Back to Journals » International Journal of Nanomedicine » Volume 14
The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity
Authors Abu Rabe DI , Al Awak MM, Yang F, Okonjo PA , Dong X, Teisl LR, Wang P, Tang Y , Pan N, Sun YP, Yang L
Received 5 January 2019
Accepted for publication 26 February 2019
Published 23 April 2019 Volume 2019:14 Pages 2655—2665
DOI https://doi.org/10.2147/IJN.S200493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Dina I Abu Rabe,1 Mohamad M Al Awak,1 Fan Yang,2 Peter A Okonjo,1 Xiuli Dong,1 Lindsay R Teisl,2 Ping Wang,2 Yongan Tang,3 Nengyu Pan,2 Ya-Ping Sun,2 Liju Yang1
1Department of Pharmaceutical Sciences and Biomanufacturing Research Institute and Technology Enterprise (BRITE), North Carolina Central University, Durham, NC 27707, USA; 2Department of Chemistry and Laboratory for Emerging Materials and Technology, Clemson University, Clemson, SC 29634, USA; 3Department of Mathematics and Physics, North Carolina Central University, Durham, NC 27707, USA
Background: Carbon dots (CDots) have recently been demonstrated their effective visible light-activated antimicrobial activities toward bacteria. This study was to evaluate and understand the roles of the surface functionalities in governing the antimicrobial activity of CDots.
Methods: Using the laboratory model bacteria Bacillus subtilis, the photo-activated antimicrobial activities of three groups of CDots with specifically selected different surface functionalization moieties were evaluated and compared. The first group consisting of CDots with surface functionalization by 2,2-(ethylenedioxy)bis(ethylamine) (EDA) vs. 3-ethoxypropylamine (EPA), was evaluated to determine the effect of different terminal groups/charges on their photo-activated antibacterial activities. The second group consisting of CDots functionalized with oligomeric polyethylenimine (PEI) and those prepared by the carbonization of PEI – citric acid mixture, was to evaluate the effects of dot surface charges vs. fluorescent quantum yields on their antimicrobial activities. The third group consisting of CDots functionalized with PEI of 1,200 vs. 600 in average molecular weight was evaluated for the effect of molecular weight of surface passivation molecular on their antimicrobial activities.
Results: The results indicated the EDA-CDots in the first group was more effective and was attributed to the positive charges from the protonation of the amino groups (–NH2) being more favorable to interactions with bacterial cells. The evaluation of the second group CDots suggested the same surface charge effect dominating the antibacterial performance over the fluorescent quantum yields. The evaluation of the third group CDots functionalized with PEI of 1,200 vs. 600 in average molecular weight, indicated the latter was significantly more effective.
Conclusions: The results from this study highlighted the dominant role of surface functionalities in governing CDots’ light activated antimicrobial activity and should have significant implications to the further design and development of CDots as a new class of visible light-activated antibacterial agents.
Keywords: carbon dots, antimicrobial, bacteria, photo-activated, surface functionalization
Introduction
Carbon dots (CDots) are small carbon nanoparticles with surface passivation, each with a carbon nanoparticle core (pre-existing or from carbonization of organic precursors under sufficiently robust processing conditions) and a thin shell of soft materials (organic or biological species).1 They have been known for their photoexcited state properties and redox processes resembling those typically found in conventional semiconductor quantum dots (QD), with efficient photoinduced charge separation for the formation of radical anions and cations (electrons and holes), so CDots are sometimes referred to in the literature as “carbon quantum dots”, but with unique advantages such as intrinsically nontoxic in vitro and in vivo,2–4 environmentally benign, facile synthesis and low costs.5–15 The photoinduced charge separation and the subsequent radiative recombinations in CDots are responsible for the observed QD-like fluorescence properties, with bright and colorful emissions in the visible and extending into the near-IR.5–17 The same photoinduced processes have recently been demonstrated to afford CDots’ photo-activated activity in killing cancer cells18–20 and bacterial cells.13,21–27 There is now substantial experimental evidence suggesting that CDots represent a new class of effective photo-activated antimicrobial agents, particularly under visible/natural light.
For a mechanistic understanding of CDots’ photo-activated antibacterial function, several possible mechanisms that are similar to the action mechanisms of other nanoparticles are being considered, including the adhesion to cell membrane that alters the membrane structure/permeability, the generation of reactive oxygen species (ROS) that oxidize protein/lipids, penetration inside the cell and nucleus to dysfunction various cell functions, and modulation of cell signaling.28 Among the more likely is that under light illumination CDots generate ROS, such as singlet oxygen, superoxide and/or hydroxyl radical, to kill bacteria.21 In any single or a combination of mechanisms for the action, however, there might be two requirements for CDots to be effective in their antibacterial action: one is that CDots are likely required to be in close interactions with bacterial cells, namely in proximity or adherent to the bacterial cells; the other is that CDots should have highly effective photo-induced properties for its photo-activated antibacterial function.
Considering the core–shell structures of CDots, the surface passivation/functionalization (shell) is particularly important, because it determines several properties of CDots, especially the surface charge status/characteristics and optical properties. In view of the interactions of nanomaterials with biological entities, surface functionalization is generally one of the most important properties affecting the interactions between them and consequently the pathway of cellular uptake, intracellular trafficking and cytotoxicity of the nanomaterials.29,30 Since many biological entities have surface charges, their interactions with nanomaterials are largely dependent on the electrostatic forces/interactions between them, so that the surface charges on the nanomaterials could be a major factor affecting the interactions. More specifically, in consideration of the negatively charged bacterial cell surfaces, the surface charges on CDots (on the surface passivation layer) can be a major factor that affects the interactions between CDots and bacterial cells. On the other hand, the surface passivation layer formed by different molecules also largely determines CDots’ physical, optical and photo-induced properties, such as thickness of the passivation layer, fluorescence quantum yield of the CDots, and their photo-induced antibacterial functions. For such a purpose, CDots are structurally versatile, amenable to modifications that make their surface functionalities favorable to more effective interactions with bacterial cells, and more potent antibacterial actions.
In this work, the influence of CDots’ surface functionalization on their antibacterial function was investigated in order to identify and understand those dot configurations that are more effective in antibacterial actions. Specially designed and synthesized CDots that are structurally similar but differ in surface functionalization, particularly including CDots with small passivation molecules of different terminal groups/charges, CDots with passivation polymers of tunable surface charges vs fluorescent quantum yields, and CDots with passivation polymers of different molecular weights, were evaluated and compared for their visible light-activated antibacterial functions against the laboratory model bacteria Bacillus subtilis. Major impacts/implications of the findings to the selection and further development of CDots as a new class of photo-activated effective antibacterial agents are highlighted and discussed.
Experimental section
CDots
Small carbon nanoparticles were harvested from the commercially acquired carbon nano-powders (US Research Nanomaterials, Inc.) in procedures similar to those reported previously.31,32 In a typical experiment, a sample of carbon nano-powders (2 g) was refluxed in concentrated nitric acid (8 M, 200 mL) for 48 hr. The reaction mixture was cooled back to room temperature, and centrifuged at 1,000 g to discard the supernatant. The residue was re-dispersed in deionized water, dialyzed in a membrane tubing (molecular weight cut-off ~500) against freshwater for 48 hr, and then centrifuged at 1,000 g to retain the supernatant. Upon the removal of water, small carbon nanoparticles were recovered and used in the functionalization reaction with different surface passivation molecules.
For the group of CDots with small passivation molecules of different terminal groups/charges, 2,2-(ethylenedioxy)bis(ethylamine) (EDA, Sigma-Aldrich, St Louis, MO, USA)31 or 3-ethoxypropylamine (EPA, TCI America)32,33 were used to functionalize the carbon nanoparticles to yield EDA-CDots or EPA-CDots, respectively. Detailed procedures for the synthesis of the two CDots were reported previously.31–33
For another group of CDots with polymer surface passivation, oligomeric polyethylenimine (PEI of molecular weight ~1,20034 was used to functionalize the small carbon nanoparticles in the thermally induced functionalization reaction to yield PEI-CDots.13 Similarly, PEI with molecular weight ~600 (denoted as PEI600 to be differentiated from the PEI of molecular weight ~1,200 above) was used for the functionalization to obtain PEI600-CDots for comparison.
In a different synthesis, a mixture of the PEI and citric acid (CA) was used as precursor in the one-pot hydrothermal processing to obtain the PEI/CA-CDots, for which the detailed experimental procedure and product characterization were largely the same as those reported previously.22 A serial of PEI/CA-CDots were specially synthesized by varying the composition in the precursor mixture and the processing conditions, with the resulting dots denoted as PEI/CA-CDots-1, PEI/CA-CDots-2 and PEI/CA-CDots-3.
All these CDots were characterized by using NMR, microscopy and optical spectroscopy techniques, from which the results were consistent with those of similarly prepared samples reported previously. According to atomic force microscopy and transmission electron microscopy results on dot sizes, both EDA-CDots and EPA-CDots were 4–5 nm in average diameter, with the latter being on average slightly smaller,31,32 whereas PEI-CDots were 4–6 nm in average diameter,13 and PEI/CA-CDots were around 10 nm in average diameter.22
Bacterial culture
Bacillus subtilis culture was grown in 10 mL nutrient broth (Fisher Scientific, Pittsburgh, PA, USA) by inoculating the broth with a single colony of a plated culture on a Luria–Bertani (LB) agar plate, and incubated overnight at 37°C, respectively. Freshly grown B. subtilis cells were washed three times with PBS (1X, pH 7.4) (Fisher Scientific) and then re-suspended in PBS or otherwise stated solutions for further experimental uses.
Treatment of bacterial cells with CDots
Treatment of bacterial cells with CDots was performed in 96-well plates. Each well was added with 150 µL bacteria cell suspension and 50 µL of different CDots with desired concentrations. The final bacterial cell concentration in each well was about ~106–107 CFU/mL, and the concentration of CDots was varied as needed while the final pH remained constant at 7.4. Treatments with CDots at each concentration were run in triplicates. The plates were exposed to visible light with wavelength ranging from 400 to 800 nm from a 36 W 12 V light bulb using a light box and optical set group (Arbor Scientific, Ann Arbor, MI, USA), from 10 cm above the surface of the plate for 1 hr or as stated otherwise.
Surface plating method to determine viable cell number
The actual cell concentration in the suspension was determined by the traditional surface plating method. Briefly, the bacterial suspensions were serially diluted (1:10) with PBS. Aliquots of 100 μL appropriate dilutions were surface-plated on LB agar plates. After incubation at 37°C for 24 h, the number of colonies on the plates were counted, and the viable cell numbers were calculated in colony forming units per milliliter (CFU/mL) for all the treated samples and the controls.
Evaluation of CDots’ photo-activated antibacterial efficiency
After the treatment, the viable cell numbers in the control and treated samples were determined by the traditional plating method as described above. The logarithmic value of the reduction in viable cell number in the CDots treated samples in comparison to the controls was used to evaluate the efficiency of bactericidal function of the CDots. The greater the viable cell reduction, the higher the CDots’ antibacterial activity.
Statistical analyses
Statistical analyses of experimental results were performed using the general linear model procedure of the SAS System 9.2 (SAS Institute Inc., Cary, NC, USA), with P<0.05 being considered as significantly different.
Results and discussion
CDots of different surface functionalities
The structure of CDots (Figure 1A) consists of a carbon nanoparticle core, which is largely amorphous for the dots in this study, and a soft shell that affords a variety of choices for surface functionalities. The effectiveness in surface passivation associated with the different functionalities determines the photoexcited state properties of the corresponding CDots, especially the fluorescence quantum yields, as already demonstrated repeatedly in the literature.5–15 On the photo-activated antibacterial activities of CDots, which share the same excited state processes with fluorescence emissions, their correlation with observed fluorescence quantum yields has been reported.21 The inactivation process of bacteria via the photo-activated antibacterial activity of CDots is illustrated in Figure 1B. In consideration of the negatively charged bacterial surface, the surface functionalities of CDots may also play an important role in their interactions with bacterial cells, and consequently their antimicrobial effectiveness and efficiency against the bacterial cells. For an evaluation on the effects of such interactions, a group of CDots with different surface functionalities was selected, as shown in Figure 1A. Their antibacterial efficiencies were evaluated and compared in correlation with the surface properties of the CDots.
Among the selected CDots of different surface passivation molecules, EDA-CDots31,33 and EPA-CDots32,33 were compared as a pair (Figure 1). EDA = 2,2ʹ-(ethylenedioxy)bis(ethylamine) and EPA = 3-ethoxypropylamine are both small amino molecules, and both CDots were synthesized by the same chemical functionalization of pre-processed and selected small carbon nanoparticles under amidation reaction conditions (for the formation of amide bonds between the nanoparticle-bound carboxylic acid moieties and the amine groups in EDA and EPA, Figure 1).31–33 However, since EDA is a diamine, different from EPA, the corresponding EDA-CDots and EPA-CDots are different in surface functionalities, with terminal –NH2 and –CH3 groups, respectively (Figure 1A). The different surface functionalities are reflected in the observed basicities of the two CDots in deionized water, with the resulting aqueous solutions of pH around 10 for the EDA-CDots vs around 7.5 for the EPA-CDots. As a result, the former is positively charged at physiological pH (PBS buffer of pH 7.4 in this study), but not the latter, enabling a comparison for the probing and understanding of the effects due to different surface charge status. Equally or more significantly, with EDA and EPA both being small molecules, the EDA-CDots and EPA-CDots are structurally ultra-compact,31–33 different from the other dots of larger oligomeric surface functionalities tested in this study, thus also valuable to an understanding of the effects due to dot surface properties other than the charge status.
On the selection of the CDots with PEI13 for surface functionalization, the PEI molecules were branched oligomers of average molecular weight ~1,200 and ~600, corresponding to the dot samples denoted as PEI-CDots and PEI600-CDots, respectively. The PEI oligomers contain both primary and secondary amines (Figure 1A), which are also present in the surface functionalities of the PEI-CDots and PEI600-CDots. These amine moieties make the dot samples in deionized water basic, and they are protonated to become cationic at physiological pH (PBS buffer of pH 7.4 in this study). Thus, these CDots were selected for an evaluation on the expected more favorable interactions of the cationic ammonium groups with negatively charged bacterial surfaces and the consequences in terms of their enhanced antibacterial activities.
It should be pointed out that the selected CDots described above were synthesized by the chemical functionalization of pre-existing carbon nanoparticles, which were of the same chemical compositions in terms of carbons in different hybridizations. Structurally according to results from the X-ray powder diffraction analysis, the carbon nanoparticles were largely amorphous, as already reported in our previous study.35
PEI/CA-CDots, synthesized by the thermal carbonization of PEI-CA mixtures,22 were selected for comparisons in reference to PEI-CDots. As already reported in the literature,22 the PEI/CA-CDots are characterized by excellent fluorescence properties, with the observed fluorescence quantum yields beyond 50% in the visible spectral region overlapping that of green fluorescence protein,13 and by some unique structural features, including surface functionalities. Unlike the PEI-CDots and PEI600-CDots discussed above, PEI/CA-CDots were from the thermal carbonization of PEI–CA mixtures, in which the basic PEI and acidic CA were likely in zwitterionic pairs. As reported previously,22 the zwitterionic pairs were likely carried over in the carbonization processing to dominate the dot surface structure (Figure 1A), consistent with the observed neutral pH in the aqueous solution of the PEI/CA-CDots corresponding to an amine/acid ratio close to 1 in the PEI– CA precursor mixture.22 Such a sample is denoted as PEI/CA-CDots-1. Two more PEI/CA-CDots samples were prepared from precursor mixtures in which PEI (thus amine groups) was in excess, denoted as PEI/CA-CDots-2 and PEI/CA-CDots-3, with their solutions in deionized water of pH ~8.6 and ~9.7, respectively. Thus, the PEI/CA-CDots-3 contained more amine moieties on the dot surface because of the larger PEI excess in the precursor mixture, somewhat closer to the PEI-CDots discussed above, so that the three PEI/CA-CDots samples and the PEI-CDots served as a nice series for comparisons of their interactions with bacterial cells in PBS buffer and the associated photo-activated bactericidal functions of the CDots.
EDA-CDots vs EPA-CDots – surface charge effects
In this study, Gram-positive laboratory model bacteria, B. subtilis, was used to evaluate the antimicrobial efficiencies of CDots with different surface functionalities. EDA-CDots and EPA-CDots were systematically evaluated for their antibacterial function against B. subtilis cells for probing the surface charge effect. EDA and EPA are small molecules, with molecular weights 148 and 103 g/mol, respectively, and they are structurally similar but their corresponding CDots differ in terms of terminal groups on the dot surface, –NH2 in EDA-CDots vs –CH3 in EPA-CDots (Figure 1A). The former can be positively charged at physiological pH as -NH3+, but not the latter. The observed fluorescent quantum yields of the EDA-CDots and EPA-CDots used in the study were both ~20%. Relevant characteristics of EDA-CDots and EPA-CDots used in this comparison experiment are listed in Table 1.
 | Table 1 Relevant characteristics of EDA-CDots and EPA-CDots |
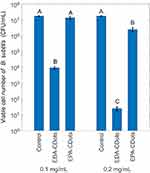
Figure 2 shows the antibacterial activity of EPA-CDots and EDA-CDots at 0.1 and 0.2 mg/mL to B. subtilis cells in terms of viable cell reduction upon treatments under light illumination for 1 hr. At 0.1 mg/mL, the treatment with EPA-CDots barely caused any reduction in viable cell number to B. subtilis cells, while the treatment with EDA-CDots was much more effective, resulting in about 3.26 log reduction in viable cells. When CDots’ concentration increased to 0.2 mg/mL, 1 hr treatment with EPA-CDots resulted in about 0.84 log reduction in viable cell number to B. subtilis cells, while treatment with EDA-CDots resulted in about 5.8 log reduction in viable cell number under the same condition. As expected, at both tested CDots concentrations, the results consistently showed that EDA-CDots exhibited much greater antibacterial activity to B. subtilis cells compared to EPA-CDots. Such results highlighted the important role of surface charge on CDots for their interactions with bacterial cells and the execution of their antibacterial function. The positively charged end groups (–NH3+) on EDA must be favored by the negatively charged bacterial surface, thus stronger binding-like interactions between EDA-CDots and the bacterial cells to result in a higher “local concentration” of EDA-CDots on bacterial surface, thus more effective in antibacterial actions against the bacterial cells.
Noticeably, similarly different effectiveness between EDA-CDots and EPA-CDots was found in their antiviral function,36 where EDA-CDots were more effective than EPA-CDots in inhibiting norovirus virus-like particles binding to histo-blood group antigen receptors, due primarily to the difference in surface charge status between the two CDots. In addition, similar surface charge effect has been reported on silver nanoparticles’ antimicrobial activity, where positively and negatively charged silver nanoparticles exhibited the highest and lowest bactericidal activities, respectively.37 As such, there have been recent studies on inducing charges onto the surface of silver nanoparticles for higher antimicrobial efficacy,38–40 The results reported here suggest that the same strategy may be exploited in the design and preparation of CDots with higher antibacterial efficacy.
Effects of surface charge and photoexcited state properties – comparisons between PEI-CDots and PEI/CA-CDots
Because of the significant presence of amine moieties on the surface of PEI-CDots, these dots are positively charged at physiological pH (in PBS buffer), thus expected to have favorable interactions with negatively charged bacterial surface. In PEI/CA-CDots-1, on the other hand, the surface functionalities are dominated by zwitterionic pairs, thus close to neutral pH in aqueous solution and without the surface positive charges in PBS buffer. However, these dots are highly fluorescent, with the observed fluorescence quantum yields beyond 50%,22 indicative of the photoexcited state properties being more favorable to photodynamic effects and consequently more effective antibacterial activity to bacterial cells, as already demonstrated experimentally.21 The evaluation was to determine the consequences of these two apparently opposing effects on antibacterial activities of the CDots. For the comparison, the PEI-CDots of a fluorescence quantum yield ~12% and the PEI/CA-CDots-1 of a much higher quantum yield of ~60% were used. Relevant characteristics of the two-dot samples are listed in Table 2.
 | Table 2 Relevant characteristics of PEI-CDots and PEI/CA-CDots |
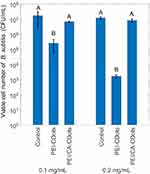
Figure 3 shows the viable cell reductions of B. subtilis upon the treatment with PEI-CDots and PEI/CA-CDots-1 at 0.1 and 0.2 mg/mL for 1 hr under light illumination, in bacterial samples containing ~107 CFU/mL cells. While the treatment with 0.1 mg/mL PEI-CDots resulted in about 1.82 log reduction in viable cells of B. subtilis cell, the treatment with PEI/CA-CDots-1 did not cause any significant reduction in viable cell number. At a higher dots concentration of 0.2 mg/mL, the treatment with PEI-CDots significantly increased the viable cell reduction to ~3.87 log, whereas the PEI/CA-CDots-1 with the increased concentration still did not inactivate B. subtilis cells. A conclusion from the results on these dots at different concentrations is that PEI-CDots are more effective in inactivating B. subtilis cells than the PEI/CA-CDots-1 at the same concentration and under the same treatment condition, despite the fact that the PEI/CA-CDots-1 had a much higher fluorescence quantum yield, thus suggesting that the dot–cell interactions are more important in the two opposing effects discussed above.
More specifically on the different surface characteristics between PEI-CDots and PEI/CA-CDots-1, the former has a significant population of amine moieties, as reflected by the basic pH value (around 9, vs about neutral for PEI/CA-CDots-1).22 It should be pointed out that the pH in the mixture of bacterial cells with CDots in the PBS buffer was around 7.4, where the –NH2 terminal groups are protonated as -NH3+. The strong ionic interactions enhance the adhesion/attachment of CDots on the bacterial membrane and allow more effective execution of photo-activated antibacterial function on the bacterial cells. This is despite the fact that the PEI/CA-CDots-1 are of significantly higher fluorescent quantum yield than PEI-CDots,22 as according to previous studies CDots of higher fluorescence quantum yields are generally more effective in their light-activated antibacterial activities.21 Therefore, the results presented here suggest that the surface charge of CDots plays a critical role and dominates the interactions between CDots and bacterial cells (adhesion/attachment) which are essential for CDots’ photo-activated antibacterial function on bacterial cells.
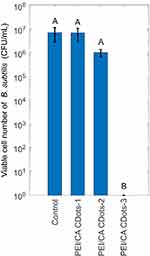
In further investigation on the two opposing effects discussed above toward an optimal balance between the effects to achieve high effectiveness of CDots’ antibacterial function, PEI/CA-CDots-1, PEI/CA-CDots-2 and PEI/CA-CDots-3 were compared. The three dot samples have different surface populations of amine moieties, in addition to the PEI/CA zwitterionic pairs, as reflected by their different pH values in aqueous solutions (~7.5, ~8.6 and ~9.7, respectively). Their observed fluorescent quantum yields were similar, all around 60%. Relevant characteristics of the three PEI/CA-CDots samples are listed in Table 3. Figure 4 shows the viable cell reductions of B. subtilis upon the treatment with the three PEI/CA-CDots samples all at 0.1 mg/mL for 1 hr under light illumination in bacterial samples containing ~6.9 x 106 CFU/mL cells, along with the untreated control samples. The results confirmed an expected trend in the effectiveness of the three dot samples, which correlated well with the increasing –NH2 population on the dot surface from the sample −1 to the sample −3, and thus suggesting the dominating role of the charge status governing the interactions of the dots with bacterial cells. It can be seen in Figure 4 that there was almost no effect with PEI/CA-CDots-1 treatment on the viable cell reduction, while PEI/CA-CDots-2 resulted in 0.83 log reduction in viable cell reduction. More dramatic demonstration of the same effect was found in the treatment with PEI/CA-CDots-3, which possess even more –NH2 groups on the dot surface, with almost a complete inactivation of the cells (~6.8 log viable cell reduction). Such tremendously effective photo-activated antibacterial function must be due to the combined effects of a sufficient population of –NH2 groups on the dot surface to enable favorable interactions of the CDots with cells, thus a higher local concentration of CDots on the cell surface, and the favorable photoexcited state properties to the generation of ROS, as reflected by the observed high fluorescence quantum yield of the dot sample. Clearly, the highly effective antibacterial function achieved by PEI/CA-CDots-3 highlights a very useful strategy for designing effective photo-activated antibacterial CDots, such that the CDots must have surface functionalities favorable to bacterial surface adhesion for effective interactions with the bacterial cells, and at the same time the CDots should possess excellent optical properties for photo-activation process to produce antibacterial species.
 | Table 3 Relevant characteristics of the serial of PEI/CA-CDots |
PEI-CDots vs PEI600-CDots – effect of PEI molecular weight
Two CDots with surface functionalization by PEI oligomers of different molecular weights, ~1,200 in PEI-CDots and ~600 in PEI600-CDots, were evaluated for effects of the PEI molecular weight difference on their photo-activated antibacterial function against B. subtilis. Figure 5A shows the viable cell reduction of B. subtilis cells after the 1 hr treatments with PEI-CDots and PEI600-CDots at 0.1 mg/mL with light illumination. With bacterial samples containing ~107 CFU/mL cells, the treatment with PEI-CDots resulted in ~1.82 log reduction of B. subtilis cells, whereas the treatment with PEI600-CDots inactivated almost all the cells, namely >7 log reduction in viable cell number, which demonstrated that PEI600-CDots were much more effective in activating the bacterial cells. Both PEI-CDots and PEI600-CDots exhibited common concentration-dependent antibacterial activity against B. subtilis (Figure 5B). Increased concentration of PEI-CDots at 0.3 mg/mL could inactivate all cells in the samples containing ~107 CFU/mL, whereas PEI600-CDots could do the same with a much lower concentration of only 0.05 mg/mL. The results again suggest that PEI600-CDots are considerably more effective than PEI-CDots in their photo-activated antibacterial activities under the same treatment conditions.
The remarkable difference in the antibacterial activities between PEI-CDots and PEI600-CDots points to both complications and opportunities in the comparison and understanding of CDots with different surface functionalities toward the design and development of dot structures with much enhanced bactericidal function. On these two CDots specifically, they are structurally rather similar, except for probably their difference in the thickness of the surface passivation layer, correlated with the size of PEI (~1,200 in average molecular weight) vs that of PEI600. The latter may correspond to a thinner surface passivation layer, thus would allow the photo-generated ROS to act more effectively on the bacterial cells for the observed more effective antibacterial function. While more studies are needed to look into the mechanistic details behind the results, the observations reported here do highlight the important role of the surface functionalization of CDots, which should be emphasized in the further design and development for highly effective photo-activated antibacterial CDots.
Conclusions
Through the comparison of the antibacterial efficiencies of the selected CDots with designed surface passivation moieties, this study demonstrated that the surface charge status on CDots dominated the interactions between CDots and bacterial cells. CDots with surface passivation terminal groups of –NH2 was more favored in interacting with negatively charged bacterial cells compared to those with terminal groups of –CH3 or with zwitterionic pairs of CA and amine groups, indicating that positively charged surface molecules on CDots would enhance the interactions with bacterial cells and thus the photo-activated antimicrobial activity of CDots. Besides the surface charge status, the optical properties of CDots reflected by high fluorescence quantum yields are desirable for CDots to exhibit highly effective antimicrobial function. In addition, the thickness of polymer passivation layer on CDots may also affect its antimicrobial function, with CDots of a thinner passivation layer exhibiting more effective antimicrobial activity. Despite the likely complexity in the details of the action and still a lack of clear mechanistic understanding of the observed activities, the results in this study highlighted the critical role of the surface functionalization in governing the overall performance of CDots’ antimicrobial function and provided useful information for further design of highly effective antimicrobial CDots.
Acknowledgments
The research was supported by NSF grants DMR# 1701399 (L.Y. and Y.T.) and DMR#1701424 (Y.-P.S.), and the NIH grant R15GM114752.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun YP, Zhou B, Lin Y, et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006;128(24):7756–7757. doi:10.1021/ja062677d
2. Luo PJG, Sahu S, Yang ST, et al. Carbon “quantum” dots for optical bioimaging. J Mater Chem B. 2013;1(16):2116–2127. doi:10.1039/c3tb00018d
3. Luo PJG, Yang F, Yang ST, et al. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 2014;4(21):10791–10807. doi:10.1039/c3ra47683a
4. Yang ST, Wang X, Wang HF, et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J Phys Chem C. 2009;113(42):18110–18114. doi:10.1021/jp9085969
5. Ding CQ, Zhu AW, Tian Y. Functional surface engineering of C-Dots for fluorescent biosensing and in vivo bioimaging. Accounts Chem Res. 2014;47(1):20–30. doi:10.1021/ar400023s
6. Fernando KAS, Sahu S, Liu YM, et al. Carbon quantum dots and applications in photocatalytic energy conversion. Acs Appl Mater Int. 2015;7(16):8363–8376. doi:10.1021/acsami.5b00448
7. Georgakilas V, Perman JA, Tucek J, Zboril R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem Rev. 2015;115(11):4744–4822. doi:10.1021/cr500304f
8. Hutton GAM, Martindale BCM, Reisner E. Carbon dots as photosensitisers for solar-driven catalysis. Chem Soc Rev. 2017;46(20):6111–6123. doi:10.1039/c7cs00235a
9. LeCroy GE, Yang ST, Yang F, et al. Functionalized carbon nanoparticles: syntheses and applications in optical bioimaging and energy conversion. Coordin Chem Rev. 2016;320:66–81. doi:10.1016/j.ccr.2016.02.017
10. Namdari P, Negahdari B, Eatemadi A. Synthesis, properties and biomedical applications of carbon-based quantum dots: an updated review. Biomed Pharmacother. 2017;87:209–222. doi:10.1016/j.biopha.2016.12.108
11. Peng ZL, Han X, Li SH, et al. Carbon dots: biomacromolecule interaction, bioimaging and nanomedicine. Coordin Chem Rev. 2017;343:256–277. doi:10.1016/j.ccr.2017.06.001
12. Roy P, Chen PC, Periasamy AP, Chen YN, Chang HT. Photoluminescent carbon nanodots: synthesis, physicochemical properties and analytical applications. Mater Today. 2015;18(8):447–458. doi:10.1016/j.mattod.2015.04.005
13. Hu Y, Al Awak MM, Yang F, et al. Photoexcited state properties of carbon dots from thermally induced functionalization of carbon nanoparticles. J Mater Chem C. 2016;4(44):10554–10561. doi:10.1039/C6TC03666J
14. Yuan FL, Li SH, Fan ZT, Meng XY, Fan LZ, Yang SH. Shining carbon dots: synthesis and biomedical and optoelectronic applications. Nano Today. 2016;11(5):565–586. doi:10.1016/j.nantod.2016.08.006
15. Zuo PL, Lu XH, Sun ZG, Guo YH, He H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta. 2016;183(2):519–542. doi:10.1007/s00604-015-1705-3
16. Wang J, Tang L, Zeng G, et al. 0D/2D interface engineering of carbon quantum dots modified Bi2WO6 ultrathin nanosheets with enhanced photoactivity for full spectrum light utilization and mechanism insight. Appl Catal B. 2018;222:115–123. doi:10.1016/j.apcatb.2017.10.014
17. Deng Y, Tang L, Feng C, et al. Construction of plasmonic ag and nitrogen-doped graphene quantum dots codecorated ultrathin graphitic carbon nitride nanosheet composites with enhanced photocatalytic activity: full-spectrum response ability and mechanism insight. ACS Appl Mater Inter. 2017;9(49):42816–42828. doi:10.1021/acsami.7b14541
18. Havrdova M, Hola K, Skopalik J, et al. Toxicity of carbon dots – effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon. 2016;99:238–248. doi:10.1016/j.carbon.2015.12.027
19. Juzenas P, Kleinauskas A, Luo PG, Sun YP. Photoactivatable carbon nanodots for cancer therapy. Appl Phys Lett. 2013;103(6). doi:10.1063/1.4817787
20. Markovic ZM, Ristic BZ, Arsikin KM, et al. Graphene quantum dots as autophagy-inducing photodynamic agents. Biomaterials. 2012;33(29):7084–7092. doi:10.1016/j.biomaterials.2012.06.060
21. Al Awak MM, Wang P, Wang SY, Tang YA, Sun YP, Yang LJ. Correlation of carbon dots’ light-activated antimicrobial activities and fluorescence quantum yield. RSC Adv. 2017;7(48):30177–30184. doi:10.1039/C7RA05397E
22. Hou XF, Hu Y, Wang P, et al. Modified facile synthesis for quantitatively fluorescent carbon dots. Carbon. 2017;122:389–394. doi:10.1016/j.carbon.2017.06.093
23. Lim SY, Shen W, Gao ZQ. Carbon quantum dots and their applications. Chem Soc Rev. 2015;44(1):362–381. doi:10.1039/c4cs00269e
24. Meziani MJ, Dong XL, Zhu L, et al. Visible-light-activated bactericidal functions of carbon “Quantum” dots. Acs Appl Mater Inter. 2016;8(17):10761–10766. doi:10.1021/acsami.6b01765
25. Ristic BZ, Milenkovic MM, Dakic IR, et al. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials. 2014;35(15):4428–4435. doi:10.1016/j.biomaterials.2014.02.014
26. Sattarahmady N, Rezaie-Yazdi M, Tondro GH, Akbari N. Bactericidal laser ablation of carbon dots: an in vitro study on wild-type and antibiotic-resistant Staphylococcus aureus. J Photochem Photobiol B. 2017;166:323–332. doi:10.1016/j.jphotobiol.2016.12.006
27. Stankovic NK, Bodik M, Siffalovic P, et al. Antibacterial and antibiofouling properties of light triggered fluorescent hydrophobic carbon quantum dots langmuir-blodgett thin films. ACS Sustain Chem Eng. 2018;6(3):
28. Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7. doi:10.3389/fmicb.2016.01831
29. Liu J-H, Anilkumar P, Cao L, et al. Cytotoxicity evaluations of fluorescent carbon nanoparticles. Nano Life. 2010;01(01n02):153–161. doi:10.1142/S1793984410000158
30. Zhao F, Zhao Y, Liu Y, Chang XL, Chen CY, Zhao YL. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7(10):1322–1337. doi:10.1002/smll.201100001
31. LeCroy GE, Sonkar SK, Yang F, et al. Toward structurally defined carbon dots as ultracompact fluorescent probes. ACS Nano. 2014;8(5):4522–4529. doi:10.1021/nn406628s
32. Yang F, LeCroy GE, Wang P, et al. Functionalization of carbon nanoparticles and defunctionalization-toward structural and mechanistic elucidation of carbon “Quantum” dots. J Phys Chem C. 2016;120(44):25604–25611. doi:10.1021/acs.jpcc.6b08171
33. Liu YM, Wang P, Fernando KAS, et al. Enhanced fluorescence properties of carbon dots in polymer films. J Mater Chem C. 2016;4(29):6967–6974. doi:10.1039/C6TC01932C
34. Lin Y, Rao AM, Sadanadan B, Kenik EA, Sun YP. Functionalizing multiple-walled carbon nanotubes with aminopolymers. J Phys Chem B. 2002;106(6):1294–1298. doi:10.1021/jp013501v
35. Ge L, Pan NY, Jin JR, et al. Systematic comparison of carbon dots from different preparations-consistent optical properties and photoinduced redox characteristics in visible spectrum and structural and mechanistic implications. J Phys Chem C. 2018;122(37):21667–21676. doi:10.1021/acs.jpcc.8b06998
36. Dong XL, Moyer MM, Yang F, Sun YP, Yang LJ. Carbon Dots’ antiviral functions against noroviruses. Sci Rep-UK. 2017;7:519. doi: 10.1038/s41598-017-00675-x.
37. Abbaszadegan A, Ghahramani Y, Gholami A, et al. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater. 2015. doi:10.1155/2015/720654
38. Lee KJ, Browning LM, Nallathamby PD, Xu XH. Study of charge-dependent transport and toxicity of peptide-functionalized silver nanoparticles using zebrafish embryos and single nanoparticle plasmonic spectroscopy. Chem Res Toxicol. 2013;26(6):904–917. doi:10.1021/tx400087d
39. Silva T, Pokhrel LR, Dubey B, Tolaymat TM, Maier KJ, Liu XF. Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: comparison between general linear model-predicted and observed toxicity. Sci Total Environ. 2014;468:968–976. doi:10.1016/j.scitotenv.2013.09.006
40. Van Phu D, Quoc LA, Duy NN, et al. Study on antibacterial activity of silver nanoparticles synthesized by gamma irradiation method using different stabilizers. Nanoscale Res Lett. 2014;9(1):162. doi: 10.1186/1556-276X-9-162.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.