Back to Journals » Drug Design, Development and Therapy » Volume 15
Nephroprotective Effect of Adropinin Against Streptozotocin-Induced Diabetic Nephropathy in Rats: Inflammatory Mechanism and YAP/TAZ Factor
Authors Guo L, Jiang B, Li D, Xiao X
Received 9 December 2020
Accepted for publication 26 January 2021
Published 16 February 2021 Volume 2021:15 Pages 589—600
DOI https://doi.org/10.2147/DDDT.S294009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Ling Guo, Bei Jiang, Dengren Li, Xiaoyan Xiao
Department of Nephrology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong Province, 250012, People’s Republic of China
Correspondence: Xiaoyan Xiao
Department of Nephrology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, No. 107 Wenhuaxi Road, Jinan, Shandong Province, 250012, People’s Republic of China
Email [email protected]
Background: Diabetic Nephropathy remains a major cause of morbidity and mortality in patients suffering from renal dysfunction. This study accessed the nephroprotective role of Adropinin against streptozotocin (STZ) induced diabetic nephropathy in rats and scrutinizes the possible mechanism of action.
Methods: STZ (45 mg/kg) dose was used for inducing diabetic nephropathy (DN) and rats were divided into different groups and received the dose-dependent treatment of Adropinin. Blood glucose level, body weight, tissue weight, antioxidant, renal, hepatic parameters, and cytokines were determined. At the end of the experimental study, renal histopathology was performed.
Results: Adropinin significantly (P< 0.001) boosted plasma insulin levels and reduced the blood glucose level. Adropinin considerably increased body weight and reduced kidney weight and kidney hypertrophy. Adropinin significantly (P< 0.001) reduced urine outflow, microalbumin, total protein, blood urea nitrogen (BUN), uric acid and increased the creatinine, creatinine clearance. Adropinin significantly (P< 0.001) reduced the indole sulfate level in the serum, kidney and reduced in the urine. Adropinin significantly (P< 0.001) reduced the total cholesterol, triglyceride, low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL) and increased the level of high-density lipoprotein (HDL). Adropinin significantly (P< 0.001) increased the level of antioxidant enzymes such as glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and reduced the level of malonaldehyde (MDA), 8-hydroxy-2ʹ -deoxyguanosine (8-OHdG). Adropinin significantly (P< 0.001) reduced the level of interleukin-1β (IL-1β), interleukin-6 (IL-6), transforming growth factor beta (TGF-β) and increased the level of interleukin-10 (IL-10), respectively. Adropinin treatment showed improvement in renal histopathology.
Conclusion: We can say that Adropinin showed the nephroprotective effect against the STZ-induced diabetic nephropathy rats via inflammatory and antioxidant pathway.
Keywords: nephropathy, diabetes mellitus, adropinin, renal, antioxidant, inflammation
Introduction
Diabetes mellitus (DM) is well-known endocrine disease induced via acquired or inherited lack of insulin production in pancreas tissue. According to the report, 300 million people are affected by DM until 2025.1 The most common pathological features of DM complications are diabetic nephropathy, neuropathy, cardiovascular disorder and retinopathy. Especially, diabetic nephropathy (DN) is a common complication of DM. Around, 30–40% patients with DM develop diabetes-linked renal disease. Renal disease is the main complication of both types of diabetes (type I and type II) even if diabetes under control. DN remains a major cause of morbidity and mortality in patients suffering from renal dysfunction.2,3 DN induces end-stage renal disease, which is categorized via a series of renal structure dysfunction including glomerulosclerosis, membrane thickening, mesangial expansion, basement membrane thickening, inflammatory reaction and oxidative stress. Therefore, urgent need to find a more potential drug for treating diabetic nephropathy and diabetes mellitus.2,4
Metabolic derangements (hyperinsulinemia, hyperglycemia and hyperlipidemia), oxidative stress, systemic hypertension, advanced glycation end products (AGEs) and glomerular hypertension enhance the risk of diabetic kidney disease.5,6 It is well documented that over-production of free radicals in persistent hyperglycemia conditions is a significant targeting factor for generate/activate of all pathways involved in the diabetic mellitus complication pathogenesis. Hyperglycemia not only boosts the reactive oxygen species (ROS) production but also attenuates antioxidative mechanisms via the glycosylation of antioxidative enzymes presented as previously reported.5 Oxidative stress play an important role in the expansion of DN and support the antioxidant theory contribute the significant role in the improvement of diabetes condition and related complications.7 Antioxidant containing compound plays an effective role in treating diabetes and diabetic nephropathy. The Hippo signaling pathway is an evolutionarily preserved kinase cascade that plays multiple roles in embryonic development, regulating organ size, apoptosis and cell proliferation. Downstream and Hippo kinase target pathway effector Yes-associated protein (YAP) and its paralog TAZ are target to treat renal disease.8
Oxidative stress is an imbalance condition between the ROS and cellular anti-oxidative capability resultant induces the dysregulation of an endogenous antioxidant system.9 Thus, amelioration of imbalanced condition by scavenging ROS and boost the endogenous antioxidant system make create a difference in the pathology of disease. Nuclear factor erythroid-2 related factor-2 (Nrf2) (n”collar basic leucine zipper protective transcription factor) plays an important role in the regulation of antioxidant gene and cytoprotective triggered via oxidative stress.10,11 When the intracellular oxidative stress increases, Keap1 starts the secretion of Nrf2 translocates into the nucleus, and further activates the cytoprotective genes. Excess generation of ROS starts the disruption of Nrf2/Kelch-like ECH-associated protein-1 (Keap1) complex that contributes to the activation of Nrf2.12 Nrf2 related genes such as Phase II detoxifying enzymes [NAD (P)H: quinine oxidoreductase 1 (NQO1)] and intracellular redox-balancing proteins [modifier subunit (GCLM) and glutamate-cysteine ligase], which boost the excretion of toxicants. Most of the researchers targeting the Nrf2 contain HO-1 and SOD and CAT (key antioxidant enzymes) to treat the disease. The nuclear factor kappa B (NF-κB) is a regulator of inflammatory disorder, is need for the transcription of sufficient cytokines and oxidative stress.11 NF-κB is a redox-sensitive transcription factor, which regulates several genes in response to inflammatory reactions. When the tissue is exposed to the stimuli, an inhibitor of κB (IκB) is phosphorylated via IκB kinase (IKK) to secrete the NF-κB into the nucleus and increase the level of inflammatory-related genes.12 Inflammatory genes such as inflammatory-related protein [inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2)] and cytokines [chemokines adhesion molecules (E-selectin) and tumor necrosis factor-α (TNF-α)] play an important role in the expansion of diabetic nephropathy.13 Based on the biological function of NF-κB and Nrf2, both are considered a novel target to treat DN.
Adropin, encoded by the gene associated with energy homeostasis (Enho), has been proposed as a secreted protein. Energy intake and dietary nutrients can affect gene expression and Adropin circulating levels.14 During the metabolic homeostasis, Adropin significantly enhances dyslipidemia and glucose homeostasis in obesity rodent. Moreover, Adropin has been reducing the PDK4 expression and enhance the glucose usage. Since NAFLD and metabolic disorders are closely related and it has been well proofed that ROS plays a significant role in the expansion of SS to NASH,14,15 in this experimental study, we hypothesized that Adropin may exert beneficial effects against ROS in diabetic nephropathy. In the current experimental protocol, we scrutinized the nephroprotective effects of adropinin on STZ-induced diabetic nephropathy and explored the possible mechanism.
Materials and Methods
Reagents
Metformin (Met) was purchased from Sigma Aldrich Co (St Louis, U.S.A). Plasma insulin, blood glucose, superoxide dismutase (SOD), malondialdehyde (MDA) and catalase (CAT) commercial kits were procured from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Pro-inflammatory cytokine kits such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) were estimated using the ELISA kits were procured from the Nanjing KeyGEN Biotech. Co. Ltd, Nanjing, China. All the antibodies used in the current experimental study were obtained from the Cell Signaling Technology, Danvers, USA.
Animals
Adult Sprague-Dawley (SD) rats (200–220 g, male; aged 4–8 weeks old) were used in the current experimental study. The rats were procured from the Jiangning Qinglongshan Animal Cultivation Farm (Nanjing, China) and kept under standard laboratory condition like temperature 22 ± 1°C, 75% relative humidity and 12-h day and night light cycle. The rats received the standard diet pellet and water ab libitum. All the experimental study was conducted accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and approved from the Qilu Hospital, Cheeloo College of Medicine, Shandong University (QLH20200069SH).
Induction of Diabetes
Single intraperitoneal injection of streptozotocin (45 mg/kg dose) was used for inducing diabetes.16 Briefly, STZ was prepared via dissolved in the 0.1 M citrate buffer (pH=4.5) and rats were kept free from any treatment for 7 days. After 7 days, the blood glucose level of all group rats was estimated and rats having more than 360 mg/dL were considered the diabetic rats.17 The rats having the blood glucose level more than the 360 mg/dL further used for the experimental study.
Experimental Group
After successfully induce diabetes, the rats were divided into four groups and each group contain the 10 rats. Group I: vehicle control and received a phosphate buffer (pH=4.5) for 28 days; Group II: STZ-induced diabetes group rats received a single injection of STZ (45 mg/kg); Group III: STZ-induced diabetes received the Adropinin (50 mg/kg, body weight) for 28 days and Group IV: STZ-induced diabetes received the Metformin (600 µg/kg, body weight), respectively. All group rats were received oral treatment after 7 days and the selection of the dose based on the previous literature. Adropinin (50 mg/kg, b.w.)18 and metformin (600 µg/kg, b.w.) dose were selected based on previous literature.19 Metformin (600 µg/kg, b.w.) dose exhibited toxicity and in the current experimental study, we used the safer dose of metformin to control blood glucose. During the experimental protocol, all group rats were checked every day for dose-related toxicity and clinical abnormality. The body weight and blood glucose levels were estimated at regular time intervals. The blood sample was collected from the animal via tail vein and estimated the blood glucose level by using the glucometer (ACCU-CHEK).
Blood Collection
The blood sample was collected from the animal by puncturing the retro-orbital and estimating the different biochemical parameters. The collected blood sample was centrifuged at 10 x g rpm for 15 min to separate the serum. The serum sample was stored at −80°C for further use.
Plasma Insulin
The plasma insulin level was estimated using the commercial kits Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Estimation of 3-Indoxyl Sulfate
3-indoxyl sulfate concentration in the plasma, kidney and urine was estimated using the high-performance liquid chromatography following the previously reported method with minor modification.20
Urine Collection
For collecting urine sample, the metabolic cage was used. All the experimental rats were transferred into the metabolic cage for 24 h and collected the urine and calculated the volume of urine. The urine samples were centrifuged at 900Xg rpm for 15 min at 4°C and the supernatant was collected for estimating other parameters. Urine samples were frozen at −80°C for further analysis.
Urine Parameters
Urine parameters such as microalbumin, total protein and creatinine were estimated using the TBA-200FR NEO urine chemistry analyzer (Toshiba, Tochigi-Ken, Japan).
Renal Parameters
Serum sample was used for the determination of renal parameters such as creatinine, albumin and blood urea nitrogen (BUN) using the procedure of standard kits (ACCUREX, Biomedical Pvt. Ltd).
Lipid Profile
Standard kits were used for estimating lipid parameters such as total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) were estimated using standard kits (Span diagnostic, India). Low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) were estimated using the previously reported formula.9,21,22
Antioxidant Parameters
Serum was used for the determination of antioxidant parameters such as glutathione (GSH), malondialdehyde (MDA), catalase (CAT) and superoxide dismutase (SOD) by using the previous reported method with minor modification.9,21,22 The oxidative DNA damage parameter such as 8-hydroxy-2ʹ-deoxy-guanosine (8-OHdG) was estimated using the ELISA kit (Cell Biolabs, USA).
Pro-Inflammatory Cytokines and Anti-Inflammatory Mediators
The cytokines such as tumor necrosis factor-α (TNF-α) (EK0525), interleukin-1β (IL-1β) (EK0392), interleukin-6 (IL-6) (M00102-2), interleukin-10 (IL-10) (EK0416) and anti-inflammatory mediator include TGF-β1 (EK0513) were estimated using the manufacture method (Boster Biological Tech, USA).
Inflammatory Mediators
Inflammatory mediators such as nuclear kappa B factor (NF-κB) (P00284) were estimated using the ELISA kits (Boster Biological Tech, USA) following the manufacture instruction.
Histopathology
At the end of the experimental study, all group rats scarified via cervical dislocation. The renal tissue was immediately removed and preserved in the formalin (40%) for the histopathological observation. The 5 mm tissue was processed for preparing the renal histopathological slide.
Statistical Analysis
Graphpad prism 7 software was used for the statistical analysis. One-way ANOVA followed by Dennett’s tests was used for the comparison of the groups. P<0.05 value was considered as the significant.
Result
Effect of Adropinin on Blood Glucose Level, Food Intake, Water Intake and Urine Output
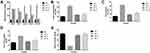
STZ-induced DN rats exhibited increased level of blood glucose level, water intake, food consumption and urine volume. Adropinin significantly (P<0.001) reduced blood glucose levels (Figure 1A), water intake (Figure 1B), food consumption (Figure 1C) and urine volume (Figure 1D). Adropinin treatment group blood glucose level, food intake and water consumption reached almost near to the without treated group rats. Metformin (600 µg/kg) treatment significantly reduced blood glucose level, water intake, food consumption and urine volume.
Figure 1E shows the plasma insulin levels in the group of rats. STZ-induced DN rats showed reduced plasma insulin level. Adropinin treatment significantly (P<0.001) increased the plasma insulin level almost near to the normal group rats. A similar momentum was observed in the metformin-treated group rats.
Effect of Adropinin on Body Weight and Organ Weight
Body weight reduction is commonly observed during diabetic nephropathy. STZ-induced DN rats showed a reduction in body weight compared to other treated and untreated group rats. Adropinin and metformin-treated group rats showed improvement in body weight (Figure 2A).
During the diabetic nephropathy, increased the weight of renal tissue. A similar result was observed in the STZ-induced DN group rats. STZ-induced DN group rats received adropinin and metformin significantly (P<0.001) reduced the renal weight (Figure 2B) and renal hypotrophy (Figure 2C).
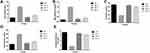
Effect of Adropinin on Urine Output, Microalbumin, Creatinine, Total Protein and Creatinine Clearance
Figure 3 shows the level of urine output, microalbumin, creatinine, total protein and creatinine clearance of the different groups of rats. STZ-induced DN rats demonstrated an increased level of urine output, creatinine, creatinine clearance and reduced level of microalbumin, total protein. The Adropinin and metformin-treated group rats showed reduced levels of urine output, creatinine, creatinine clearance and increase level of microalbumin, total protein (Figure 3A–E).
Effect of Adropinin on HbA1c, BUN and Uric Acid
Figure 4A–C showed increased levels of HbA1c, BUN and uric acid, during diabetic nephropathy. STZ-induced DN group rats exhibited an increased level of HbA1c, BUN and uric acid as compared to treated and untreated group rats. Adropinin and metformin-treated group rats significantly (P<0.001) reduced the levels of HbA1c, BUN and uric acid.
Effect of Adropinin on Indole Sulfate
During the nephropathy, increased the level of indole sulfate in serum, kidney and urine. STZ-induced DN rats showed an increased level of indole sulfate in the serum (Figure 5A), kidney (Figure 5B) and reduced in the urine (Figure 5C). Adropinin and metformin treatment significantly (P<0.001) down-regulated the indole sulfate level in the serum, kidney tissue and increased in the urine.
Effect of Adropinin on Lipid Parameters
The lipid alteration is another complication observed during the diabetes mellitus. STZ-induced DN rats showed the boosted level of TC, TG, LDL, VLDL and reduced levels of HDL in the serum of rats. Adropinin and metformin treatment significantly (P<0.001) down-regulated the level of TC (Figure 6A), TG (Figure 6B), LDL (Figure 6D), VLDL (Figure 6E) and up-regulated the level of HDL (Figure 6C).
Effect of Adropinin on Antioxidant Parameters
STZ-induced group rats exhibited increased level of TBARS, 8-OhdG and reduced level of GSH, CAT, SOD in the renal tissue. Adropinin and metformin treatment significantly (P<0.001) suppressed the level of TBARS (Figure 7A), 8-OhdG (Figure 7E) and increased level of GSH (Figure 7C), CAT (Figure 7B), SOD (Figure 7D) in the renal tissue.
Effect of Adropinin on Cytokines Parameter
Pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-10 and TGF-β1 altered during the diabetic nephropathy. STZ-induced DN rats demonstrated the increased cytokines TNF-α (Figure 8A), IL-1β (Figure 8B), IL-6 (Figure 8C), TGF-β1 (Figure 8E) and reduced level IL-10 (Figure 8D) as compared treated and untreated group rats. Adropinin and metformin treatment significantly (P<0.001) increased the level of IL-10 and reduced the level TNF-α, IL-1β, IL-6, TGF-β1.
Effect of Adropinin on Inflammatory Parameter
Figure 9 shows the effect of NF-κB on the different groups of rats. STZ-induced DN group rats showed the increased level of NF-κB and adropinin and metformin significantly (P<0.001) reduced the level of NF-κB.
 |
Figure 9 Effect of adropinin on NF-κB in STZ-diabetic nephropathy rats. Treatment group was compared with diabetic control, ***p<0.001 using one-way ANOVA with Dunnett’s test. |
Effect on Renal Histopathology
Normal control group rat did not exhibit any abnormal changes in the renal histopathology of rats. In the normal control group rats showed the normal glomerular size, basement membrane thickness and architecture. STZ-induced DN rats showed the enlargement of glomerular size along with the dysregulation of architecture. It also showed the degeneration of tubules with increased basement membrane thickness. Adropinin treatment considerably reduced the glomerular size and reduced the thickness of basement membrane. Metformin-treated group rats renal histopathology showed the similar result as showed in the adropinin treated group rats (Figure 10).
 |
Figure 10 Showed the renal histopathology of STZ-diabetic nephropathy rats. (A) normal control, (B) STZ-induced DN, (C) STZ treated with adropinin and (D) STZ treated with metformin. |
Discussion
Streptozotocin-induced diabetes mellitus induce the injury in the β-cells of islets of Langerhans due to decrease in the insulin secretion, which further leads to the induction of diabetes and related complications especially diabetic nephropathy.10 In the current experimental study, STZ-induced rats exhibited typical characteristics of DM and body weight loss along with increase in the renal tissue weight. During the DM, reduced body weight is a common complication and in this study, we observed reduced body weight in the STZ-induced rats. According to Kumar et al, body weight decreased occurred after the STZ treatment due to dehydration and catabolism of protein and fats.22,23 Adropinin treatment considerably increased body weight, suggesting a reduction of dehydration and catabolism of protein and fats. Adropinin exhibited remarkable augmented body weight probably due to its potential effect in maintaining the wasting of muscle ie, gluconeogenesis’s reversal and reduction of kidney weight.STZ induced DN rats showed enhanced kidney weight compared to without treated and other group rats. Adropinin treatment considerably reduced the kidney weight. The above finding suggests that the adropinin treatment may prevent kidney hypertrophy.
Hyperglycemia involves in glucose overproduction via excessive hepatic gluconeogenesis and glycogenolysis and reduced utilization via tissue and glucose control can protect the incidence and expansion of DN.24 Plasma insulin and blood glucose level boosted during diabetes, while adropinin treatment reduced the level of insulin and blood glucose levels, indicating that adropinin exerts a potential effect on diabetes.25,26 In the meantime, the histopathological study of STZ boosted the renal section revealed vascular wall thickening, necrosis or degeneration, interstitial inflammation and moderate degrees of glomerular dilation. While, adropinin treatment considerably altered these changes in renal histopathology (data not shown). Collectively, the results showed that adropinin could thwart STZ challenged diabetic rats. A higher level of glucose in the blood reacts with hemoglobin to produce glycosylated hemoglobin. Higher glycosylated hemoglobin levels observed in the STZ-induced rats show the poor glycemic control.
Kidney abnormalities progress by altering renal hemodynamics, leading to glomerulosclerosis, renal dysfunction and proteinuria.27,28 An imbalance of nitrogen coupled with reduced protein synthesis starts the generation of non-protein nitrogenous compounds include creatinine and BUN during diabetic nephropathy conditions.16 The boosted level of uric acid, creatinine and BUN in the serum of diabetic rats specifies progressive renal injury, an index of altered GFR in diabetic nephropathy.29,30 STZ-induced DN rats showed increased levels of urine markers such as protein, urine volume and albumin. The clearance of creatinine is determined by serum and urine levels of creatinine, which is an indication of renal function changes.31 During the diabetic condition, the high creatinine level observed in the blood and decreased level of creatinine in the urine was commonly observed; suggest an alteration in renal function.32 Adropinin remarkably enhanced renal function via increased serum creatinine, creatinine clearance and reduced total protein, micro-albumin, urine output.
Oxidative stress plays a major role in diabetes and its complications such as diabetic nephropathy.28,30 Imbalance between the endogenous antioxidant and oxidants is considered oxidative stress. Oxidative stress increased the production of reactive oxygen species (ROS), which could interact with polyunsaturated fatty acids and start the generation of lipid peroxidation (LPO) in the renal tissue and as a result start the toxicity or damage the renal tissue.33 ROS degrades the polyunsaturated fatty acid membrane and generates malondialdehyde (MDA) and 4-hydroxylnoneal (4-HNE). MDA (unstable aldehyde) commonly induces oxidative stress via generate the covalent protein adduct, which serves as a hallmark of oxidative stress in tissue injury.34 Endogenous free radical scavenging antioxidant enzymes such as SOD and CAT are the first defensive line against oxidative damage in mammalian systems (Kumar et al, 2018, 2013b). SOD catalyzes the conversion/reduction/oxidation of superoxide radicals to H2O2 and molecular oxygen. Other endogenous antioxidant enzymes such as CAT, involved in the secretion of H2O2 and guard the tissue from the highly reactive hydroxyl radical (OH).35 Studies have suggested that a reduction in the level of CAT accelerated renal injury during the diabetic nephropathy via peroxisomal dysfunction. Various researches suggest that MDA and SOD are closely related to the DN.11 In the current experimental study, we have observed that reduced levels of SOD and increased level of MDA in the STZ-induced DN and adropinin considerably altered the endogenous antioxidant parameter and suggested the antioxidant effect.
An inflammatory reaction plays a crucial role in the expansion of diseases. During the inflammatory reaction, the level of cytokines and inflammatory mediators is boosted. Inflammatory cytokines (IL-1β, IL-6 and TNF-α) are involved in the expansion of diabetic nephropathy.36 Cytokines such as TNF-α exhibited a cytotoxic effect on epithelial, glomerular and mesangial cells. TNF-α also induces direct renal injury via generating the free radicals.37 An IL-6 (another cytokine) boost hastens mesangial cell proliferation, fibronectin level, alter the extracellular matrix dynamics and enhances the endothelial permeability.37,38 Cytokines such as IL-1β implicated in the development of irregularities in intraglomerular hemodynamics related to prostaglandin synthesis.39,40 In this study, we found that the adropinin considerably reduced the cytokines in the tissue and serum, suggesting the anti-inflammatory effect.
It is well proofed that the NF-κB is a crucial target for the treatment of oxidative stress and inflammatory reactions.41 NF-κB mediates the expansion of renal dysfunction and takes part in the attenuation of oxidative stress during the DN. The NF-κB activation is necessary for phosphorylation and degradation of IκB.42 NF-κB induction creates the imbalance between cytokines and oxidative stress.43 The NF-κB boosted during DN that leads to the up-regulation in the level of pro-inflammatory cytokines. Our experimental study suggests that the adropinin significantly reduced the level of NF-κB and suggested the anti-inflammatory effect.
Conclusion
In conclusion, our experimental data clearly indicated that adropinin showed a nephroprotective effect via attenuating the inflammatory and oxidative stress condition possible via NF-κB signaling pathway. Further studies are needed to investigate adropinin clinical use.
Abbreviations
STZ, streptozotocin; DN, diabetic nephropathy; IL-1β, interleukin-1β; IL-6, interleukin-6; TGF-β, transforming growth factor beta; IL-10, interleukin-10; DM, diabetes mellitus; AGEs, advanced glycation end products; ROS, reactive oxygen species; YAP, Yes-associated protein; Nrf2, nuclear factor erythroid-2 related factor-2; NF-κB, nuclear factor kappa B; IκB, inhibitor of κB; IKK, IκB kinase; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; TNF-α, tumor necrosis factor-α; Met, metformin; BUN, blood urea nitrogen; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein; GSH, glutathione; MDA, malonaldehyde; CAT, catalase; SOD, superoxide dismutase; 8-OHdG, 8-hydroxy-2ʹ-deoxy-guanosine.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author (Xiaoyan Xiao). The data are not publicly available due to [restrictions eg their containing information that could compromise the privacy of research participants].
Funding
The current research was funded by {Key R & D project of Shandong Province (Public welfare tackling of key scientific and technical problems), 2019GSF108111 role and mechanism of Yap / TAZ in diabetic nephropathy}.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nasiry D, Khalatbary AR, Ahmadvand H, et al. Protective effects of methanolic extract of Juglans regia L. leaf on streptozotocin-induced diabetic peripheral neuropathy in rats. BMC Complement Altern Med. 2017;17:476. doi:10.1186/s12906-017-1983-x.
2. Nasiry D, Khalatbary AR, Ahmadvand H, et al. Juglans regia L. Leaf extract attenuates diabetic nephropathy progression in experimental diabetes: an immunohistochemical study. Iran J Med Sci. 2019;44:44–52. doi:10.30476/ijms.2019.40624.
3. Nasiry D, Khalatbary AR, Ahmadvand H, et al. Therapeutic potential of Juglans regia L. Leaf extract against diabetic retinopathy in rat. Iran J Basic Med Sci. 2017;20:1275–1281. doi:10.22038/IJBMS.2017.9465.
4. Javidanpour S, Fatemi Tabtabaei SR, Siahpoosh A, et al. Comparison of the effects of fresh leaf and peel extracts of walnut (Juglans regia L.) on blood glucose and β-cells of streptozotocin-induced diabetic rats. Vet Res Forum. 2012;3:251–255.
5. Yuan D, Liu XM, Fang Z, et al. Protective effect of resveratrol on kidney in rats with diabetic nephropathy and its effect on endoplasmic reticulum stress. Eur Rev Med Pharmacol Sci. 2018;22:1485–1493. doi:10.26355/eurrev_201803_14497.
6. Mohammad BI, Kareem ZA, Hadi NR, et al. Aliskiren retards the progression of renal disease in diabetes mellitus: an experimental study in rats. World J Pharm Res. 2014;2(6):1999–2011.
7. Shivanna N, Naika M, Khanum F, et al. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J Diabetes Complications. 2013;27:103–113. doi:10.1016/j.jdiacomp.2012.10.001.
8. Boopathy GTK, Hong W. Role of Hippo pathway-YAP/TAZ signaling in angiogenesis. Front Cell Dev Biol. 2019;7:49. doi:10.3389/fcell.2019.00049.
9. Kumar V, Ahmed D, Gupta PS, et al. Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of Melastoma malabathricum Linn. leaves in streptozotocin induced diabetic rats. BMC Complement Altern Med. 2013;13. doi:10.1186/1472-6882-13-222.
10. Wang S, Yang Z, Xiong F, et al. Betulinic acid ameliorates experimental diabetic-induced renal inflammation and fibrosis via inhibiting the activation of NF-κB signaling pathway. Mol Cell Endocrinol. 2016;434:135–143. doi:10.1016/j.mce.2016.06.019.
11. Xie R, Zhang H, Wang XZ, et al. The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats. Food Funct. 2017;8:299–306. doi:10.1039/c6fo01601d.
12. Yang WJ, Li YR, Gao H, et al. Protective effect of the ethanol extract from Ligusticum chuanxiong rhizome against streptozotocin–induced diabetic nephropathy in mice. J Ethnopharmacol. 2018;227:166–175. doi:10.1016/j.jep.2018.08.037.
13. Surh YJ, Kundu JK, Na HK, et al. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135(12):2993S–3001S. doi:10.1093/jn/135.12.2993s.
14. Ganesh Kumar K, Zhang J, Gao S, et al. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity. 2012;20:1394–1402. doi:10.1038/oby.2012.31.
15. Lovren F, Pan Y, Quan A, et al. Adropin is a novel regulator of endothelial function. Circulation. 2010;122(11 Suppl):S185–S192. doi:10.1161/CIRCULATIONAHA.109.931782.
16. Navale AM, Paranjape A. Antidiabetic and renoprotective effect of Anogeissus acuminata leaf extract on experimentally induced diabetic nephropathy. J Basic Clin Physiol Pharmacol. 2018;29:359–364. doi:10.1515/jbcpp-2017-0190.
17. Yu LY, Shi WL, Guo XG. Cardio-protective role of gingerol along with prominent anti-diabetic cardiomyopathy action in a streptozotocin-induced diabetes mellitus rat model. Cell J. 2017;19(3):469–475. doi:10.22074/cellj.2017.4509.
18. Ghoshal S, Stevens JR, Billon C, et al. An endocrine link between the biological clock and cholesterol homeostasis. Mol Metab. 2018;8:51–64. doi:10.1016/j.molmet.2017.12.002.
19. Salma B, Janhavi P, Muthaiah S, et al. Ameliorative efficacy of the cassia auriculata root against high-fat-diet + STZ-induced type-2 diabetes in C57BL/6 mice. ACS Omega. 2021;6(1):492–504. doi:10.1021/acsomega.0c04940.
20. Kundu A, Dey P, Sarkar P, et al. Protective effects of Croton hookeri on streptozotocin-induced diabetic nephropathy. Food Chem Toxicol. 2020;135:110873. doi:10.1016/j.fct.2019.110873.
21. Kumar V, Anwar F, Ahmed D, et al. Paederia foetida Linn. leaf extract: an antihyperlipidemic, antihyperglycaemic and antioxidant activity. BMC Complement Altern Med. 2014;14:76. doi:10.1186/1472-6882-14-76
22. Kumar V, Ahmed D, Verma A, et al. Umbelliferone β-D-galactopyranoside from Aegle marmelos (L.) corr. An ethnomedicinal plant with antidiabetic, antihyperlipidemic and antioxidative activity. BMC Complement Altern Med. 2013;13:273. doi:10.1186/1472-6882-13-273
23. Kumar V, Sharma K, Ahmed B, et al. Deconvoluting the dual hypoglycemic effect of wedelolactone isolated from Wedelia calendulacea: investigation via experimental validation and molecular docking. RSC Adv. 2018;8:18180–18196. doi:10.1039/c7ra12568b.
24. Dias LD, Casali KR, Leguisamo NM, et al. Renal denervation in an animal model of diabetes and hypertension: impact on the autonomic nervous system and nephropathy. Cardiovasc Diabetol. 2011;10:33. doi:10.1186/1475-2840-10-33.
25. Murali R, Karthikeyan A, Saravanan R. Protective effects of d-limonene on lipid peroxidation and antioxidant enzymes in streptozotocin-induced diabetic rats. Basic Clin Pharmacol Toxicol. 2013;112(3):175–181. doi:10.1111/bcpt.12010.
26. Yokozawa T, Cho EJ, Park CH, et al. Protective effect of proanthocyanidin against diabetic oxidative stress. Evid Based Compl Altern Med. 2012;2012:1–11. doi:10.1155/2012/623879.
27. Jiang T, Huang Z, Lin Y, et al. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:850–860. doi:10.2337/db09-1342.
28. Xu Z, Wei Y, Gong J, et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014;57(1):204–213. doi:10.1007/s00125-013-3093-8.
29. Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi:10.2337/db08-0057.
30. Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84(21–22):705–712. doi:10.1016/j.lfs.2009.02.026.
31. Sharma S, Anjaneyulu M, Kulkarni SK, et al. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology. 2006;76:69–75. doi:10.1159/000089720
32. Nisha R. Biochemical evaluation of creatinine and urea in patients with renal failure undergoing hemodialysis. J Clin Pathol Lab Med. 2017;1(2):1–5.
33. Calabrese V, Mancuso C, Sapienza M, et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12:299–306. doi:10.1379/CSC-270.1
34. Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi:10.1016/j.taap.2006.01.003.
35. Zhu L, Wei T, Chang X, et al. Effects of salidroside on myocardial injury in vivo in vitro via regulation of Nox/NF-κB/AP1 pathway. Inflammation. 2015;38:1589–1598. doi:10.1007/s10753-015-0134-0
36. Lim AKH, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. doi:10.1155/2012/146154.
37. King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79:1527–1534. doi:10.1902/jop.2008.080246.
38. Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi:10.1681/ASN.2007091048.
39. Sharma K. Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int Suppl. 2014;4(1):113–117. doi:10.1038/kisup.2014.21.
40. Kanasaki K, Taduri G, Koya D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol (Lausanne). 2013;4:7. doi:10.3389/fendo.2013.00007.
41. Mohamed AK, Bierhaus A, Schiekofer S, et al. The role of oxidative stress and NF-κB activation in late diabetic complications. BioFactors. 1999;10:157–167. doi:10.1002/biof.5520100211.
42. Mezzano S, Aros C, Droguett A, et al. NF-κB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19:2505–2512. doi:10.1093/ndt/gfh207.
43. Ahangarpour A, Oroojan AA, Khorsandi L, et al. Preventive effects of betulinic acid on streptozotocin-nicotinamide induced diabetic nephropathy in male mouse. J Nephropathol. 2016;5(4):128–133. doi:10.15171/jnp.2016.24.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.