Back to Journals » Research and Reports in Neonatology » Volume 10
Neonatal (and Infant) Coarctation of the Aorta: Management Challenges
Authors Rao PS
Received 29 October 2019
Accepted for publication 31 January 2020
Published 18 February 2020 Volume 2020:10 Pages 11—22
DOI https://doi.org/10.2147/RRN.S189545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Schelonka
P Syamasundar Rao
Department of Pediatrics, University of Texas-Houston McGovern Medical School, Children’s Memorial Hermann Hospital, Houston, TX, USA
Correspondence: P Syamasundar Rao
Emeritus Chief of Pediatric Cardiology, UT-Houston McGovern Medical School, 6410 Fannin, UTPB Suite # 425, Houston, TX 77030, USA
Tel +1 713-500-5738
Fax +1 713-500-5751
Email [email protected]
Abstract: Surgical correction of coarctation of the aorta was described in the mid-1940s and balloon angioplasty was introduced in the early 1980s. Several types of surgical methods were devised to treat native coarctation, but eventually, resection and end-to-end anastomosis became a standard approach with the use of extended end-to-end anastomosis for babies with hypoplasia of the isthmus and/or transverse aortic arch. Balloon angioplasty was considered as a substitute for surgical correction and was so used for some time, but because of high rate of recurrence in the neonate and young infant, most centers have reverted back to surgical correction as a primary mode of treatment of aortic coarctation in the neonate. Further research into feasibility of using stents in the management of coarctation in neonates and young infants are necessary. It is generally agreed that balloon angioplasty is the treatment of choice for post-surgical aortic recoarctations.
Keywords: coarctation of the aorta, balloon angioplasty, surgical correction, neonate, stents, post-surgical recoarctation
Introduction
Coarctation of the aorta (CoA) is a congenital cardiac anomaly with obstruction to blood flow in the descending aorta; it comprises of narrowed aortic segment with localized medial thickening and infolding of the media and superimposed neo-intimal tissue.1,2 A shelf-like structure or a membranous curtain-like structure may be seen with either an eccentric or a central opening. Both discrete and long segment forms have been found; the discrete form is more common. The classic CoA is located in the thoracic aorta distal to the origin of the left subclavian artery (LSA) and is juxtaductal. Varying degrees of hypoplasia of the transverse aortic arch (aortic segment between the origin of the right innominate artery and LSA) and of the isthmus of the aorta (aorta between the origin of the LSA and ductus arteriosus) are present, particularly in the symptomatic neonates and infants. The prevalence of CoA is 5% to 8% among children with congenital heart disease (CHD).1–3 It may be an isolated lesion or may be seen in association with other CHDs.1 CoA is seen with clinically important CHD particularly in the neonate and these include patent ductus arteriosus, ventricular septal defect (VSD), and aortic stenosis. Bicuspid aortic valve may be seen in two-third of infants with CoA. Mitral valve anomalies are less frequent than those of the aortic valve. At times, CoA is a complicating feature of a more complex cyanotic CHDs, such as double inlet left ventricle, Taussig–Bing anomaly, tricuspid atresia with transposition of the great arteries, and hypoplastic left heart syndrome. This review deals largely with isolated CoA. The pathologic, pathophysiologic, clinical, chest x-ray, electrocardiographic, echo-Doppler, and angiographic features of CoA were reviewed elsewhere1–5 by the author and will not be repeated here.
Since the original reports of surgical repair by Crafoord and Nylin6 and Gross7 in mid-1940s, surgery has been used in the management of the neonate with CoA. However, following more recent description of balloon angioplasty, balloon dilatation techniques have been utilized in the treatment of CoA as a substitute for surgery. The technique of balloon angioplasty described by Dotter and Judkins8 and Gruntzig and his associates9–11 was applied by Sos,12 Singer,13 and Sperling14 and their colleagues to enlarge narrowed aortic segments in a postmortem specimen, and in infants with post-surgical recoarctation and native coarctation, respectively. Subsequently, a number of investigators that participated in the Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry15,16 including our group17–20 used the balloon angioplasty technique to address CoA. In this review, the author will address the indications and timing of intervention, available medical, surgical and transcatheter management options, and the techniques and results of these procedures. Then, a presentation of comparison of surgical with balloon therapy, selection of therapy, and future directions will be made.
Indications and Timing of Intervention
Indications for intervention either by surgical or transcatheter therapy are significant hypertension and/or congestive heart failure (CHF) along with a peak-to-peak systolic pressure gradient of more than 20 mmHg across the coarctation site. Neonates and infants who are symptomatic should have relief of the aortic obstruction as soon as the infant is stabilized whereas infants who are not symptomatic may have relief of the aortic obstruction electively. Careful evaluation by echo-Doppler studies may help in this assessment. Very narrow coarcted segment (by two-dimensional [2D] echo) and high Doppler flow velocity with diastolic extension of the Doppler signal and with either normal or decreased left ventricular (LV) systolic function are indications for immediate intervention. Modest 2D narrowing with mild increase in Doppler velocity without diastolic extension and spontaneously closed ductus arteriosus and normal LV systolic function do not need intervention in the neonatal period. However, in the presence of wide-open ductus arteriosus, it is difficult to evaluate the degree of aortic obstruction.
Medical Management
As mentioned above, significant hypertension and/or CHF are indications for intervention. In the presence of CHF, conventional anti-congestive measures including diuretics and digitalis should be promptly instituted. In the presence of hypertension, it is better to relieve the obstruction promptly rather than attempting to “treat” hypertension with antihypertensive drugs. In neonates with severe aortic obstruction, initial medical management includes the infusion of PGE1 to bypass the coarctation (Figure 1). The recommended dosage for PGE1 is 0.05 to 0.1 mcg/kg/minute intravenously. Adverse effects include apnea, hyperthermia, flushing, and muscular twitching. The side effects have not created significant problems, but development of apnea is of concern and should be watched for. We usually begin PGE1 at the suggested dose of 0.05 to 0.1 mcg/kg/minute, but will quickly decrease the dosage stepwise to 0.02 to 0.03 mcg/kg/minute once the desired effect is achieved. This may avoid the need for endotracheal ventilation because of apnea.
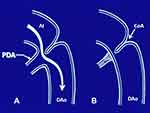 |
Figure 1 Diagrammatic portrayal of the flow from the isthmus to the descending aorta in coarctation of the aorta with ductus open (A) and closed (B). When the ductus is open, the posterior shelf of aorta is bypassed and symptoms of obstruction are relieved. Adapted with permission from Rao PS, Solymar L. Transductal balloon angioplasty for coarctation of the aorta in the neonate: Preliminary observations. Am Heart J. 1988;116(6, Part 1):1558–1563.70 doi: 10.1016/0002-8703(88)90,743–0. |
Surgical Management
Surgical repair of aortic coarctation was first described in 1945 by Crafoord & Nylin and Gross. Four major types of repair were used in the past and include resection and end-to-end anastomosis, subclavian flap angioplasty, prosthetic patch angioplasty, and tubular bypass grafts. These procedures are generally performed under general anesthesia via lateral thoracotomy. If other associated defects such as VSD need repair concurrently, the procedure can be performed via median sternotomy. Resection of the coarcted segment with end-to-end anastomosis was initially performed for this lesion. Because of high recurrence rates observed during the original series, Waldhausen and associates21 introduced and advocated subclavian flap angioplasty. However, subsequent studies did not demonstrate significant advantage of subclavian flap angioplasty over resection and end-to-end anastomosis. While prosthetic patch angioplasty was preferred by some surgeons, because of frequent development of aneurysm during late follow-up, this procedure is not commonly used at present. Tubular bypass grafts do not grow as the baby grows and therefore, are not generally used. Consequently, resection and end-to-end anastomosis have become the main surgical method to address aortic coarctations. Babies with hypoplasia of the isthmus and/or transverse aortic arch may require an extended end-to-end anastomosis.22
Results of Surgery
Surgical results in neonates and infants were examined by the author in early 1990s.20,23,24 Forty-nine papers published between 1980 and 1991 reporting on surgery in 3 to 191 neonates and infants less than one-year-old in the respective papers, who had surgery between 1953 and 1990 were reviewed. The operative mortality varied between 0% and 50% with a mean of 19% (617 of 3292). The late mortality was reported to be between 3% and 59% with an average of 18% (483 of 2648). Re-coarctation rates ranged between 0% and 100% with mean of 17% (421 of 2540). However, more recent reports25–27 have documented better results with initial mortality rates of 0.5%, 2.4%, and 1.6%; late mortality rates of 8.3%, 1.4%, and 1.1%; and recoarctation rates of 2.2%, 6%, and 22.6%, indicating substantial improvement of surgical results. Young age at surgery, presence of hypoplasia of the aortic arch, and short body length24,27 were identified as risk factors for recoarctation. While surgery has indeed improved the prognosis of patients with aortic coarctation, significant problems as reviewed above, morbidity associated with surgery, paradoxical hypertension, paraplegia (though rare), aneurysm formation, and vascular complications related subclavian flap repair remain.
Balloon Angioplasty
The usual approach for balloon angioplasty of aortic coarctation is retrograde femoral arterial route. Because of potential for damage to the femoral artery, particularly in the newborn, trans-umbilical arterial28–30 and anterograde transvenous approaches31 (via a transposed aorta or through the VSD, when possible) have been attempted to prevent injury to the femoral artery. When femoral arterial route is utilized, low profile balloons (for example, Tyshak II balloons) which may be introduced through 4-French sheaths or Mini-Tyshack that can be positioned via 3-French sheaths32 should be used.
If balloon angioplasty is planned, infusion of PGE1 should not be initiated because wide-open ductus may interfere with effectiveness of balloon dilatation. Hemodynamic data and selective cine-angiography are secured to confirm the clinical and echocardiographic diagnosis of aortic coarctation and to establish suitability for balloon coarctation angioplasty. Measurements of the coarcted segment and other aortic measurement are made in two orthogonal views and averaged. At this point, a # 4-French multipurpose catheter is passed across the aortic coarctation segment into the ascending aorta or into right subclavian artery. When umbilical arterial route is used, the catheter in the umbilical artery is exchanged over a suitable-sized guidewire with a 4-French multipurpose catheter and the catheter passed across the coarctation segment. At this juncture, a 0.021 to 0.025 inch J-tipped guidewire is positioned via the catheter already in place into the ascending aorta (or right subclavian artery) and the catheter removed leaving the guidewire in place. When an anterograde femoral venous approach is used, a 4-French multipurpose catheter is positioned in the descending aorta either via the transposed aorta or through the VSD and the tip of the guidewire is positioned in the descending aorta distal to the coarcted segment of the aorta.
The size of the balloon selected for balloon angioplasty should be two or more the times size of the coarcted aortic segment, but not more than the diameter of the descending aorta at the level of the diaphragm. The author normally selects a balloon which is midway between the diameter of the aortic isthmus (or transverse aortic arch) and the size of the descending aorta at the diaphragm. The selected balloon dilatation catheter is placed across the aortic coarctation over the guide wire already in place and the balloon is filled (Figure 2A) with diluted contrast material at an inflation pressure up to three to five atmospheres, based on the balloon manufacturer’s recommendations. The author usually monitors the pressure of balloon inflation with commercially available pressure gauges. The balloon is inflated for roughly 5 s or until the waist of the balloon is abolished (Figure 2B). The balloon is inflated two to four times, 5 mins apart. The balloon catheter is replaced with an angiographic catheter and an aortic arch cine angiogram and pressure pullback gradients across the coarctation site are recorded. If there is no adequate relief of narrowing (pressure gradient less 20 mmHg and angiographic improvement) is achieved, the diameter of the balloon is increased such that it is similar to the diameter of the descending aortic at the diaphragm.33 It is important to leave a guidewire across the coarctation site during catheter exchanges and no manipulation of tips of the guide wires or catheters across the site of freshly dilated coarcted segments should be performed.
 |
Figure 2 Selected cine angiographic frames in 20° left anterior oblique view showing a balloon dilatation catheter across the aortic coarctation with waisting (arrow) of the balloon (A) during the early phases of balloon inflation which was abolished (B) on further inflation of the balloon. Reproduced from Doshi AR, Rao PS. Coarctation of aorta-management options and decision making. Pediatr Therapeut. 2012;S5:006.47 Abbreviations: C, central venous catheter; GW, guide wire. |
Results of Balloon Angioplasty
Decrease in peak-to-peak systolic pressure gradients across the aortic coarctation, increase in diameter of the coarcted segment by angiography (Figure 3A and B), improvement of signs and symptoms of CHF, and reduction of systemic hypertension occur following balloon angioplasty. These encouraging results were reviewed in detail elsewhere.23,32,34-37 However, the problem in the newborn is significant incidence of recurrence.32,34,38,39 Risk factors for development of recoarctation are 1. Age less than 12 months, 2. Aortic isthmus less than 2/3 the size of the ascending aorta immediately proximal to the right innominate artery, 3. Coarcted aortic segment less than 3.5 mm before dilation, and 4. coarcted aortic segment less than 6 mm after angioplasty. It was further noted that presence of two or more risk factors is associated with high rate of recoarctation; the larger the number of risk factors, the greater is the probability for recoarctation.39–41 These risk factors for recurrence are not too dissimilar to those found for post-surgical recoarctation. Furthermore, the event-free rates are remarkably lower in neonates (1 to 30 days) compared to those in infants (1 to 12 months) and children (1 to 15 years) (Figure 4).39 However, there may be palliative benefit with balloon angioplasty; the results of neonatal and young infant group from the author’s personal experience32,34 will be presented in support of this concept.
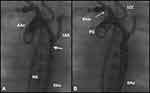 |
Figure 3 Selected cine frames from aortic cine angiograms in 20° left anterior oblique projection demonstrating narrowed coarcted aortic segment (arrow) before balloon angioplasty (A) which increased after balloon angioplasty (B). Moderate hypoplasia of distal transverse aortic arch and isthmus is also present. Modified from Rao PS. Role of interventional cardiology in neonates: Part II – Balloon angioplasty/valvuloplasty. Congenital Cardiol Today. 2008;6(1):1–14.71 Abbreviations: AAo, ascending aorta; DAo, descending aorta; LCC, left common carotid artery; LSA, left subclavian artery; NG, nasogastric tube; PG, pigtail catheter. |
 |
Figure 4 Actuarial event-free survival curves of neonates (<30 days), infants (1 to 12 months) and children (1 to 15 years) who had undergone balloon angioplasty of aortic coarctation are drawn. The event-free survival rates are better for children group than for the neonatal and infant groups (p < 0.001). Modified from Rao PS, Galal O, Smith PA, Wilson AD. Five-to-nine-year follow-up results of balloon angioplasty of native aortic coarctation in infants and children. J Am Coll Cardiol. 1996;27:462–470.39 doi: 10.1016/0735-1097(95)00479–3. |
Results of Balloon Angioplasty of Coarctation of the Aorta in the Neonate and Young Infant
The ideal management strategy of the neonate and young infant with native aortic coarctation is controversial. We examined our experience with balloon angioplasty in neonates and infants less than 3 months to test our hypothesis that balloon angioplasty provides successful palliation, defined as avoidance of surgical intervention for 4 weeks along with control of CHF.34 During this study, we also sought to detect differences in the results of the trans-umbilical arterial (UA), trans-femoral arterial (FA) and trans-femoral venous anterograde (FVA) routes that we used to perform balloon angioplasty. Fifty-one neonates and infants less than 3 months with coarctation presenting with heart failure, hypertension or both underwent balloon angioplasty during a 6.5-year period ending June 2001. Balloon angioplasty of coarctation was performed via UA (n = 16), FA (n = 26), and FVA (n = 9) routes. Associated defects were present in all three groups and were similar. Immediate results showed reduction of peak-to-peak gradients across the coarctation (40 ± 17 mmHg vs 5.4 ± 6.1 mmHg; p < 0.001) (Figure 5, left panel), increase in diameter of the coarcted segment (2.2 ± 0.5 mm vs 5.6 ± 0.8 mm; p < 0.001), and improvement in symptomatology. There was no difference in pressure gradient reduction between UA, FA, and FVA groups (Figure 5, right panels).
 |
Figure 5 Bar graph showing reduction (p < 0.001) of peak-to-peak systolic pressure gradients (in mmHg) across the aortic coarctation after balloon angioplasty. The fall in the gradients was seen for the entire group (left panel) and for all the three subgroups, namely trans-umbilical arterial (UA), trans-femoral arterial (FA) and trans-femoral venous anterograde (FVA). Mean + standard deviation (SD) are shown. N represents number of subjects in each group. Modified from Rao PS, Jureidini SB, Balfour IC, Singh GK, Chen SC. Severe aortic coarctation in infants less than 3 months: Successful palliation by balloon angioplasty. J Intervent Cardiol. 2003;15(6):202–208.34 |
Surgical intervention to relieve aortic obstruction was needed in 4 infants at 5, 21, 24 and 28 days after balloon angioplasty. Thus, effective palliation was achieved in the remaining 47 infants (92%). During follow-up (5 months to 5.5 years; median, 3 years), most patients did well with good angiographic improvement (Figure 6A–C), but, twenty-two infants (~50%) were assessed to have recoarctation (defined as peak-to-peak gradient >20 mmHg) and required repeat balloon (n = 14) or surgical (n = 8) therapy 2 to 10 months (median, 3 months) after initial balloon dilatation.
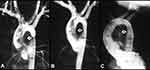 |
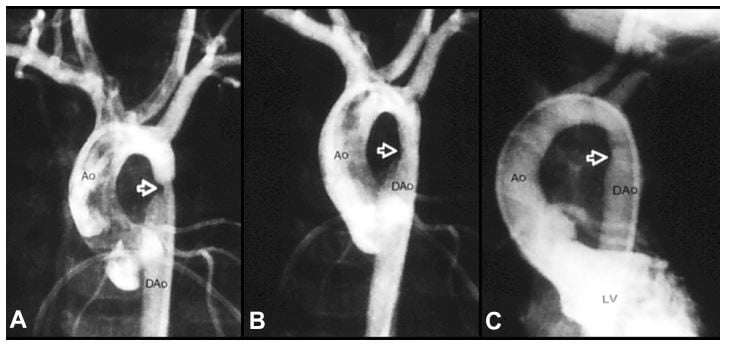
Figure 6 Selected aortic (Ao) and left ventricular (LV) cine-angiographic frames of a one-month-old baby prior to (A), immediately after (B) and 1 year following (C) balloon angioplasty are shown. The coarcted aortic segment (arrowhead) shown in “A” improved remarkably after angioplasty (B) which continues to be wide open at follow-up (C). Reproduced from Rao PS. Coarctation of the aorta. In: Secondary Forms of Hypertension, Ram CVS (Ed), Seminars in Nephrology, Kurtzman NA (Ed), (W)B. Saunders, Philadelphia, PA. Seminars in Nephrology. 1995;15(2):81–105.1 Abbreviation: DAo, descending aorta. |
The reason for re-intervention was hypertension in all patients. Repeat balloon angioplasty resulted in reduction of peak-to-peak systolic pressure gradients from 54 ± 20 mmHg to 9 ± 7 mmHg (p < 0.001). At a median follow-up of 3 years (range, 0.5 to 5.5 years), blood pressures remained low (98 ± 11 mmHg) with an arm/leg blood pressure gradient of 4 ± 6 mmHg.
Comparison of the UA, FA, and FVA groups revealed similar effectiveness immediately after the procedure (Figure 5 right panels). The recoarctation rates at follow-up were also similar (p > 0.05) in all the three groups (Figure 7, left panel). The recoarctation rates were related to age at angioplasty than to the route of through which the procedure was performed (Figure 7, right panel); sixteen of 22 (72%) developed recoarctation in babies ≤30 days while only six of 22 (28%) babies 30 to 90 days old had recoarctation (p < 0.001) at follow-up. However, femoral artery complications were found in only the FA group.
 |
Figure 7 Bar graph demonstrating recoarctation rates during follow-up after balloon angioplasty of native coarctation in infants. The rate of recurrence is not related (p > 0.05) to the route through which balloon angioplasty was performed (left panel). However, when the patients were divided into neonates (≤ 30 days) and infants between 31 and 90 days, the rate of recurrence was significantly higher (p <0.001) in neonates than in infants (right panel). Number of subjects with recurrence/number of subjects in that particular group is shown on the top of each bar. The data indicate that age at angioplasty plays a major role in recoarctation and not the route of balloon angioplasty. Modified from Rao PS, Jureidini SB, Balfour IC, Singh GK, Chen SC. Severe aortic coarctation in infants less than 3 months: Successful palliation by balloon angioplasty. J Intervent Cardiol. 2003;15(6):202–208.34 Abbreviations: d, days; FA, femoral artery; FVA, femoral venous, anterograde; UA, umbilical artery. |
On the basis of these data, we concluded that effective palliation is achieved with balloon angioplasty in all 3 groups, femoral artery complications are seen only in the FA group and balloon angioplasty is an excellent alternative to surgical intervention in the management of native CoA in the neonate and young infant.32,34
Comparison of Surgery with Balloon Angioplasty
Comparisons of surgical vs balloon therapy from the published reports and from the author's retrospective study as well as comparison of complications will be reviewed in this section.
Comparison of Results from Published Reports
At first, a comparison of safety and efficacy of balloon angioplasty with surgical correction of native aortic coarctation was made from the published reports at the time of that writing.23 The author examined papers published from 1979 to 1991 which reported on the results of surgery in infants younger than 1 year and publications from 1982 to 1991 that reported on balloon angioplasty. The surgical group consisted of nearly 600 infants while the balloon group was much smaller, around 70 patients. The prevalence of associated defects was 70% in both groups (p > 0.1). Initial mortality (13.5% in the surgical group vs 7% in the balloon group) and late mortality (12.8% in the surgical group vs 1.5% in the balloon group) were higher (p < 0.001) in the surgical than balloon group while the recoarctation rates (59 of 517 [11.4%] in the surgical group vs 11 of 67 [16.0%] in the balloon group) were similar (p > 0.05).
Comparison of Surgical and Balloon Therapy in a Retrospective Study
The author compared the efficacy and safety of balloon angioplasty with those of surgery in babies ≤3 months.42 Data of 29 babies ≤3 months of age who were treated for aortic coarctation during the decade ending 1992 at the University of Wisconsin Hospitals were examined. Fourteen infants had surgery and 15 had balloon angioplasty. The surgical and balloon cohorts were similar (p > 0.1) with regard to age, weight, and prevalence (7 of 14 vs 8 of 15) and type of concomitant cardiac defects. Immediate (1 of 14 vs 1 of 15) and late (3 of 13 vs 3 of 14) mortality and immediate (Figure 8) and follow-up (Figure 9, left panel) residual gradients were similar (p > 0.1) in both groups. Babies with gradients ≥20 mmHg at follow-up (6 of 13 vs 7 of 14) and necessity for re-intervention (6 of 13 vs 7 of 14) were also similar (p > 0.1) in both groups. Results of repeat balloon angioplasty in babies with recurrence were also similar (p > 0.1) in both groups (Figure 9, middle and right panels). However, morbidity (durations of hospital stay, endotracheal intubation, and mechanical ventilation) and complication rates (0.27 events/patient vs 0.86 events/patient) were lower (p < 0.05 to <0.001) in the balloon than in the surgical group (Figure 10).
 |
Figure 8 Bar graph shows comparison of pressure gradient reduction immediately after surgical and balloon therapy. Note significant reduction in peak-to-peak pressure gradient in both the surgical (p < 0.005) and balloon (p < 0.001) groups. This fall in pressure gradients in both groups is not significantly different (p = 0.28) form each other. Mean + standard error of mean (SEM) are shown. PRE, prior to; POST, following. Modified from Rao PS, Chopra PS, Koscik R, Smith PA, Wilson AD. Surgical versus balloon therapy for aortic coarctation in infants ≤3 months old. J Am Coll Cardiol. 1994;23(6):1479–1483.42 doi: 10.1016/0735-1097(94)90395–6. |
 |
Figure 9 Bar graph shows comparison of residual pressure gradients at follow-up (left panel) after surgical (SURG) and balloon (BALL) therapy and of the results of repeat balloon angioplasty (middle and right panels, respectively). Note similar (p > 0.1) residual gradients at follow-up (left panel). Also, note that there is a significant (p < 0.01) fall in pressure gradients in both groups (middle and right panels) after repeat balloon angioplasty at follow-up (FU). Mean + standard error of mean (SEM) are shown. Modified from Rao PS, Chopra PS, Koscik R, Smith PA, Wilson AD. Surgical versus balloon therapy for aortic coarctation in infants ≤3 months old.J Am Coll Cardiol. 1994;23:1479–1483.42 doi: 10.1016/0735-1097(94)90395–6. Abbreviations: PRE, prior to repeat balloon angioplasty; POST, after repeat balloon angioplasty. |
 |
Figure 10 Bar graph showing comparison of morbidity and complication rates after surgical (SURG) and balloon (BALL) therapy. The duration of hospitalization (p < 0.05) and duration of mechanical (Mech.) ventilation (Vent.) (p < 0.01) are longer for the surgical than for the balloon group. The complication rate was higher (p < 0.05) in the surgical than in the balloon group. Mean + standard error of mean (SEM) are shown. Modified from Rao PS, Chopra PS, Koscik R, Smith PA, Wilson AD. Surgical versus balloon therapy for aortic coarctation in infants ≤3 months old. J Am Coll Cardiol. 1994;23:1479–1483.42 doi: 10.1016/0735-1097(94)90395–6. |
In summary, this study demonstrated that the degree of relief of aortic obstruction and the frequency with which re-intervention is required are similar between surgical and balloon therapy groups, but the morbidity and complication rates were higher with the surgical than with balloon therapy. The authors concluded that balloon angioplasty may be an acceptable alternative to surgery in the treatment of symptomatic aortic coarctation in infants ≤3 months.42
Comparison of Complications
A comparison of complications associated with both these procedures, namely, paradoxical hypertension, paraplegia, aneurysms and femoral artery occlusion were made. Paradoxical hypertension which is reported with surgery is rare with balloon angioplasty and, if present, very mild and inconsequential following balloon angioplasty.37,39 Similarly paraplegia, though rare after surgery, is practically unheard of after balloon angioplasty. Aneurysms are described following coarctation surgery of all types; nearly 30% patients had aneurysms;43 this is much higher than that seen by us39 and others after balloon angioplasty. The rate of femoral artery occlusion after balloon angioplasty may be higher than that seen with surgery but, vascular complications do occur in the left upper limb following coarctation repair with subclavian flap aortoplasty.
Summary
In summary, comparison between surgical and balloon management of CoA indicates nearly equal effectiveness, the mortality rates are also similar and are more related to the associated cardiac defects, but surgery requires longer hospital stay and greater morbidity with attendant higher costs and has higher complication rates. Balloon angioplasty may be an effective alternative to surgery for the relief of CoA.
Stent Therapy
Stents have been used to treat CoA in adolescents and adults, but not often used in neonates and young infants. Consequently, stents will not be discussed in this review. Historical aspects, types of stents available, method of implantation and results related to CoA were reviewed elsewhere2,44-50 for the interested reader.
Selection of Therapy
Effective use of balloon angioplasty procedure was documented by several investigators, including our own group. But, frequent recurrence if the procedure is performed in the neonatal period32,34,38,39 and common occurrence of varying degrees of transverse aortic arch and isthmus hypoplasia in the newborn, most cardiologists and surgeons prefer surgery at this time. However, balloon angioplasty of coarctation has a beneficial role in critically ill young babies, particularly if averting anesthesia or aortic cross-clamping needed for surgical approach is valuable in the overall management of the infant. Such distinctive circumstances are babies with shock-like syndrome,29 severe myocardial dysfunction along with hypertensive cardiomyopathy,51 prior spontaneous cerebral hemorrhage,35 and biliary atresia awaiting liver transplantation.35,52 A midway approach by using stents in young patients was proposed53,54 as an alternative to balloon and surgical therapies, but the author is not in favor of use of stents in neonates, infants and young children, as detailed in editorials published55,56 concurrent with these papers.
Post-Surgical Aortic Recoarctation
Recurrence of coarctation following surgery has been documented after all types of surgical repair such as resection with end-to-end anastomosis, subclavian flap angioplasty, prosthetic patch repair, subclavian artery turn-down method and interposition tube grafts, as reviewed elsewhere.19,24,43 The younger the baby at the time surgery, the greater is the likelihood of recoarctation. The technique of Gruntzig’s balloon angioplasty9–11 was first applied to treat recoarctations following surgery by Singer and his colleagues.13 Subsequently, a number of other cardiologists including the author16,24,36,57-60 performed the balloon angioplasty procedure for the post-surgical recoarctations and showed improvement. It is generally agreed that balloon angioplasty is the first option in the treatment of postsurgical aortic recoarctation. The indications are same as those used for native aortic coarctation, ie, signs of CHF and/or elevated blood pressure along with a peak-to-peak systolic pressure gradient more than 20 mmHg across the coarctation. However, it should be understood that the need to perform balloon angioplasty for recoarctation during the neonatal period is uncommon.
Future Directions
Development of recoarctation both after surgery23,24,27,43 and after balloon angioplasty23,34,36,38-40 is well known. While recoarctation following surgery is independent of the type of surgical repair, the age at surgery and hypoplasia of the aortic arch were identified as risk factors for recoarctation. Risk factors for post-balloon angioplasty re-stenosis were also identified as young age and narrowed isthmus and very small coarcted segment;39–41 these are not too dissimilar to those found for post-surgical recoarctation. Examination of biophysical characteristics61 revealed reduced recoil of the coarcted aortic segment in patients with recoarctation indicating that the elastic properties of the aortic wall may not have been preserved. Cystic medial necrosis62,63 or extension of ductal tissue into the aortic wall64,65 may be the cause for this abnormality. However, true pathophysiologic mechanisms responsible for recoarctation at cellular level have not yet been identified. Once such a knowledge comes to light, suitable treatment algorithms to address the pathophysiology and methods of prevention of restenosis may be developed. In the meanwhile, maintaining coarcted segments open mechanically with stents appears to be an attractive option. However, the stents, which are mostly metallic, do not enlarge as the child grows; this is particularly true in the neonate and young infant, as has been articulated elsewhere.32,55,56,66 Although re-dilatation of the stents is feasible, such re-expansion may not achieve adult size. Accordingly, alternative solutions32,34,55,56,66 may have to be developed and will be reviewed.
Biodegradable Stents
Biodegradable stents are constructed by polyesters, polycarbonates, bacterial-derived polymers, or corrodible metals. The purpose of these stents is to keep the coarcted aortic segment open initially which then dissolve over the next few months. The remaining scaffolding may provide a favorable ratio of the normal aortic tissue to abnormal tissue which may prevent significant re-narrowing. This concept was tested in some animal models. However, clinical trials involving large number of patients to provide information on the feasibility, safety and efficacy of this approach are awaited. Successful use of a bio-absorbable metal stent in a neonate with critical recoarctation67 is encouraging. Other issues such as radial strength, body’s inflammatory response, possible toxicity of the dissolving stent material, and others should also be investigated. Furthermore, stent delivery systems should also be made small enough to be practical for use in the neonate and young infant.
Growth Stents
Modifying the existing stents by an “open-ring” or by suturing laser-cut longitudinal halves of metallic stents with biodegradable sutures was adopted by some investigators. Trials in animal models and in small number of infant aortic coarctations68 appear encouraging. However, the overall results68 do not appear to be satisfactory mostly secondary to the need for multiple re-interventions. Clinical trials with this type of stent modification in larger numbers of neonates and young children are required to demonstrate the usefulness of this method and to confirm the efficacy of this concept.
Dilatable Stents
Use of stents such as Valeo Biliary Pre-mounted Re-dilatable Stents (Edwards Life Sciences, California, USA)69 which can be dilated up to 20 mm is another alternative and should be explored in the future. Availability of such stents, especially if they can be introduced through small sheaths may resolve growth issues related to stents in growing children.
Summary
CoA in neonates and infants tend to have high recurrence whether treated by surgery or by balloon angioplasty. While causes of these recurrences have been studied, the true cellular mechanisms for recurrence have not been identified. Consequently, stents may resolve such a problem; however, the stents do not expand as the infant grows. Therefore, innovative approaches such as bio-absorbable, growth, and dilatable stents should be investigated.
Conclusion
Of the available options, namely, surgery, balloon angioplasty and stents, the current evidence suggests that surgical correction is the best for treating neonates and young infants with native coarctation of the aorta. Further research on the utility of bio-degradable, growth, and dilatable stents in addressing aortic coarctation are necessary. Balloon angioplasty is the treatment of choice for post-surgical aortic recoarctations.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Rao PS Coarctation of the aorta. In: Secondary Forms of Hypertension, Ram CVS editors.
2. Rao PS, Seib PM. Coarctation of the aorta. Medscape Reference. Available from: http://emedicine.medscape.com/article/895502-overview.
3. Rao PS. Diagnosis and management of acyanotic heart disease: part I - obstructive lesions. Indian J Pediatr. 2005;72:496–502.
4. Rao PS. Congenital heart defects – A review. In: Rao PS, editor. Congenital Heart Disease - Selected Aspects, ISBN 978-953-307-472-6; Rijeka, Croatia: InTech;January 2012:3–44. Chapter 1.
5. Rao PS. Coarctation of the aorta. In: Rao PS, Vidyasagar D, editors. Perinatal Cardiology: A Multidisciplinary Approach. Minneapolis, MN: Cardiotext Publishing;2015. Chapter 38.
6. Crafoord O, Nylin G. Congenital coarctation of the aorta and its surgical treatment. J Thorac Cardiovasc Surg. 1945;14:347–361.
7. Gross RE. Surgical correction for coarctation of the aorta. Surgery. 1945;18:673–678.
8. Dotter CT, Judkins MP. Transluminal treatment of arteriosclerotic obstruction: description of a new technique and a preliminary report of its application. Circulation. 1964;30:654–670. doi:10.1161/01.CIR.30.5.654
9. Grüntzig A. Die perkutane Rekanalisation chronischer arterieller Verschlüsse (Dotter-Prinzip) mit einem neuen doppellumigen Dilatationskatheter. [Percutaneous recanalization of chronic arterial occlusions (Dotter principle) with a new double lumen dilatation catheter (author’s translation)]. Rofo. 1976;124:80–86. German. doi:10.1055/s-0029-1230286
10. Gruntzig AR. Transluminal dilatation of coronary artery stenosis. Lancet. 1978;1:263. doi:10.1016/S0140-6736(78)90500-7
11. Gruntzig AR, Kuhlmann V, Vetter W, Lutolf V, Meier B, Siegenthaler W. Treatment of renovascular hypertension with percutaneous transluminal dilatation of a renal artery stenosis. Lancet. 1978;1:801–802. doi:10.1016/S0140-6736(78)93000-3
12. Sos T, Sniderman KW, Rettek-Sos B, Strupp A, Alonso DR. Percutaneous transluminal dilatation of coarctation of the thoracic aorta-postmortem. Lancet. 1979;2:970–971. doi:10.1016/S0140-6736(79)92675-8
13. Singer MI, Rowan M, Dorsey TJ. Transluminal aortic balloon angioplasty for coarctation of the aorta in the newborn. Am Heart J. 1982;103:131–132. doi:10.1016/0002-8703(82)90539-7
14. Sperling DR, Dorsey TJ, Rowen M, Gazzaniga AB. Percutaneous transluminal angioplasty of congenital coarctation of the aorta. Am J Cardiol. 1983;51:562–564. doi:10.1016/S0002-9149(83)80097-6
15. Tynan M, Finley JP, Fontes V, Hess J, Kan J. Balloon angioplasty for treatment of native coarctation: results of valvuloplasty and angioplasty of congenital anomalies registry. Am J Cardiol. 1990;65:790–792. doi:10.1016/0002-9149(90)91389-N
16. Hellenbrand WE, Allen HD, Golinko RJ, et al. Balloon angioplasty of aortic recoarctation: results of valvuloplasty and angioplasty of congenital anomalies registry. Am J Cardiol. 1990;65:793–797. doi:10.1016/0002-9149(90)91390-R
17. Rao PS, Mardini MK, Najjar HN. Relief of coarctation of the aorta without thoracotomy: the experience with percutaneous balloon angioplasty. Ann Saudi Med. 1986;6:193–203. doi:10.5144/0256-4947.1986.193
18. Rao PS. Transcatheter treatment of pulmonary stenosis and coarctation of the aorta: experience with percutaneous balloon dilatation. Br Heart J. 1986;56:250–258. doi:10.1136/hrt.56.3.250
19. Rao PS. Balloon angioplasty for coarctation of the aorta in infancy. J Pediat. 1987;110:713–718. doi:10.1016/S0022-3476(87)80008-2
20. Rao PS, Thapar MK, Galal O, Wilson AD. Follow-up results of balloon angioplasty of native coarctation in neonates and infants. Am Heart J. 1990;120:1310–1314. doi:10.1016/0002-8703(90)90241-O
21. Waldhausen JA, Nahrwold DL. Repair of coarctation of the aorta with a subclavian flap. J Thorac Cardiovasc Surg. 1966;51:532–533. doi:10.1016/S0022-5223(19)43319-9
22. van Heurn LW, Wong CM, Spiegelhalter DJ, et al. Surgical treatment of aortic coarctation in infants younger than three months: 1985 to 1990. Success of extended end-to-end arch aortoplasty. J Thorac Cardiovasc Surg. 1994;107:74–85. doi:10.1016/S0022-5223(94)70456-2
23. Rao PS. Balloon angioplasty of native aortic coarctation. In: Rao PS, editor. Transcatheter Therapy in Pediatric Cardiology. New York, NY: Wiley-Liss; 1993:153–196.
24. Rao PS. Balloon angioplasty of aortic recoarctation following previous surgery. In: Rao PS, editor. Transcatheter Therapy in Pediatric Cardiology. New York, NY: Wiley-Liss; 1993:197–212.
25. Wood AE, Javadpour H, Duff D, Oslizlok P, Walsh K. Is extended arch aortoplasty the operation of choice for infant aortic coarctation? Results of 15 years’ experience in 181 patients. Ann Thorac Surg. 2004;77:
26. Wright GE, Nowak CA, Goldberg CS, Ohye RG, Bove EL, Rocchini AP. Extended resection and end-to-end anastomosis for aortic coarctation in infants: results of a tailored surgical approach. Ann Thorac Surg. 2005;80:1453–1459. doi:10.1016/j.athoracsur.2005.04.002
27. Hager A, Schreiber C, Nutzl S, Hess J. Mortality and restenosis rate of surgical coarctation repair in infancy: a study of 191 patients. Cardiology. 2009;112:36–41. doi:10.1159/000137697
28. Attia IM, Lababidi Z. Transumbilical balloon coarctation angioplasty. Am Heart J. 1984;116:1623–1624. doi:10.1016/0002-8703(88)90752-1
29. Rao PS, Wilson AD, Brazy J. Transumbilical balloon coarctation angioplasty in a neonate with critical aortic coarctation. Am Heart J. 1992;124:1622–1624. doi:10.1016/0002-8703(92)90082-7
30. Rao PS. Role of interventional cardiology in neonates: part II - Balloon angioplasty/valvuloplasty. Neonatol Today. 2007;2(10):1–12.
31. Alyousef S, Khan A, Nihill M, Lababidi Z, Mullins C. Perkutane transvenose antegrade ballonangioplastic bei aortenisthmusstenose. Herz. 1988;13:36–40.
32. Rao PS. Current status of balloon angioplasty for neonatal and infant aortic coarctation. Progress Pediat Cardiol. 2001;14:35–44. doi:10.1016/S1058-9813(01)00114-X
33. Rao PS. Balloon angioplasty of native coarctations (Letter). Am J Cardiol. 1990;66:1401. doi:10.1016/0002-9149(90)91185-9
34. Rao PS, Jureidini SB, Balfour IC, Singh GK, Chen SC. Severe aortic coarctation in infants less than 3 months: successful palliation by balloon angioplasty. J Invasive Cardiol. 2003;15:202–208.
35. Rao PS. Should balloon angioplasty be used as a treatment of choice for native aortic coarctations? J Invasive Cardiol. 1996;8:301–308.
36. Rao PS, Chopra PS. Role of balloon angioplasty in the treatment of aortic coarctation. Ann Thorac Surg. 1991;52:621–631. doi:10.1016/0003-4975(91)90961-O
37. Rao PS. Should balloon angioplasty be used instead of surgery for native aortic coarctation? (Editorial). Br Heart J. 1995;74:578–579. doi:10.1136/hrt.74.6.578
38. Reddington AN, Booth P, Shore DF, Rigby ML. Primary balloon dilatation of coarctation in neonates. Br Heart J. 1990;64:277–281. doi:10.1136/hrt.64.4.277
39. Rao PS, Galal O, Smith PA, Wilson AD. Five-to-nine-year follow-up results of balloon angioplasty of native aortic coarctation in infants and children. J Am Coll Cardiol. 1996;27:462–470. doi:10.1016/0735-1097(95)00479-3
40. Rao PS, Thapar MK, Kutayli F, Carey P. Causes of recoarctation following balloon angioplasty of unoperated aortic coarctations. J Am Coll Cardiol. 1989;13:109–115. doi:10.1016/0735-1097(89)90557-3
41. Rao PS, Koscik R. Validation of risk factors in predicting recoarctation following initially successful balloon angioplasty of native aortic coarctations. Am Heart J. 1995;130:116–121. doi:10.1016/0002-8703(95)90245-7
42. Rao PS, Chopra PS, Koscik R, Smith PA, Wilson AD. Surgical versus balloon therapy for aortic coarctation in infants ≤ 3 months old. J Am Coll Cardiol. 1994;23:1479–1483. doi:10.1016/0735-1097(94)90395-6
43. Pinzon JL, Burrows PE, Benson LS, et al. Repair of coarctation of the aorta in children: postoperative morphology. Radiology. 1991;180:199–203. doi:10.1148/radiology.180.1.2052694
44. Rao PS. Stents in the treatment of aortic coarctation (Editorial). J Am Coll Cardiol. 1997;30:1853–1855. doi:10.1016/s0735-1097(97)00407-5
45. Rao PS. Stents in the management of congenital heart disease in the pediatric and adult patients. Indian Heart J. 2001;53:714–730.
46. Rao PS. Coarctation of the aorta. Curr Cardiol Rep. 2005;7:425–434. doi:10.1007/s11886-005-0060-0
47. Doshi AR, Rao PS. Coarctation of aorta-management options and decision making. Pediatr Therapeut. 2012;S5:006. doi:10.4172/2161-0665.S5-006
48. Sahu R, Rao PS. Transcatheter stent therapy in children: an update. Pediatr Therapeut. 2012;S5:001. doi:10.4172/2161-0665.S5-001
49. Rao PS. Stents in the management of vascular obstructive lesions associated with congenital heart disease. In: Vijayalakshmi IB, editor. Cardiac Catheterization and Imaging (From Pediatrics to Geriatrics). New Delhi, India: Jaypee Publications; 2015:573–598.
50. Rao PS. Percutaneous management of aortic coarctation. In: Vijayalakshmi IB, editor. Cardiac Catheterization and Imaging (From Pediatrics to Geriatrics). New Delhi, India: Jaypee Publications; 2015:433–471.
51. Saluhuddin N, Wilson AD, Rao PS. An unusual presentation of coarctation of the aorta in infancy: role of balloon angioplasty in the critically ill infant. Am Heart J. 1991;122:1772–1775. doi:10.1016/0002-8703(91)90300-7
52. Rao PS. Balloon angioplasty for native aortic coarctation in neonates and infants. Cardiol Today. 2005;9:94–99.
53. Francis E, Gayathri S, Vaidyanathan B, Kannan BRJ, Krishna Kumar R. Emergency balloon dilation or stenting of critical coarctation of aorta in newborn and infants: an effective interim palliation. Ann Pediatr Card. 2009;2:111–115. doi:10.4103/0974-2069.58311
54. Mohan UR, Danon S, Levi D, Connolly D, Moore JW. Stent implantation for coarctation of the aorta in children. JACC Cardiovasc Interv. 2009;2:877–883. doi:10.1016/j.jcin.2009.07.002
55. Rao PS. Transcatheter interventions in critically ill neonates and infants with aortic coarctation. Ann Pediatr Card. 2009;2:116–119. doi:10.4103/0974-2069.58312
56. Rao PS. Stents in the management of aortic coarctation in young children (Editorial). J Am Coll Cardiol. 2009;2:884–886. doi:10.1016/j.jcin.2009.07.001
57. Kan JS, White RL
58. Hess J, Mooyaart EL, Busch HJ, et al. Percutaneous transluminal balloon angioplasty in restenosis of coarctation of the aorta. Br Heart J. 1986;55:459–461. doi:10.1136/hrt.55.5.459
59. Rao PS, Wilson AD, Chopra PS. Immediate and follow-up results of balloon angioplasty of postoperative recoarctation in infants and children. Am Heart J. 1990;120:1315–1320. doi:10.1016/0002-8703(90)90242-P
60. Siblini G, Rao PS, Nouri S, Ferdman B, Jureidini SB, Wilson AD. Long-term follow-up results of balloon angioplasty of postoperative aortic recoarctation. Am J Cardiol. 1998;81:61–67. doi:10.1016/S0002-9149(97)00805-9
61. Rao PS, Waterman B. Relation of biophysical response of coarcted aortic segment to balloon dilatation with development of recoarctation following balloon angioplasty of native coarctation. Heart. 1998;79:407–411. doi:10.1136/hrt.79.4.407
62. Isner JM, Donaldson RF, Fulton D, Bhan I, Payne DD, Cleveland RJ. Cystic medial necrosis in coarctation of the aorta: a potential factor contributing to adverse consequences observed after percutaneous balloon angioplasty of coarctation sites. Circulation. 1987;75:689–695. doi:10.1161/01.CIR.75.4.689
63. Ho SY, Somerville J, Yip WCL, Anderson RH. Transluminal balloon dilatation of resected coarcted segments of thoracic aorta histologic study and clinical implications. Int J Cardiol. 1988;19:99–105. doi:10.1016/0167-5273(88)90195-7
64. Elzenga NJ, Gittenberger-de Groot AC, Oppenheimer-Dekker A. Coarctation and other obstructive aortic arch anomalies: their relationship to the ductus arteriosus. Int J Cardiol. 1986;13:289–308. doi:10.1016/0167-5273(86)90116-6
65. Russell GA, Berry PJ, Watterson K, Dhasmana JP, Wisheart JD. Patterns of ductal tissue in coarctation of the aorta in the first three months of life. J Thorac Cardiovasc Surg. 1991;102:596–601.
66. Rao PS. Future directions in the management of aortic coarctation in young patients. Pediat Therapeut. 2014;4:e125. doi:10.4172/2161-0665.1000e125
67. Schranz D, Zartner P, Michel-Behnke I, Akintürk H. Bioabsorbable metal stents for percutaneous treatment of critical recoarctation of the aorta in a newborn. Catheter Cardiovasc Interv. 2006;67:671–673. doi:10.1002/(ISSN)1522-726X
68. Ewert P, Peters B, Nagdyman N, Miera O, Kühne T, Berger F. Early and mid-term results with the Growth Stent–a possible concept for transcatheter treatment of aortic coarctation from infancy to adulthood by stent implantation? Catheter Cardiovasc Interv. 2008;71:120–126. doi:10.1002/(ISSN)1522-726X
69. Shepherd E, Connolly GM, Morgan G. Using the Valeo dilatable stent in coarctation stenting for small children: expanding the inclusion criteria for coarctation stenting? BMJ Case Rep. 2013;2013. doi:10.1136/bcr-2013-202095
70. Rao PS, Solymar L. Transductal balloon angioplasty for coarctation of the aorta in the neonate: preliminary observations. Am Heart J. 1988;116(6,Part 1):1558–1563. doi:10.1016/0002-8703(88)90743-0
71. Rao PS. Role of interventional cardiology in neonates: part II - Balloon angioplasty/valvuloplasty. Congenit Cardiol Today. 2008;6(1):1–14.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
