence rate, Figure 2B promotes an awareness of the peak mortality rate in the < 10-year age group. However, it is worth noting that almost equal male-to-female mortality rate existed in the < 10-year age group in contrast to the predominant male incidence at the same age course (Figure 2A and B).
Of the 198 cases, 149 (75.25%) suffered from intracranial metastases, 169 (85.35%) had intraspinal NM and 87 had coexistence of intra-cranial/spinal metastases accounting for 43.94%. The evidence grouped by anatomical regions suggested that a majority of intracranial NM were located at the whole leptomeninges, followed by the ventricular system. And intraspinal NM was found more preferentially in the whole leptomeninges and cauda equine (Figure 2C and D). However, the most common primary site was stationed at the fourth ventricle because of the high proportion of medulloblastoma cases (supplementary Figure 1A). The median interval from the primary tumor to the first time NM was 18.4 months (range 0 ~ 156.0 months). Among them, 58 (29.29%) patients with primary tumors presented the concurrent NM, of which the most common metastatic sites ranged by the fourth ventricle, sellar and parasellar regions. As shown in Figure 2E, it was statistically significant that the median interval of NM receiving primary tumor removal and following chemotherapy presented approximately 2.5 times longer than those with only stereotactic surgery (SRS) treatment, which highlighted the following therapy after SRS as soon as possible in order to prevent the metastatic progression.
Considering the wide variations in different age arrays or primary tumors as mentioned above, we continued to deepen the subgroup analysis in terms of age and pathology. As shown in Supplementary Table 1, cases were grouped based on age. Metastatic sites in the children group were more eccentric to leptomeninges. Additionally, the duration from primary to metastatic lesions in the pediatric group was the shortest among the three populations. And the concurrent NM with primary tumors was also relatively common in children group, of which medulloblastoma was the most common subset, indicating the poorest prognosis in this period. On the other hand, the most five common pathological subgroups of NM were further analyzed including demographic and metastatic parameters (Supplementary Table 2). The male-to-female ratio was highest in patients with GCTs, supporting the evidence that male adolescents progressed more frequently to disseminated status (Supplementary Tables 1 and 2). Only the NM from ependymoma exhibited a tendency in females. The median duration from initial GCTs to NM was only 8.9 months, dramatically shorter than others. Medulloblastoma and ependymoma disseminated more dominantly to the whole leptomeninges, whereas the GCTs, glioma and PPTs showed particular preferences to the ventricular system. Given the significant differences between low- and high-grade glioma, we further divided gliomas into two subgroups. As shown in Supplementary Table 3, based on the shorter duration to NM and a higher percentage of coexisting NM, high-grade gliomas significantly tended to disseminate along the neuraxis.
Clinical Manifestations On NM Onset
Among the entire participants, 38 (19.19%) cases presented intracranial symptoms and signs and 65 (32.83%) cases suffered from intraspinal presentations (Table 1). Of others, 58 (29.29%) patients had contaminant NM with primary tumors and 48 (24.24%) asymptomatic patients were diagnosed incidentally during the following MRI monitor. A variety of clinical manifestations indicated that neuraxis was infiltrated multifocally by NM. As listed in Table 1, the most common symptom was pain including headache (9/38 cases) and back pain (22/65 cases). Parenchymal and ventricular metastases led to epilepsy (8/38 cases) and ataxia (5/38 cases). Attentively, a consensus had been reached that there was difficulty in distinguishing metastatic from primary manifestations because of the multifocal lesions. Moreover, given a part of patients showing minimal symptoms, suspicious screening should be performed for early diagnosis.
Diagnostic Establishment Of NM On MRI
Referring to the NCCN guidelines and Response Assessment in Neuro-oncology Group,21 the identification of NM in our center depended mainly on the Gd-enhanced MRI. As mentioned above, 318 positive MRI results were detected including 149 intracranial NM and 169 intraspinal lesions.
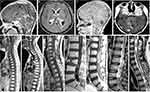
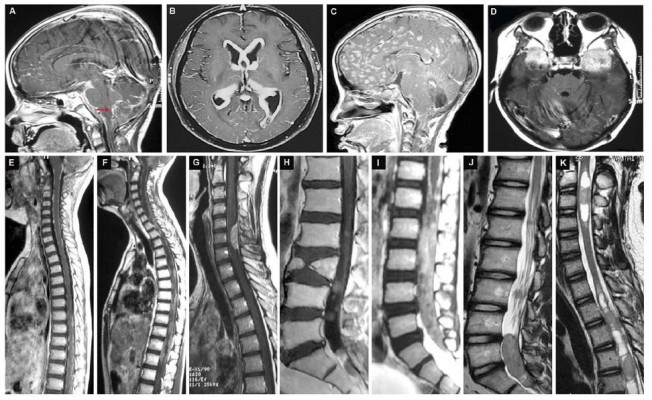
Based on the typical presentations of MRI, we classified the intracranial NM as miliary, procumbent, nodal and massive subtypes (Figure 3). The standards were described as follows:
- the miliary subtype should be identified as the NM on the surface of leptomeninges or ventricular walls in the miliary or filament manner (red arrow) (Figure 3A);
- the procumbent subtype presented as the spreadable and crawled plaque attaching internal walls of lateral ventricles, whose diameter was more than 1 cm (Figure 3B);
- the character of nodal NM should be defined as the lesions being less than 1 cm in the nodular shape, which are often located in subarachnoid spaces and ventricles (Figure 3C);
- the massive feature was that the diameter of NM with round or oval shape presented larger than 1 cm, which is usually sited in the subarachnoid cisterns and ventricles (Figure 3D).
Accordingly, the most common intracranial subtype was the massive one (Supplementary Figure 1B). There existed a dramatic difference in the coexisting NM rate between the miliary group and the three others (54.05% vs. 23.21%, supplementary Figure 1B), indicating that the postoperative tumors progressed more commonly to NM in the nodal, procumbent and massive manner. Previously, we had constructed the classification and stage of brain-to-spine NM,22 which included the leptomeningeal type (L type) and nerve root type (N type, Figure 3E–J). Apart from spreading in the spinal subarachnoid space, 7 cases of the intramedullary metastases (IM type) in the central canal of the spinal cord were involved in the current database (Figure 3K). The NM in the miliary subgroup occurred most frequently, followed by the nodal subset (Supplementary Table 4 and Supplementary Figure 1B). Similar to the intracranial findings, the postoperative intraspinal NM usually showed the nodal and massive features (N type, Supplementary Figure 1B).
Treatment For NM
Among the whole cases, 28 (14.14%) intracranial and 15 (7.58%) intraspinal cases underwent operation, 38 (19.19%) patients received chemotherapy alone, 20 (10.10%) patients received radiation alone and 110 (55.56%) patients were treated with combined chemoradiotherapy (Table 1).
Operation
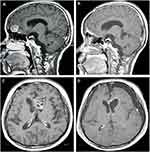
Of the 28 intracranial NM, 24 were removed targeting the bulky masses and 4 cases only received the biopsies. The anterior skull base and lateral ventricles were most commonly involved in the operation regions (Table 1, Figure 4). Besides, 2/3 of intraspinal NM with NI type (or the nodal shape) underwent operation. Taken together, the operative removal for NM was most frequently performed in the nodal subset, followed by the massive subset (Figure 4).
Chemotherapy
A total of 148 (74.75%) cases received the intravenous chemotherapy in this study. Regimens are listed in Supplementary Table 5. For the disseminated medulloblastoma, the most commonly applied plan was a triple regimen consisting of ifosfamide (IFO), carboplatin (CBP) and teniposide (VM-26). Cisplatin (DDP) combined with pemetrexed (PEM) and high-dosage methotrexate (HD MTX) was utilized as the rescue strategy in 8 patients. Among the patients with GCTs, the triple therapeutic strategy containing IFO, CBP and etoposide (VP-16) was most commonly applied accounting for 10.61%. HD MTX was mainly adopted for the NM infiltrating the ventricular system. For the metastatic glioma, 23 patients received temozolomide (TMZ) combined with two or three other regimens, such as irinotecan (CTP-11), nimustine (ACNU) and endostatin. In the ependymal tumors, the triple plan (IFO + CBP + VP-16) was also available for 3/4 patients. For the PPTs, IFO + CBP + VP-16 strategy was utilized most frequently (Supplementary Table 5).
Radiation
Radiation was performed in order to reduce the tumor volumes, control the symptoms and banish the CSF obstructions. The plans are shown in Supplementary Table 5. In the current study, the craniospinal irradiation (CSI) was performed most commonly, and CSI was also the most frequently concurrent application with chemotherapy (61/110, 55.45%). Patients with disseminated medulloblastoma received most frequently the combined chemo-/radiotherapy.
Survival And Prognosis Factors Of NM Patients
The median OS was 11.6 months (range, 0.5 ~ 83.0 months). As shown in Figure 5A, patients with glioma showed the poorest survival (p < 0.001). Moreover, patients suffering from the coexisting NM showed a shorter median OS than the non-coexisting group (p < 0.01), which suggested the deteriorating condition among the primary patients with concomitant NM (Figure 5B). The poor OS was detected in both intracranial and intraspinal nodal subgroups (Figure 5C and D). Figure 5E shows that comprehensive management (OP + CT + RT) contributed to better survival by analyzing the comparable data of different treatment options (p < 0.01).
Furthermore, Cox proportional hazard regression model revealed that younger age (< 15 years), concomitant NM with primary tumors, interval to NM (< 1 year), LM and the nodal subset were significantly associated with the poor prognosis (Supplementary Table 6). The 12-month survival rate was 44.32%, and of these 39 patients, 10 (25.64%) patients lived for more than 36 months. The mean duration to NM, the incidence rate of GCTs and the ratio of LM exhibited higher levels in patients who survived more than 12 months (Supplementary Table 7). The ratio of operation alone to comprehensive treatment was higher among patients with < 12-month survival, emphasizing the importance of interdisciplinary management (Supplementary Table 7).
Discussion
Though numerous studies regarding the cancer metastases in CNS have been previously published, those are limited to systemic cancers or LM from a fraction of CNS tumors, which makes the comprehensive understanding of neuraxis dissemination difficult. Leptomeninges refers to the inner two membranous layers enveloping the brain: the arachnoid membrane and the pia mater.23,24 Thus, LM only represents the involvement of subarachnoid space and leptomeninges without extension to the whole nervous system. Moreover, it is important to note that the concept of neuraxis in the current study contains leptomeninges, ventricular system and cerebrospinal parenchyma. Having realized that, we continuously extracted three disseminating routes of cancer cells along the neuraxis, which include (1) the closely direct invasion arising from contiguous tumor deposits to the leptomeninges or subependymal tissues, (2) the centripetal migration through perineural or perivascular spaces, and (3) the spreading to CSF during operation because of the drops from primary tumors.
NM are diagnosed in 1 ~ 2% of patients with brain tumors,25 which is consistent with our data on the incidence rate in about ten years. Both malignant and benign tumors can involve the neuraxis including 32 histopathological subgroups in the current study. Metastases as the terminal stage usually occur in progressive cancers. However, NM from CNS tumors often occur concomitantly with primary tumors and even coexistence is highly suggestive of poor prognosis. Despite that metastases occupy 27.5% of pediatric patients with some common cancers according to the United States Surveillance, Epidemiology and End Results database,26 our findings reveal that disseminated lesions from CNS tumors in children group are about 48.48%. It is extensively different from the systematic metastases occurring more prevalently in the older-age group population.27
Previous analyses have reported that the median survival is 3 to 7 months among patients with LM arising from systematic cancers.13 In the current series, patients with CNS neoplasms achieved the median OS in 11.6 months, because we excluded the cases who only received supportive care. In addition, the poor survival of patients suffering from concomitant NM with primary tumors provides the evidence that NM dramatically contribute to the malignant progression of brain tumors. Similarly, Chamberlain MC and Brower JV also discussed that the coexistent bulky metastases with LM resulted in the short median survival, which was consistent with our findings on NM.28,29
The diagnosis of NM remains challenging without one single tool presenting sufficiently sensitive to figure out metastases. In the current study, we highlight the radiographic classification based on MRI presentations, which is non-aggressive and intuitionistic for patients and is established well for the prognostic assessment. Importantly, the sensitivity and specificity of enhanced MRI can reach approximately 70% and 77%, respectively.30 A few previous studies only classified intracranial metastases of glioma.31,32 Herein, all kinds of NM cases are submitted to the classification system by interpreting the MRI characters (Figure 1). Gd-enhanced neuraxis MRI is a preferred radiographic method for establishing a diagnosis of NM. The findings indicative of NM include the leptomeningeal enhancement extending basal cisterns in the procumbent, nodal or massive manners, and intradural-enhancing nodules, especially in the cauda equina. Gururangan et al reported three patterns of NM on MRI including parenchymal, subependymal and combinations, emphasizing the need for screening patients with diffuse pontine glioma for NM at the time of recurrence. Based on the Gururangan’s classification, we reanalyzed our data and grouped the NM into the parenchymal, subependymal and combinations. Fifteen (7.5%) had parenchymal metastases, 81 (40.9%) had leptomeningeal metastases, 62 (31.3%) had subependymal metastases, and 40 (20.2%) suffered from a combination of two or more patterns. MRI features in both Gururangan’s study and our work will provide more meaningful refers to facilitate the precise diagnosis.
However, the CSF examination data are not analyzed in the current workup such as lumbar pressure, cell counts and concentration of protein. The reasons are listed as follows. (1) These indexes vary at different levels, and the low sensitivity and specificity of CSF cytology restrict the diagnostic value in clinical practice.33 (2) It is unnecessary for the recurrent or metastatic patients with confirmed pathology to make a differential diagnosis via CSF cytological analysis. In future study, we are collecting the CSF to identify the ctDNA of malignant cells and analyze the association to the genome of NM, which will make sense to detect the underlying molecular mechanisms involved in tumorigenesis of NM.
Although the treatment for NM remains palliative for a lot of patients, it is clear that effective management can protect patients from further neurological deterioration. Survival analysis based on the different therapeutic strategies in the current study emphasized the importance of tumor resection. Therefore, the surgical indications for NM in our center are determined as follows:
- To identify the histopathology of tumors
- NM are relatively safe for operation or biopsy, given the high risk of dissemination that comes from the resection of weeny primary tumors.
- NM are the large uni-focus, when primary tumors have been reduced significantly or disappeared after experiential chemo-/radiotherapy.
- To relive the massive effect
- NM is insensitive to the chemo-/radiotherapy, when primary tumors are under control.
- NM are grouped as the nodal or massive subsets based on MRI features.
- NM locate at the relatively safe anatomical regions, such as the anterior skull base and cerebellopontine angle.
Most patients need a combination of surgery, radiation therapy, systemic chemotherapy or intrathecal chemotherapy. However, when the prognosis is poor, palliative care should be taken into consideration with the primary aim of stabilizing patients’ neurological status. Intrathecal chemotherapy is the mainstream treatment for patients with NM. The standard intrathecal chemotherapy regimens contain methotrexate, cytarabine and thiotepa.34 Intraventricular injections via catheters are typically and commonly comfortable for patients. Furthermore, the implanted reservoir usually facilitates cytotoxic drug delivery. In addition, several chemotherapeutic reagents are suitable for intravascular chemotherapy for patients with LM. Patients with LM form systematic cancers, such as lung cancer, breast cancer or lymphoma may receive HD MTX.35 The PCV regimen or TMZ is usually selected for patients with NM from primary CNS tumors. Although LM or NM of melanomas appears to be difficult to be treated, there are some indications for the activity of TMZ.36
Due to the promising effects of intravenous chemotherapy in this study, we summarize some experiences as follows. (1) The systematic application of anti-neoplastic regiments varies depending on the histological and genetic type of NM, and the triple scheme of IFO + CBP + VP-16/VM-26 is recommended for NM except glioma. (2) DDP combined with PEM and HD MTX plays a critical role in suppressing the refractory NM as the rescue strategy. (3) HD MTX should be adapted by patients with NM in the ventricular system. (4) NM residues after the full cycle of chemotherapy can be submitted to locoregional management, such as operation, cyberknife or intensity-modulated radiation.
Conclusion
In summary, NM is one of the most complicated diseases concerning diagnostic inefficiency, controversial therapies and poor prognosis. First, a number of CNS tumors have the potential of developing as NM. Secondly, prognostic factors including younger age, coexistence of NM with primary tumors, shorter interval from primary disorder to metastases, high-grade glioma, leptomeningeal seeding and the nodal subtype significantly correlate with poor outcomes. Thirdly, Gd-MRI findings contribute to promote the diagnostic accuracy, and classification based on MRI patterns provides referential clues to make therapeutic decisions and evaluate prognosis. Finally, interdisciplinary management with respect to operation and chemo-/radiotherapy is recommended, thereby improving outcomes.
Abbreviations
CNS, central nervous system; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography; GCTs, germinal cell tumors; PPTs, pineal parenchymal tumors; CSF, cerebrospinal fluid; NCCN, National Comprehensive Center Network; CT, chemotherapy; RT, radiotherapy; OP, operation; PCNSL, primary central nervous system lymphoma.
Ethical Approval And Consent To Participate
All procedures for clinical medical research about human subjects were approved by the Research Ethics Committee of our institute and the written informed consent was obtained from each patient (or the children guardians) included in the current study.
Availability Of Data
Please contact the corresponding author for the data requests.
Acknowledgments
This work was supported by the Beijing Municipal Science & Technology Commission Grant (Z151100004015165, to M Zhang), the 65th China Postdoctoral Science Foundation (2019M653940, to H Liu), National Natural Science Foundation of China (81902975, to H Liu) and Scientific Research Common Program of Beijing Commission of Education Grant (KM201610025027, to C Gu). Acknowledgment should be owed to I Nakano at the Department of Neurological Surgery of The Ohio State University and ZJ Yang at the Fox Chase Cancer Center for the thoughtful comments. The authors also would like to thank K Yao at the Department of Neuropathology of Sanbo Brain Hospital Capital Medical University for the pathological estimates as well as MW Zhu at the Department of Neuroradiology of Sanbo Brain Hospital Capital Medical University for the neuroimaging interpretations.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. M Zhang and H Liu conceived the study. J Zhang, C Li and H Wang contributed to the collection of samples and data. H Liu, Y Liu and Y Sun performed the data analyses. H Liu and M Zhang drafted the manuscript, as well as C Yu, C Gu, and H Zhang gave critical revision to it.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
2. Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Oncol. 2006;5:443–452.
3. Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancers: review and update on management. Cancer. 2018;124:21–35. doi:10.1002/cncr.30911
4. Hyun JW, Jeong IH, Joung AR, et al. Leptomeningeal metastasis: clinical experience of 519 cases. Eur J Cancer. 2016;56:107–114. doi:10.1016/j.ejca.2015.12.021
5. Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin N Am. 2003;21(5):25–66. doi:10.1016/S0733-8619(02)00032-4
6. Bordignon KC, Neto MC, Ramina R, et al. Patterns of neuraxis dissemination of gliomas: suggestion of a classification based on magnetic resonance imaging findings. Surg Neurol. 2006;65:472–477. doi:10.1016/j.surneu.2005.08.019
7. Gangadhar K, Santhosh D. Radiopathlogical evaluation of primary malignant skull tumors: a review. Clin Neurol Neurosurg. 2012;114:833–839. doi:10.1016/j.clineuro.2012.05.041
8. Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74:1449–1454. doi:10.1212/WNL.0b013e3181dc1a69
9. Zhang MS, Wang ZY, Zhang JP, et al. Metastases in cerebellopontine angle from the tumors of the central nervous system. J Clin Neurosci. 2017;42:84–90. doi:10.1016/j.jocn.2017.04.011
10. Barajas RF, Cha S. Metastasis in adult brain tumors. Neuroimage Clin N Am. 2016;26:601–620. doi:10.1016/j.nic.2016.06.008
11. Faria AV, Azevedo GA, Zanardi VA, Ghizoni E, Queiroz LS. Dissemination patterns of pilocytic astrocytoma. Clin Neurol Neurosurg. 2006;108:568–572. doi:10.1016/j.clineuro.2005.01.006
12. Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumors. Lancet Oncol. 2010;11:871–879. doi:10.1016/S1470-2045(10)70034-6
13. Herrlinger U, Foschler H, Kuker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurolog Sci. 2004;223:167–178. doi:10.1016/j.jns.2004.05.008
14. Umemura S, Tsubouchi K, Yoshioka H, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: okayama lung cancer study group. Lung Cancer. 2012;77:134–139. doi:10.1016/j.lungcan.2012.03.002
15. Assi HI, Mahmoud T, Saadeh FS, Darsa HE. Management of leptomeningeal metastasis in breast cancer. Clin Neurol Neurosurg. 2018;172:151–159. doi:10.1016/j.clineuro.2018.07.001
16. Tremont-Lukats IW, Bobustuc G, Lagos G, Lolas K, Kyritsis AP, Puduvalli VK. Brain metastasis from prostate carcinoma: the M.D. Anderson Cancer Center experience. Cancer. 2003;15:363–368. doi:10.1002/cncr.11522
17. Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, Wit MD. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol. 2010;136:1729–1735. doi:10.1007/s00432-010-0831-x
18. Waki F, Ando M, Takashima A, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol. 2009;93:205012. doi:10.1007/s11060-008-9758-3
19. Nabors LB, Porthow J, Ammirati M, et al. Central nervous system cancers, version 1. 2015. J Natl Compr Canc Netw. 2015;13:1191–1202. doi:10.6004/jnccn.2015.0148
20. Cheng HY, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:e43–e55. doi:10.1016/S1470-2045(17)30689-7
21. Chamberlain MC, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19:484–492. doi:10.1093/neuonc/now183
22. Zhang MS, Ou YW, Zhang HW, et al. Leptomeningeal metastases from central nervous system tumors: a study of classification and stage in the spinal cord of 58 patients. Chin Sci Bull. 2012;57:2914–2919. doi:10.1007/s11434-012-5262-4
23. Sanan A, van Loveren HR. The arachnoid and the myth of Arachne. Neurosurgery. 1999;45:152–155. doi:10.1097/00006123-199907000-00034
24. Haines DE, Frederickson GR. The meninges. In: Al-Mefty O, editor. Meningiomas. New York: Raven Press; 1991:9–25.
25. Chamberlain MC. Neoplastic meningitis. J Clin Oncol. 2005;23:3605–3613. doi:10.1200/JCO.2005.01.131
26. Perkins SM, Shinohara ET, DeWee T, Frangoul H. Outcome for children with metastatic solid tumors over the last four decades. PLoS One. 2014;9:e1000396. doi:10.1371/journal.pone.0100396
27. US Department of Health and Human Service, US National Institute of Health, US National Cancer Institute, Adolescent and Young Adult Oncology Progress Report Group. Closing the gap: research and care imperative for adolescents and young adults with cancer; 2015.
28. Brower JV, Saha S, Rosenberg SA, Hullett CR, Robins HI. Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci. 2016;27:130–137. doi:10.1016/j.jocn.2015.11.012
29. Groves MD. New strategies in the management of leptomeningeal metastasis. Arch Neurol. 2010;67:305–312. doi:10.1001/archneurol.2010.18
30. Chamberlain MC, Glantz M, Grove MD, Wilson WH. Diagnostic tools for neoplastic meningitis: detecting disease, identifying patient risk, and determining benefit of treatment. Semin Oncol. 2009;36:S35–S45. doi:10.1053/j.seminoncol.2009.05.005
31. Gururangan S, McLaughlin CA, Brashear J, et al. Incidence and patterns of neuraxis metastases in children with diffuse pontine glioma. J Neurooncol. 2006;77:207–212. doi:10.1007/s11060-005-9029-5
32. Chamberlain MC, Soffietti R, Raizer J, et al. Leptomeningeal metastasis: a response assessment in neuro-oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;16:1176–1185. doi:10.1093/neuonc/nou089
33. O’Meara WP, Borkar SA, Stambuk HE, Lymberis SC. Leptomeningeal metastasis. Curr Probl Cancer. 2007;31:367–424. doi:10.1016/j.currproblcancer.2007.07.001
34. Melisko ME, Glantz M, Rugo HS. New challenges and opportunities in the management of brain metastases in patients with ErbB2-positive metastatic breast cancer. Nat Clin Pract Oncol. 2009;6:25–33. doi:10.1038/ncponc1243
35. Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561–1567. doi:10.1200/JCO.1998.16.4.1561
36. Biasco G, Pantaleo MA, Casadei S. Treatment of brain metastases of malignant melanoma with temozolomide. N Engl J Med. 2010;345:621–622. doi:10.1056/NEJM200108233450817
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.