Back to Journals » Risk Management and Healthcare Policy » Volume 8
Necrotizing fasciitis: risk factors of mortality
Authors Khamnuan P, Chongruksut W , Jearwattanakanok K, Patumanond J , Yodluangfun S, Tantraworasin A
Received 18 November 2014
Accepted for publication 5 January 2015
Published 16 February 2015 Volume 2015:8 Pages 1—7
DOI https://doi.org/10.2147/RMHP.S77691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Frank Papatheofanis
Patcharin Khamnuan,1,2 Wilaiwan Chongruksut,3 Kijja Jearwattanakanok,4 Jayanton Patumanond,5 Suttida Yodluangfun,6 Apichat Tantraworasin3
1Clinical Epidemiology Program, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand; 2Department of Nursing, Phayao Hospital, Phayao, Thailand; 3Department of Surgery, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand; 4Department of Surgery, Nakornping Hospital, Chiang Mai, Thailand; 5Clinical Epidemiology Unit, Clinical Research Center, Faculty of Medicine, Thammasat University, Pathum Thani, Thailand; 6Department of Nursing, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand
Background: Necrotizing fasciitis (NF) is a serious infection of skin and soft tissues that rapidly progresses along the deep fascia. It becomes a fatal soft tissue infection with high mortality rate if treatment is delayed. Early diagnosis for emergency surgical debridement and broad-spectrum antibiotic therapy were the optimal treatments to reduce the mortality rate of NF.
Objective: The aim of this study was to identify risk factors that increased the mortality rate in patients with NF under routine clinical practices.
Methods: A retrospective cohort study was performed at three general hospitals located in northern Thailand. All medical records of patients with surgically confirmed NF treated between January 2009 and December 2012 were reviewed. Clinical predictors for mortality were analyzed using multivariable risk regression analysis.
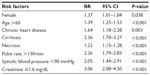
Results: Of a total of 1,504 patients with a diagnosis of NF, 19.3% (n=290) died in hospital and 80.7% (n=1,214) survived. From multivariable analysis, being female (risk ratio [RR] =1.37, 95% confidence interval [CI] =1.01–1.84); age >60 (RR =1.39, 95% CI =1.25–1.53); having chronic heart disease (RR =1.64, 95% CI =1.18–2.28), cirrhosis (RR =2.36, 95% CI =1.70–3.27), skin necrosis (RR =1.22, 95% CI =1.15–1.28), pulse rate >130/min (RR =2.26, 95% CI =1.79–2.85), systolic BP <90 mmHg (RR =2.05, 95% CI =1.44–2.91), and serum creatinine ≥1.6 mg/dL (RR =3.06, 95% CI =2.08–4.50) were risk factors for mortality.
Conclusion: Prognostic factors for mortality in NF patients included being female; age >60; or having chronic heart disease, cirrhosis, skin necrosis, pulse rate >130/min, systolic BP <90 mmHg, and serum creatinine ≥1.6 mg/dL. Thus, disease progression to mortality may occur in such patients presenting one of these risk factors. Further examination or close monitoring for systemic involvement may be advantageous to reduce morbidity and mortality.
Keywords: clinical predictors, risk factor, mortality, necrotizing fasciitis
Introduction
Necrotizing fasciitis (NF) is commonly known as flesh-eating disease. The infection involves necrosis of the subcutaneous tissue and fascia first described by Wilson in the 1950s.1 NF is a disease characterized by a rapidly progressing destruction of tissue and systemic toxicity, and delayed treatment can lead to an infection with a high mortality rate.2 The incidence of NF has been varyingly reported worldwide, 0.4 cases per 100,000 population in Canada3 and 1.3 cases per 100,000 population in Florida, USA.4 The mortality rates reported of 15% to 36% in Ohio, USA (1989–1994);5 Taiwan (1995–2006);6 Chicago, USA (1999–2002);7 and the Philippines (2004–2007).8 In northern Thailand, Hongladaromp et al9 reported the incidence of NF was 7.45 cases per 100,000 population, and the mortality rate ranged from 5.9% to 22.1%.9,10
Emergency surgical debridement and broad-spectrum antibiotic therapy remain the most appropriate treatments to reduce the mortality rate of NF. Delayed recognition and treatment can cause the disease to progress and increases the risk of poor outcomes, so it is very important to diagnose the disease at an early stage and treat rapidly. Previous studies have reported independent risk factors for mortality among NF patients including being female,11 having advanced age,6,7,11–13 diabetes mellitus (DM),4 heart disease,14,15 liver cirrhosis,6,12,15 serum creatinine level 2 mg/dL,6,11,12,14 white blood cell count >30,000/mm3,14 hypoalbuminemia,8 presence of hemorrhagic bleb,16,17 and skin necrosis.17 All studies presented varying prevailing epidemiology and microbiology in each place.
The aim of this study was to evaluate risk factors for mortality in a large group of patients with NF from three general hospitals in northern Thailand undergoing routine clinical practices. From clinical findings the identified risk factors can then be used as predictors in order to assist surgeons into taking preventative measures at an early stage of NF. However, some clinical data were unavailable for this study such as anaerobic bacteria culture and some laboratory findings that might be possible risk factors to predict mortality.
Patients and methods
This study was a retrospective cohort study. The medical records of patients with surgically confirmed NF were registered between January 2009 and December 2012 at three general hospitals located in northern Thailand (Chiang Rai, KamphaengPhet, and Phayao Provinces).
NF was defined by the presence of extensive necrosis involving at least the fascia and subcutaneous tissue, including myonecrosis.18 The gray necrotic fascia and myonecrosis were detected intraoperatively by surgeons and used to identically follow Practice Guidelines for the Diagnosis of Skin and Soft Tissue Infections by the Infectious Diseases Society of America.19 The definition of mortality was death at admission or 28 days after surgical treatment. Clinical data and demographic characteristics including sex, age, body mass index (BMI), education, occupation, underlying disease, vital signs within the first day of admission and after 48–72 hours were retrieved from inpatient charts. Important data associated with the investigation and treatment of NF (wound appearance, site of infection, organisms involved, and laboratory data within the first day of admission and after 48–72 hours, surgical intervention, and outcome) were also extracted from the medical records.
In total 1,504 patients were enrolled in this study. All cases were assessed and treated with broad-spectrum antibiotics by emergency physicians. Patients were investigated, evaluated, and provided proper emergency surgical treatment. The surgical interventions included incision, drainage, debridement of the necrotic fascia or muscle, and amputation. All debris tissue was dissected, and in some of the cases tissue culture was performed. Anaerobic organism cultures were not performed because of the limitation of the culture process. Aerobic organism cultures were performed in some of the cases, but not all, depending on surgeon request. Although we have a standard practice guideline for taking tissue cultures or pus cultures in all infected specimens, some surgeons did not follow this guideline. The reasons were shortage of culture instruments or culture transferring process (loss of specimen). Furthermore, the data recording process in each provincial hospital was not good enough. Some of the important data such as tissue culture, blood culture, or pus culture might be lost.
Patients were divided into two groups: mortality and survival. Continuous variables were analyzed by Student’s t-test or Wilcoxon rank sum test and assessed as mean or median with standard deviation or interquartile range, depending on the distribution of data. Differences in proportion were analyzed by Fisher’s exact test and assessed as count and percentage. Firstly, the univariable risk regression generalized linear model and cluster hospitals were used to identify the possible independent risk factors for mortality. The clinically significant variables and all variables that have P-value less than 0.01 were included in the multivariable analysis model. Secondly, multivariable risk regression analysis with generalized linear model cluster hospitals and step-backward method were used to identify the independent risk factors for mortality. A P-value ≤0.05 was considered statistically significant.
This study was approved by the Ethics Committee of the Chiang rai Prachanukroh Hospital and Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. Date of approved 22 January 2013 is number 032/2013, Research ID: 1461/Study Code: COM-13-1461-EX.
Results
The study enrolled 1,504 patients diagnosed with NF. The mortality rate was 19.3% (n=290) and the survival rate was 80.7% (n=1,214). Among the fatalities, 92.8% (n=269) died at admission and 7.2% (n=21) died after discharge from the hospital but within 28 days after surgery. Organisms cultured from blood and wounds are shown in Table 1. Blood cultures were performed in 428 (28.6%) of 1,502 patients. A positive isolated blood culture was found in 73 patients (17.1%). No bacteria were found in 355 patients (82.9%). Wound cultures were performed in 729 (48.5%) of 1,503 patients. Positive isolated specimens comprised 469 patients (64.3%), and negative ones comprised 260 patients (35.7%). Data for two patients in blood culture and one patient in wound culture were omitted owing to a lack of medical records. Blood culture was performed in 144 patients (9.6%), wound culture was performed in 450 patients (29.9%), and both blood and wound cultures were performed in 285 patients (19.0%). Overall cultures were done in 879 patients (58.4%). Data for two patients who had blood cultures and one patient who had a wound culture were omitted owing to a lack of medical records. The number of patients who had mixed infected organism (gram positive organism and gram negative organism) in the wound culture or the blood culture were 39 patients (5.3%) and 3 patients (0.7%), respectively. The number of patients who have multiple infected organism (more than one gram positive or gram negative organism) in wound culture and blood culture are 47 patients (6.4%) and 3 patients (0.7%), respectively. Streptococcus pyogenes was the most common gram positive organism in both blood culture (3.7%) and wound culture (22.9%) in the mortality group and in both blood culture (2.1%) and wound culture (15.9%) in the survival group. The overall percentage of S. pyogenes infection was 43.2% in the mortality group and 39.2% in the survival group. Escherichia coli was the most common gram negative organism in both blood culture (3.7%) and wound culture (13.7%) in the mortality group and wound culture (5.4%) in the survival group. The overall percentage of E. coli infection was 17.8% in the mortality group and 27.4% in the survival group. However, there were no statistically significant differences in infected organism in either blood culture or wound culture between both groups (P-value >0.05).
Different characteristics between the groups included sex, age, BMI, education, occupation, underlying morbidity such as chronic heart disease, cirrhosis, hypertension, gout, and wound appearance such as erythema, hemorrhagic bleb, severe pain and site of infection, as shown in Table 2. This table demonstrates that female sex age more than 60 years, patients who have no education, or elderly patients who stay at home most of the time are risk factors for mortality in NF patients by univariable analysis. Most of the laboratory findings and vital signs either on admission or within 48–72 hours after admission of patients in the mortality group were statistically significantly worse than those in the survival group; they include higher polymorphonuclear cell (PMN) predominant, higher serum creatinine, lower bicarbonate (more acidosis), lower total serum protein, higher pulse rate, and lower systolic blood pressure. Furthermore, the number of patients in the mortality group presenting with severe sepsis was significantly higher than that in the survival group, as shown in Table 3.
The clinically significant variables such as skin necrosis and all variables that have P-value of less than 0.01 are included in the multivariable analysis model. Multivariable risk regression analysis with generalized linear model cluster hospitals and step-backward method demonstrated that the predictors of mortality among patients with NF were being female (risk ratio [RR] =1.37, 95% confidence interval [CI] =1.01–1.84, P=0.038), age >60 (RR =1.39, 95% CI =1.25–1.53, P<0.001), having chronic heart disease (RR =1.64, 95% CI =1.18–2.28, P=0.003), cirrhosis (RR =2.36, 95% CI =1.70–3.27, P<0.001), skin necrosis (RR =1.22, 95% CI =1.15–1.28, P<0.001), pulse rate >130/min (RR =2.26, 95% CI =1.79–2.85, P<0.001), systolic blood pressure <90 mmHg (RR =2.05, 95% CI =1.44–2.91, P<0.001), and serum creatinine ≥1.6 mg/dL (RR =3.06, 95% CI =2.08–4.50, P<0.001), as shown in Table 4.
Discussion
NF is an important surgical infection, and there is a high incidence of it in Thailand, especially in the northern part, because people there are mainly farmers and laborers, most of them with low education and practicing low hygiene. Caring for wounds or preventing serious infection is still a problem in northern Thailand’s health care system.
NF is of surgical urgency with a high mortality rate, even with sufficient treatment, with the reported rate of mortality varying from 6% to 36%.5–8,10 The overall mortality rate in this study was 19.3% (290 patients) of 1,504 patients. These patients had adverse outcomes, and although the cases were investigated and treated with broad-spectrum antibiotics by emergency physicians, these patients died rapidly from systemic inflammatory response syndrome (SIRS).5 However, identification of independent risk factors of death could have had some effect on survival and could have assisted surgeons to counter these risk factors as much as possible to achieve a successful outcome of treatment in all patients.
At admission time, this study identified the following risk factors for mortality: being female; age >60 years; having chronic heart disease, liver cirrhosis, skin necrosis, pulse rate >130/min, systolic blood pressure <90 mmHg, and serum creatinine level ≥1.6 mg/dL, and these findings were similar to those of many previous studies.4,7,11,12,14 In this study, sex affected the outcome of treatment and could predict mortality. Females were at greater risk for mortality. This study found that the number of females with a BMI ≥30 was significantly higher than that of males (74.6% versus 38.6%, P-value 0.004). A possible reason was that females have a greater amount of subcutaneous fat than men and are more easily prone to infection. Elliott et al11 reviewed 198 patients between March 1985 and June 1993, and also found that being female was an independent predictor of death. However, some studies have reported that sex did not influence mortality.7,14–16,20
Elderly persons with or without underlying diseases, such as chronic heart disease and liver cirrhosis, are considered as having worse prognostic factors, similar to previous studies reporting that advanced age was associated with an increased risk of mortality.6,7,12,13,21,22 This study found that being more than 60 years old increased the mortality rate significantly. However, some studies found that advanced age had no effect on mortality.5,8,16,17 NF occurred frequently in patients with underlying diseases. Diabetes was the most frequent comorbidity in NF patients. Previous studies reported that poorly controlled DM in NF patients can cause adverse outcomes.23–27 In this study, DM did not demonstrate association in terms of survival. We did not explore in detail blood glucose as this data could not be extracted from the patients medical records. One possible reason is that most of our patients might have well-controlled DM. Therefore, further study should be performed to establish the exact effect of DM on adverse outcome or survival in NF patients.
We also found that other underlying diseases such as chronic heart disease or liver cirrhosis were associated with higher mortality, as did previous studies.6,12,14,15,28 Patients with liver cirrhosis have a higher sensitivity to infection than those without. The mechanism for increased mortality in cirrhotic patients with infection may be immunologic and mechanical. Abnormalities affect immunity in patients with cirrhosis featuring cellular immunity and humoral immunity, T lymphocyte and B lymphocyte dysfunctions caused by malnutrition.29 The abnormal defensive mechanism could be caused by decreasing phagocytic activity of the reticuloendothelial system, impaired function monocyte and incomplete chemotaxis.28 Moreover, we found that skin necrosis was associated with higher mortality as in previous studies.17,30 Infection and toxin-producing bacteria can cause skin necrosis and multiple organ failure. All necrotic tissue, including fascia, must be removed by surgical debridement to reduce the bacteria, and broad-spectrum antibiotics should be administered promptly whenever NF is diagnosed.31
A pulse rate of more than 130 beats per minute and systolic blood pressure of less than 90 mmHg were also associated with increased mortality. The consequences of septic shock occurred in patients with severe systemic infection.32 Septic shock was a typical complication in patients with NF.33 In patients with septic shock, hypotension (a systolic blood pressure below 90 mmHg) was a significant risk factor for organ dysfunction and death, as reported in previous studies.3,15,16,34 Clinical suspicion must lead to promptly maintained fluid resuscitation with intravenous broad-spectrum antibiotics and aggressive surgical debridement.33 However, in patients with NF and septic shock, to maintain these changes in the circulation may lead to acute kidney failure and elevate serum creatinine.35
Referring to laboratory findings, increased serum creatinine has been an associated risk factor of death in NF, which has also been reported by many previous studies.6,11,12,14,17 Increased creatinine levels can be used to predict impaired renal function most likely associated with septic shock, and may indicate renal failure. Acute renal failure in NF patients was a life-threatening condition when treatment was delayed or not cautious enough.17 All patients with a confirmed, progressive increase in their serum creatinine level should consult with a nephrologist for dialysis.36 Regarding the hospital variable, all the three hospitals are provincial hospitals, and, therefore, the health care system is the same with no difference in hospital conditions. Also, the mortality of NF did not differ among the hospitals. Although the P-value of the hospital variable is 0.021, we set a P-value of 0.01 as statistically significant difference, and, therefore, there is no difference in mortality among the hospitals.
In the review of literature, prognostic tools to evaluate the severity of NF patients on admission included the LRINEC (laboratory risk indicator for NF) score, composed of six marker variables, including C-reactive protein, total white blood cell count, hemoglobin, serum sodium, serum creatinine, and serum glucose;37and APACHE II (acute physiology and chronic health evaluation II) scores were used as a prognostic scoring system for critical care including the patient’s vital signs (temperature, mean arterial pressure, heart rate, and respiratory rate), oxygenation (A-PaO2 [FiO2 >50%] or PaO2 [FiO2 <50%], metabolic parameters (sodium, potassium, creatinine, bicarbonate concentrations [Arterial pH or HCO3], hematocrit, white blood cell count), and Glasgow coma score and calculates a score relating to severity of the disease of a patient who was admitted to the ICU.38
The limitations of this study include the following: we could not apply either of these scoring systems (LRINEC and APACHE II), because this study was a retrospective study, some important data could have been omitted because of insufficient medical records and laboratory limitations in provincial hospitals such as unexamined serum C-reactive protein arterial blood gas in all patients. Unfortunately, some data were not available in the clinical manifestations such as non-recorded Glasgow coma score.
Conclusion
Risk factors of mortality in patients with NF included being female; age >60 years; having chronic heart disease, liver cirrhosis, skin necrosis, pulse rate >130/min, systolic blood pressure <90 mmHg, and serum creatinine level ≥1.6 mg/dL. Thus, patients presenting any clinical predictors should direct concern toward progression of disease and might be considered for early investigation or close monitoring to prevent morbidity and mortality as much as possible. In addition, health care promotion should be directed toward preventing adverse events and early detection of NF.
Acknowledgments
The authors thank the authorities of Chiang rai Prachanukroh Hospital, KamphaengPhet Hospital, and Phayao Hospital for their support. The study was supported by a grant from the Faculty of Medicine and the Graduate School, Chiang Mai University, Chiang Mai, Thailand.
Disclosure
The authors report no conflict of interest in this work.
References
Wilson B. Necrotizing fasciitis. Am Surg. 1952;18(4):416–431. | |
Cainzos M, Gonzalez-Rodriguez FJ. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433–439. | |
Kaul R, McGeer A, Low DE, Green K, Schwartz B. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med. 1997;103(1):18–24. | |
Mulla ZD, Gibbs SG, Aronoff DM. Correlates of length of stay, cost of care, and mortality among patients hospitalized for necrotizing fasciitis. Epidemiol Infect. 2007;135(5):868–876. | |
McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995; 221(5):558–563; discussion 563–555. | |
Bair MJ, Chi H, Wang WS, Hsiao YC, Chiang RA, Chang KY. Necrotizing fasciitis in southeast Taiwan: clinical features, microbiology, and prognosis. Int J Infect Dis. 2009;13(2):255–260. | |
Dworkin MS, Westercamp MD, Park L, McIntyre A. The epidemiology of necrotizing fasciitis including factors associated with death and amputation. Epidemiol Infect. 2009;137(11):1609–1614. | |
Salvador VB, San Juan MD, Salisi JA, Consunji RJ. Clinical and microbiological spectrum of necrotizing fasciitis in surgical patients at a Philippine university medical centre. Asian J Surg. 2010;33(1):51–58. | |
Hongladaromp C, Chareonsil B, Phadhana-anake O. Predictors on mortality from necrotizing fasciitis in Sawanpracharak Hospital, NakhonSawan, Thailand. Chiang Mai Med J. 2009;48(4):135–142. | |
Awsakulsutthi S. A retrospective review of necrotizing fasciitis in Thammasat University Hospital. J Med Assoc Thai. 2010;93(Suppl 7):S246–S253. | |
Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann Surg. 1996;224(5):672–683. | |
Huang KF, Hung MH, Lin YS, et al. Independent predictors of mortality for necrotizing fasciitis: a retrospective analysis in a single institution. J Trauma. 2011;71(2):467–473; discussion 473. | |
Golger A, Ching S, Goldsmith CH, Pennie RA, Bain JR. Mortality in patients with necrotizing fasciitis. Plast Reconstr Surg. 2007;119(6):1803–1807. | |
Anaya DA, McMahon K, Nathens AB, Sullivan SR, Foy H, Bulger E. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg. 2005;140(2):151–157; discussion 158. | |
Chen IC, Li WC, Hong YC, Shie SS, Fann WC, Hsiao CT. The microbiological profile and presence of bloodstream infection influence mortality rates in necrotizing fasciitis. Crit Care. 2011; 15(3):R152. | |
Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med. 2008;26(2):170–175. | |
Ruiz-Tovar J, Cordoba L, Devesa JM. Prognostic factors in Fournier gangrene. Asian J Surg. 2012;35(1):37–41. | |
Wong CH, Wang YS. The diagnosis of necrotizing fasciitis. Curr Opin Infect Dis. 2005;18(2):101–106. | |
Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52. | |
Kuo Chou TN, Chao WN, Yang C, Wong RH, Ueng KC, Chen SC. Predictors of mortality in skin and soft-tissue infections caused by Vibrio vulnificus. World J Surg. 2010;34(7):1669–1675. | |
Kalaivani V, Hiremath BV, Indumathi VA. Necrotising soft tissue infection-risk factors for mortality. J Clin Diagn Res. 2013;7(8):1662–1665. | |
Anaya DA, Bulger EM, Kwon YS, Kao LS, Evans H, Nathens AB. Predicting death in necrotizing soft tissue infections: a clinical score. Surg Infect (Larchmt). 2009;10(6):517–522. | |
Oncul O, Erenoglu C, Top C, et al. Necrotizing fasciitis: a life-threatening clinical disorder in uncontrolled type 2 diabetic patients. Diabetes Res Clin Pract. 2008;80(2):218–223. | |
Hsu JC, Shen SH, Yang TY, Chen PH, Huang KC, Tsai YH. Necrotizing fasciitis and sepsis caused by and in diabetic patients. Biomed J. Epub September 2, 2014. | |
Doran H, Catrina E, Mihalache O, Patrascu T. [The necrotizing fasciitis of the leg in diabetic patients]. Chirurgia (Bucur). 2007;102(2):169–174. Romanian. | |
Demirag B, Tirelioglu AO, Sarisozen B, Durak K. [Necrotizing fasciitis in the lower extremity secondary to diabetic wounds]. Acta Orthop Traumatol Turc. 2004;38(3):195–199. Turkish. | |
Mundigler G, Geppert A, Henk C, Girsch W, Siostrzonek P. Extensive necrotizing fasciitis in a diabetic patient. Wien Klin Wochenschr. 1998;110(12):446–448. | |
Cheng NC, Tai HC, Tang YB, Chang SC, Wang JT. Necrotising fasciitis: clinical features in patients with liver cirrhosis. Br J Plast Surg. 2005;58(5):702–707. | |
Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;124(3):1016–1020. | |
Tilkorn DJ, Citak M, Fehmer T, et al. Characteristics and differences in necrotizing fasciitis and gas forming myonecrosis: a series of 36 patients. Scand J Surg. 2012;101(1):51–55. | |
Headley AJ. Necrotizing soft tissue infections: a primary care review. Am Fam Physician. 2003;68(2):323–328. | |
Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530–538. | |
Puvanendran R, Huey JC, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55(10):981–987. | |
Tsai YH, Huang KC, Shen SH, Hsu WH, Peng KT, Huang TJ. Microbiology and surgical indicators of necrotizing fasciitis in a tertiary hospital of southwest Taiwan. Int J Infect Dis. 2012;16(3):e159–e165. | |
Su YC, Chen HW, Hong YC, Chen CT, Hsiao CT, Chen IC. Laboratory risk indicator for necrotizing fasciitis score and the outcomes. ANZ J Surg. 2008;78(11):968–972. | |
Mendelssohn DC, Barrett BJ, Brownscombe LM, et al. Elevated levels of serum creatinine: recommendations for management and referral. CMAJ. 1999;161(4):413–417. | |
Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535–1541. | |
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.