Back to Journals » OncoTargets and Therapy » Volume 12
NCOA1–ALK: a novel ALK rearrangement in one lung adenocarcinoma patient responding to crizotinib treatment
Authors Cao Q , Liu Z, Huang Y, Qi C, Yin X
Received 26 October 2018
Accepted for publication 3 January 2019
Published 7 February 2019 Volume 2019:12 Pages 1071—1074
DOI https://doi.org/10.2147/OTT.S192367
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Qi Cao,1 Zhiguang Liu,1 Yanhua Huang,1 Chuang Qi,2 Xiaowei Yin1
1Department of Respiratory and Critical Care Medicine, Changzhou Second Affiliated People’s Hospital of Nanjing Medical University, Changzhou 213003, China; 2Medical Department, 3D Medicines, Shanghai 201100, China
Abstract: The heterogeneity of ALK tyrosine-kinase inhibitor (TKI) responses poses a puzzling question to clinicians. Different variants of ALK rearrangements might be one of the mechanisms explaining this phenomenon. Therefore, identifying specific fusion forms is crucial to clinical practice. This case report assesses the clinical efficacy of an ALK-TKI in a new ALK-rearrangement variant. Next-generation sequencing was performed in tumor tissue of one lung adenocarcinoma patient, and one new fusion form of an ALK rearrangement (NCOA1–ALK) was identified. This patient had progression-free survival >18 months with crizotinib treatment. Our findings provide valuable information about responses to crizotinib in patients with this form of ALK rearrangement and better understanding of ALK-TKI applications.
Keywords: NCOA1–ALK, lung adenocarcinoma, crizotinib
Introduction
Approximately 5% of patients with non-small-cell lung cancer (NSCLC) have accompanying ALK rearrangement.1 Fortunately, compared with ALK-rearrangement-negative NSCLC, ALK-positive patients can achieve impressive clinical outcomes after ALK tyrosine-kinase inhibitor (TKI) therapy.2 Fusion genes consist of the promoter of a partner gene and the entire ALK domain, leading to continuous activation of downstream signaling pathways. Numerous fusion-partner genes have been discovered, such as KIF5B, TFG, HIP1,3 among which EML4 is the most common. However, in ALK-positive patients, the response to ALK-TKIs is heterogeneous for different individuals. Research has indicated that different fusion variants might be one of the mechanisms explaining this phenomenon.3 Even though various new types of ALK rearrangements have been discovered in clinical practice, the response to ALK-TKIs remains unknown. In the era of precision treatment, it is very important to understand the clinical significance of these unknown variants. Herein, we report one lung adenocarcinoma patient with a new fusion form of ALK rearrangement (NCOA1–ALK) who presented a long-term response to crizotinib.
Case presentation
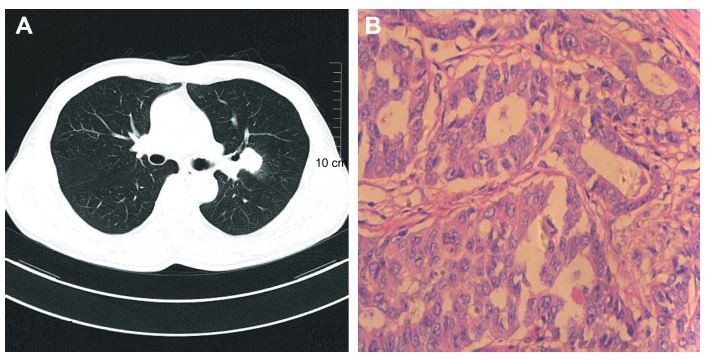
A 59-year-old man with a smoking history was admitted to our hospital for a conventional health checkup with a nodule in his upper-left lung. After pulmonary computed tomography (Figure 1A) and magnetic resonance imaging (MRI) of the head in hospital, the patient was assessed as being acceptable for surgery, without distant metastasis. Left-lung resection and lymph node-dissection were then performed. Postoperative pathology showed a diagnosis of moderately differentiated lung adenocarcinoma in his upper-left lung (Figure 1B).
Genetic testing of common driver genes, such as EGFR, ALK, ROS1, was not performed in hospital. At 3 months after surgery, four cycles of standard chemotherapy (pemetrexed and carboplatin) was administered, and this patient did not come to hospital for regular routine follow-up visits until 28 months later, with a complain of chest congestion. Pulmonary CT and MRI screening showed multiple metastases in both lungs and brain metastasis, respectively (Figure 2A and B).
Second-line chemotherapy with docetaxel (120 mg according to the patient’s surface area, 75 mg/m2, once every 3 weeks) was performed without any benefit. To explore potential targeted therapies, next-generation sequencing was performed in tumor tissue and revealed a new type of ALK arrangement (NCOA1–ALK, Figure 3A). Mutation profiling of this patient is summarized in Figure 3B, and expression of the ALK protein was confirmed by immunohistochemistry (Figure 3C). Oral crizotinib (250 mg twice a day) was administered immediately. After 2 months of crizotinib treatment, the multiple metastatic nodules in the right lung had decreased significantly (Figure 4A and B) and the brain-lesion metastases were stable (Figure 4F and G). Several follow-up visits were recorded, and the patient was still alive without disease progression in lung lesions (Figure 4C–E) or brain metastases (Figure 4H–J). The progression-free survival of this patient had exceeded 18 months.
Discussion
In this case report, a new fusion form of ALK rearrangement (NCOA1–ALK) was identified, which included inversion of NCOA1 exons 1–12 and ALK exons 20–29. This fusion gene retained the TK domain of ALK, a critical region for ALK activity. The patient was still alive, with progression-free survival >18 months after crizotinib therapy. None of the other genetic mutations displayed a potential response to ALK-TKI.
ALK-TKIs are widely used in clinical practice, and the heterogeneity of response posed a puzzling question. Numerous researchers have explored the mechanisms of the primary resistance or heterogeneous response of EGFR-TKIs for EGFR-mutation positive NSCLC patients.4 However, similar studies for ALK-TKIs have been rare. Two explanations for the heterogeneity of ALK-TKI responses have been suggested. One interpretation was that different variants of ALK rearrangement resulted in different protein stability and expression levels, and the other interpretation was that concomitant ALK rearrangement with other genetic alterations led to different amplitudes in ALK-TKI response.5
The golden evaluation criterion for ALK state is fluorescent in situ hybridization or immunohistochemistry, neither of which can identify specific fusion forms. With the notion that different variants may exert different responses to ALK-TKIs, it is crucial to identify the specific variant in different individuals, in order to achieve precise medication in the future. To a certain extent, next-generation-sequencing testing for ALK rearrangement may be an excellent method of supplementation.
To our knowledge, this is the first case to present a lung adenocarcinoma patient harboring a new fusion form of ALK rearrangements (NCOA1–ALK) showing a long-term response to crizotinib. Our findings provide valuable information on response to crizotinib in patients with this form of ALK rearrangement and better understanding of ALK-TKIs applications in future.
Informed consent
Written informed consent had been provided by the patient to have the case details and any accompanying images published. This was an observational case report and institutional approval was not required, because all treatment received by this patient is standard therapy, including the ALK-TKI (crizotinib).
Disclosure
The authors report no conflicts of interest in this work.
References
Solomon B, Varella-Garcia M, Camidge DR. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol. 2009;4(12):1450–1454. | ||
Yoshida T, Oya Y, Tanaka K, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34(28):3383–3389. | ||
Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–1019. | ||
Zhong J, Li L, Wang Z, et al. Potential resistance mechanisms revealed by targeted sequencing from lung adenocarcinoma patients with primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). J Thorac Oncol. 2017;12(12):1766–1778. | ||
Lin JJ, Shaw AT. Differential sensitivity to crizotinib: does EML4-ALK fusion variant matter? J Clin Oncol. 2016;34(28):3363–3365. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.