Back to Journals » Nature and Science of Sleep » Volume 13
Natural History of REM-OSA in Children and Its Associations with Adverse Blood Pressure Outcomes: A Longitudinal Follow-Up Study
Authors Chan KC, Au CT , Yu MW, Wing YK , Li AM
Received 27 July 2021
Accepted for publication 8 October 2021
Published 4 November 2021 Volume 2021:13 Pages 1967—1984
DOI https://doi.org/10.2147/NSS.S331389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sarah L Appleton
Kate C Chan,1 Chun T Au,1 Michelle W Yu,1 Yun K Wing,2 Albert M Li1
1Department of Paediatrics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China; 2Li Chiu Kong Family Sleep Assessment Unit, Department of Psychiatry, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
Correspondence: Albert M Li; Chun T Au
Department of Paediatrics, Faculty of Medicine, The Chinese University of Hong Kong, 6/F, Lui Che Woo Clinical Sciences Building, Prince of Wales Hospital, Shatin, N.T., Hong Kong Special Administrative Region
Tel +852-35052982
; +852-35052917
Fax +852-26360020
Email [email protected]; [email protected]
Purpose: Most respiratory events in childhood obstructive sleep apnea (OSA) take place during rapid-eye-movement (REM) sleep. This study aimed to describe the characteristics and natural history of childhood REM-OSA and to evaluate the associations between OSA subtypes and blood pressure (BP) outcomes.
Participants and Methods: This was a prospective 10-year follow-up study of a cohort established for a childhood OSA epidemiologic study. All subjects from the original cohort were invited to undergo a polysomnography (PSG) and 24-hour ambulatory blood pressure (ABP) monitoring. REM-OSA was defined with a ratio of obstructive apnea hypopnea index (OAHI) during REM sleep (OAHIREM) to OAHI during non-REM sleep (OAHINREM) ≥ 2. Natural history was observed and linear mixed models were used to assess the associations between OSA subtypes and BP outcomes.
Results: A total of 610 participants from baseline were included to study the epidemiology of REM-OSA in childhood. Among children with OSA, 65% had REM-OSA. At 10-year follow-up, 234 were included in the analysis. REM-OSA was more common at both baseline (58/92, 63%) and 10-year follow-up (34/58, 59%). For those with REM-OSA at baseline and persistent OSA at follow-up, the majority (72%) remained to have REM-OSA. Compared to those without OSA, subjects with REM-OSA had significantly higher nocturnal SBP (mean difference 2.19 mmHg, 95% confidence interval (CI): 0.12, 4.26; p = 0.039) and DBP (mean difference 1.58 mmHg, 95% confidence interval (CI): 0.11, 3.04; p = 0.035), and less nocturnal SBP dipping (mean difference − 1.84%, 95% CI: − 3.25, − 0.43; p = 0.011), after adjusting for potential confounders. This significant association between REM-OSA and nocturnal SBP dipping was observed at baseline visit only.
Conclusion: REM-OSA was found to be a stable phenotype through childhood to young adulthood, and REM-OSA was associated with higher nocturnal BP and a lesser degree of nocturnal SBP dipping in children.
Keywords: obstructive sleep apnea, children, epidemiology, stage-dependent OSA, rapid eye movement, phenotype, blood pressure
Introduction
Obstructive sleep apnea (OSA) is a common childhood sleep disorder.1 Obstructive apnea hypopnea index (OAHI) is widely used to define disease severity.2 However, sleep is a complicated process composed of different sleep stages, which are grouped under rapid-eye-movement (REM) or non-rapid-eye-movement (NREM) sleep. Physiologic changes in different sleep stages may have a differential impact on an individual’s predisposition to sleep apnea, for example, low muscle tone during REM sleep may enhance upper airway collapse.3,4 Moreover, sleep stages are believed to have different functions, for instance, REM sleep may play a role in memory formation, processing of emotional information, neuronal plasticity, and excitability.5 Therefore, respiratory disturbances in different sleep stages will have varying sequelae, which cannot be truly reflected by an overall OAHI.
In children with OSA, respiratory events predominantly occur in REM sleep. The majority of childhood OSA is REM-predominant disease with a reported prevalence ranging from 69.6% to 74.7%, while NREM-predominant disease accounts for 16.7–25.3% of childhood and adolescent OSA.6–8 It is postulated that inter-individual variations in pathophysiologic factors would predispose to airway obstruction in different sleep stages, and may explain the development of REM-predominant OSA in some individuals and NREM-predominant disease in others.9 These factors, including upper airway collapsibility and muscle responsiveness, ventilatory control stability, and arousal threshold, change with age and disease status and therefore may influence the pattern of polysomnographic subtypes across age groups.9,10 However, the natural history and clinical significance of sleep stage-dependent OSA have not been fully assessed.
The clinical significance of REM-predominant OSA remains controversial. Most data come from adult studies, which demonstrate that REM-predominant OSA is associated with excessive daytime sleepiness, non-adherence to continuous positive airway pressure, depression, hypertension, non-dipping of nocturnal blood pressure, increased insulin resistance, and impaired spatial navigational memory.4,11–17 However, results are not consistent across all studies.18–20 Paediatric studies evaluating the cardiovascular outcomes in REM-predominant OSA are scarce. In children, previous studies reported lower pulse transit time and higher blood pressure during REM than NREM sleep.21,22 On the other hand, our previous work demonstrated that children with obstructive events mainly in REM sleep had lower blood pressure than those with stage-independent OSA, and both daytime and nighttime systolic blood pressure correlated with OAHI during NREM but not REM sleep.23 Thus, it remains unclear whether REM-predominant OSA would have a better or worse cardiovascular repercussion.
Our long-term follow-up study of childhood OSA identified the natural trajectory course of OSA and its long-term effects on blood pressure as subjects entered adulthood.24,25 Nevertheless, the epidemiology of childhood stage-dependent OSA, its evolutionary course and how it modulates OSA-related complications remain unexplored. In this study, we aimed 1) to describe the epidemiology of stage-dependent OSA in childhood; 2) to describe the natural history of childhood stage-dependent OSA subtypes and 3) to investigate the associations between stage-dependent OSA and BP outcomes.
Participants and Methods
Participants
A cohort of 619 children aged 6–13 years old was established between the years 2003 and 2005 for a local epidemiology study of childhood OSA.1 Details of recruitment can be found in our previous publications.1 Subjects were recruited from 13 randomly selected primary schools. Children were classified into high-risk or low-risk of OSA groups based on our validated parent proxy OSAS screening questionnaire.1,26 All children belonging to the high-risk OSA group and randomly selected subjects at low risk of OSA were invited to participate in the baseline epidemiologic study. Exclusion criteria were having cardiovascular, renal, or neuromuscular diseases, chromosomal abnormalities, history of upper airway surgery, or the presence of an intercurrent illness within 4 weeks of the polysomnographic study. At baseline, 619 participants underwent overnight polysomnography (PSG). The epidemiology of stage-dependent OSA in childhood was investigated utilizing this cohort.
Follow-up took place at 10-year from baseline. Details of recruitment can be found in our previous publications.24 In the follow-up study, all subjects from the original cohort were invited to undergo PSG and 24-hour ambulatory blood pressure (ABP) monitoring. Subjects were excluded if they had cardiovascular, renal, or neuromuscular diseases, chromosomal abnormalities, or acute illness within 2 weeks of recruitment. Natural history of childhood stage-dependent OSA subtypes was observed from the 10-year follow-up. Participants with REM sleep less than 30 minutes at baseline and/or follow-up, and those with unknown stage-specific OAHI at baseline and/or follow-up were excluded. This study was performed in accordance with the Declaration of Helsinki. This study was approved by The Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee – approval: CRE-2013.011. All parents, guardians, or next of kin provided written informed consent for the minors to participate in this study. All adult participants provided written informed consent to participate in this study.
Data Collection and Anthropometric Measurements
Parents completed a validated personal data and sleep symptom questionnaire at baseline, and the participants did so at follow-up.26 Anthropometric measurements were performed on the day of PSG as previously described.24 Body Mass Index (BMI) was calculated as weight/height2 (kg/m2) and converted to z-scores appropriate for age and sex according to local reference.27 Overweight and obesity at baseline were defined as BMI z-scores of ≥1.036 and 1.645, corresponding to the 85th and 95th percentiles, respectively. Overweight/obesity at follow-up was defined as BMI ≥ 23.
PSG and Definitions
Nocturnal PSG was carried out at the Prince of Wales Hospital. Baseline PSG was performed, manually edited, and scored as described.1 At follow-up, Siesta 802 PSG monitor (Compumedics Telemed, Abbotsford, Victoria, Australia) was used to record the following parameters: electroencephalogram (F4/A1, C4/A1, and O2/A1), bilateral electrooculogram, electromyogram of mentalis activity, and bilateral anterior tibialis. Respiratory movements of the chest and abdomen were measured by inductance plethysmography. Electrocardiogram and heart rate were continuously recorded from two anterior chest leads. Arterial oxyhemoglobin saturation (SaO2) was measured by a finger probe oximeter. Respiratory airflow pressure signal was obtained via a nasal catheter placed at the anterior nares and connected to a pressure transducer. An oronasal thermal sensor was used to detect absent airflow. Snoring was measured by a microphone placed near the throat. Body position was monitored via a body position sensor.
Respiratory events including obstructive apneas, mixed apneas, central apneas, and hypopneas were scored based on the American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events V.2.0.28 A respiratory event was scored when it lasted ≥2 breaths irrespective of its duration for children at baseline, and 10 seconds for adolescents and adults at follow-up based on the AASM recommendations.28 Arousal was defined as an abrupt shift in electroencephalogram frequency during sleep, which may include theta, alpha, and/or frequencies greater than 16 Hz but not spindles, with 3–15 s in duration. In REM sleep, arousals were scored only when accompanied by a concurrent increase in submental electromyography amplitude.28 OAHI was defined as the total number of obstructive and mixed apneas and hypopneas per hour of sleep. Oxygen Desaturation Index (ODI) was defined as the total number of dips in arterial oxygen saturation ≥3% per hour of sleep. Arousal Index (ArI) was the total number of arousals per hour of sleep.
At baseline, childhood OSA was defined by an overall OAHI ≥ 1 event/hour. Mild childhood OSA was defined as an OAHI between 1 and 5 events/hour, while moderate-to-severe childhood OSA was defined as an OAHI of ≥5 events/hour. OSA at follow-up was defined by an OAHI of ≥5 events/hour as the subjects had already reached late adolescence or early adulthood.29 Primary snoring (PS) was defined when a subject had self-reported or parent-reported habitual snoring (at least three nights per week) in the past 12 months with an OAHI below the diagnostic cut-off for OSA. REM-predominant OSA (REM-OSA) was defined with a ratio of OAHI during REM sleep (OAHIREM) to OAHI during NREM sleep (OAHINREM) ≥ 2, while NREM-predominant OSA (NREM-OSA) as OAHIREM/OAHINREM <0.5.23 Stage-independent OSA (SI-OSA) was defined as having OAHIREM/OAHINREM between 0.5 and 2. In this study, “non REM-OSA” included both NREM-OSA and SI-OSA to compare with REM-OSA for the subjects’ characteristics and outcomes.
Twenty-Four-Hour ABP Measurement
All participants underwent 24-hour ABP monitoring carried out on the day of overnight PSG with a validated oscillometric monitor (Spacelabs 90217, Spacelabs Healthcare) as previously described.25 Recordings were included in the analysis when they had a minimum of seven successful readings during active wakefulness and at least seven successful readings during sleep.30 Hypertension at baseline was defined when systolic blood pressure (SBP) or diastolic blood pressure (DBP) at daytime, night-time, or over 24 hours was ≥95th percentile with reference to height-based local norms.31 Hypertension at follow-up was defined as SBP at daytime, night-time and 24-hour mean of ≥135, 120, and 130 mm Hg, respectively; or DBP at daytime, night-time, and 24-hour mean of ≥85, 70, and 80 mm Hg, respectively.32,33 Degree of nocturnal dipping was calculated as the percentage drop of BP from wakefulness to sleep [(wake BP−sleep BP)/wake BP×100%].34,35 Blood pressure normally follows a diurnal pattern that the average nocturnal systolic blood pressure is >10% lower than that during daytime. The phenomenon of non-dipping in nocturnal BP precedes the development of hypertension in normotensive individuals. For patients with hypertension, non-dipping is associated with increased target organ damage and a worse cardiovascular prognosis.36 It is particularly relevant in individuals with OSA because of the high likelihood of a nocturnal non-dipping pattern. Based on previous studies, non-dipping of nocturnal BP was defined as <10% nocturnal drop of BP.15,25,35
Statistical Analysis
t-test, Mann–Whitney U, and chi-square tests were used for normally distributed, skewed, and categorical data, respectively, to assess differences in baseline characteristics and BP at baseline and follow-up by baseline OSA subtypes (REM vs non-REM). Comparisons among all sleep-disordered breathing (SDB) groups (normal, PS, REM-OSA, and non REM-OSA) were performed by one-way ANOVA, Kruskal–Wallis 1-way ANOVA, and chi-square for parametric, non-parametric, and categorical variables respectively. Linear mixed models were used to assess the associations of OSA subtypes (REM-OSA vs others) with BP outcomes. Covariates and potential confounders defined as factors potentially related to childhood OSA and BP outcomes included sex, age, BMI, height, and parents’ self-reported hypertension (either parent vs none). Interaction effects among OSA subtypes, OSA severity, and visit time points were assessed by the models. Poisson regression was used to estimate the relative risks and 95% CIs of having hypertension or non-dipping of blood pressure at baseline and at follow-up by OSA subtypes (with non-OSA as the reference group). These models were adjusted for log10(NREM OAHI+1), age, sex, BMI and height, and family history of hypertension. A p-value of <0.05 was considered statistically significant for all analyses. Statistical analyses were performed using SPSS statistical software package V.25.0 for Windows.
Results
Sample Characteristics
There were 619 participants in the original cohort, characteristics of the participants have been reported in our previous publication.1 One participant with outlying OAHI (80.9 events/hour) at baseline and eight with REM sleep <30 minutes at baseline were excluded, leaving 610 subjects in the analysis to examine the epidemiology of stage-dependent OSA in childhood (Figure 1). The baseline characteristics and epidemiology of stage-dependent OSA are shown in Table 1. At baseline, REM-OSA was more common in our cohort (169/262, 64.5%). Those with REM-OSA had significantly longer REM sleep duration than non REM-OSA (101.9 ± 25.3 mins vs 93.3 ± 27.5 mins, p = 0.012). Those with REM-OSA had significantly lower ODI and AI arousal index than those with non REM-OSA. Expectedly, those with REM-OSA had significantly lower saturation nadir during REM stage than those with non REM-OSA, while those with non REM-OSA had significantly lower saturation nadir during NREM stage than those with REM-OSA. There were no significant differences in other characteristics such as age, sex, obesity, tonsillar hypertrophy, symptoms, and overall OAHI between the two OSA subtypes. In these 610 individuals, 476 performed ABP at baseline but ABP data from 58 participants were excluded because of inadequate valid BP measurements (21 normal, 10 PS, 27 OSA with 22 REM-OSA and 5 non REM-OSA). Those with REM-OSA had significantly lower nocturnal SBP dipping than those with PS (9.6 ± 5.9% vs 12.0 ± 5.7%, p = 0.021).
 |
 |
 |
Table 1 Baseline Characteristics of Subjects with Different Baseline OSA Status and Subtypes (N = 610) |
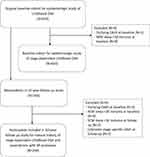 |
Figure 1 Study flow chart. |
Two hundred and forty-three (59% male) attended the 10-year follow-up visit. Only 39% of the original sample participated in the follow-up. Among the respondents, 40%, 20%, 31%, and 9% were normal control subjects, primary snorers, and individuals with mild OSA and moderate-to-severe OSA, respectively, at baseline. Comparisons between respondents and non-respondents are shown in supplementary Table 1. A higher proportion of participants with primary snoring and a lower proportion of those with moderate-to-severe OSA participated. When compared with non-respondents, respondents with mild OSA had a higher prevalence of allergic rhinitis and longer NREM sleep duration. Among those with moderate-to-severe OSA, respondents had a lower OAHI during NREM sleep than the non-respondents. Detailed characteristics of the participants have been reported in our previous publication.24
In this analysis, one participant with outlying OAHI (80.9 events/hour) at baseline, four and two with REM sleep <30 minutes at baseline and follow-up, respectively, and two with unknown stage-specific OAHI at follow-up were excluded (Figure 1). The final analysis included 234 participants, mean age at baseline and follow-up was 9.8 ± 1.8 and 20.2 ± 1.9 years, respectively. In these 234 individuals, 177 had ABP performed at baseline but ABP data from 20 participants (7 normal, 3 PS, 9 REM-OSA, and 1 non REM-OSA) were excluded due to inadequate valid measurements. Therefore, 157 participants had valid ABP data from baseline while all had valid ABP data from the follow-up visit.
In our usual practice, indications for adenotonsillectomy in children were having moderate-to-severe OSA, with ≥ grade 2 tonsillar or adenoidal hypertrophy. At baseline, all subjects who had received a diagnosis of OSA were given follow-up appointments at our outpatient clinic. As this cohort was established from a community-based sample and many were not aware of any significant sleep symptoms, few of them agreed to treatment despite the OSA diagnosis and explanation of its implications. Twenty percent of children with OSA underwent surgical treatment, while 13.5% of them received intranasal corticosteroids.24
Characteristics of the subjects at baseline and follow-up with different baseline SDB status and subtypes are shown in Table 2. At baseline, REM-OSA was more common in our cohort (58/92, 63%). Those with REM-OSA had significantly longer REM sleep duration than non REM-OSA (106.1 ± 21.9 mins vs 96.0 ± 24.8 mins, p = 0.045). Those with non REM-OSA had significantly lower saturation nadir during NREM stage than those with REM-OSA (90.8 ± 2.7% vs 92.8 ± 1.9, p < 0.001). There were no significant differences in other characteristics such as age, sex, obesity, tonsillar hypertrophy, symptoms and SpO2 nadir during REM sleep between the OSA subtypes.
 |
 |
 |
Table 2 Baseline and Follow-Up Characteristics of Subjects with Different Baseline OSA Status and Subtypes (N = 234) |
Characteristics of the subjects at follow-up by SDB status and subtypes are shown in Table 3. Here in Table 3, the subjects were categorised according to their SDB status and subtypes at follow-up. At 10-year follow-up, REM-OSA remained more common (34/58, 59%). Subjects with REM-OSA had significantly lower total OAHI (median 8.48 events/h, IQR 5.76–14.59 vs 15.22 events/h, IQR 7.68–23.20) and arousal index (median 16.45 events/h, IQR 11.85–22.47 vs 23.68 events/h, IQR 16.44–34.98) than those with non REM-OSA. There were no statistically significant differences in other clinical characteristics between the subtypes.
 |
Table 3 Characteristics at Follow-Up by SDB Status and Subtypes at 10-Year Follow-Up (N = 234) |
Natural History of OSA Subtypes
Table 4 describes the follow-up OSA status and subtypes by the subjects’ status at baseline. Overall, for those who had REM-OSA at baseline and had persistent OSA at follow-up, the majority (72%) remained to have REM-OSA regardless of the OAHI cutoff to define OSA. Among the 13 participants with REM-predominant moderate-to-severe OSA at baseline, 7 persisted to have OSA at follow-up with OAHI ≥5 events/hour, and 6 (86%) continued to have REM-OSA. Four of the 6 non-REM moderate-to-severe OSA at baseline persisted to have OSA at follow-up. In contrast, all of them evolved to be REM-OSA. Among 45 REM-predominant mild OSA at baseline, 11 had persistent OSA (OAHI ≥5 events/h) at follow-up, 7 (64%) remained REM-predominant. Among 28 non REM-predominant mild OSA at baseline, 7 (25%) had persistent OSA (OAHI ≥5 events/h) at follow-up and 5 (71%) remained as non REM-OSA. The incidence rate of OSA (with OAHI ≥5 events/h) at follow-up among those who were normal or having primary snoring at baseline was 20%. Among these adolescent/young adult-onset OSA, 15 (52%) were REM-OSA.
 |
Table 4 SDB Status and Subtypes at Baseline and Follow-Up (N = 234) |
Associations Between OSA Subtypes and BP
Associations between OSA subtypes and BP are shown in Table 5. By linear mixed model, only those with REM-OSA, but not non REM-OSA, had significant BP differences from those without OSA. When compared to non-OSA individuals, subjects with REM-OSA had significantly higher nocturnal SBP (mean difference 2.19 mmHg, 95% confidence interval (CI): 0.12, 4.26; p = 0.039) and DBP (mean difference 1.58 mmHg, 95% CI: 0.11, 3.04; p = 0.035), and less nocturnal SBP dipping (mean difference −1.84%, 95% CI: −3.25, −0.43; p = 0.011), after adjusting for age, sex, BMI, body height and parental history of hypertension. Details of the between-group differences are shown in Supplementary Table 2. There was a significant modulating effect by the visit time point (baseline vs 10-year follow-up visit) on the association between OSA subtypes and SBP dipping (p = 0.041), therefore subgroup analysis was performed and results are shown in Table 6. The significant association between REM-OSA subtype and nocturnal SBP dipping was observed at baseline visit only. Analyses were repeated for those (N = 157) who had both valid ABP measurements at baseline and follow-up, similar findings were observed (Supplementary Tables 3 and 4). In addition, when compared to non REM-OSA, those with REM-OSA also had significantly less nocturnal SBP dipping (mean difference −2.34%, 95% CI: −4.46, −0.21; p = 0.031) and significantly less DBP dipping (mean difference −2.73%, 95% CI: −5.73, −0.092; p = 0.043). Associations between OSA subtypes and BP were further evaluated using OAHI ≥5 events/h to define OSA, results are shown in Table 7. Similarly, those with REM-OSA had significantly lower nocturnal SBP dipping (mean difference −2.15%, 95% CI: −4.19, −0.12; p = 0.038) than non-OSA individuals, although other associations became insignificant.
 |
Table 5 Associations Between OSA Subtypes and Blood Pressure Among Participants from Both Visits (Pooled Data from Both Visits) |
 |
Table 6 Subgroup Analysis on the Associations Between OSA Subtypes and Nocturnal Systolic Blood Pressure Dipping Among Participants |
 |
Table 7 Associations Between OSA Subtypes and Blood Pressure Among Participants from Both Visits Using OAHI ≥5/h to define OSA(Pooled Data from Both Visits) |
At baseline and follow-up visits, the number of individuals having hypertension is shown in Tables 1 and 2. By Poisson regression, REM-OSA, when compared to non-OSA individuals, was not significantly associated with hypertension or non-dipping of nocturnal BP at both baseline and follow-up (Supplementary Tables 5–7).
Discussion
In our community-based cohort, REM-OSA was the predominant subtype (65%) of childhood OSA. REM-OSA was associated with higher nocturnal BP and a lower degree of nocturnal SBP dipping when compared to those without OSA. This association between REM-OSA and lower nocturnal SBP dipping was observed at baseline visit only in subgroup analysis. At 10-year follow-up, REM-OSA remained more common (59%). For those who had REM-OSA at baseline and had persistent OSA at follow-up, most (72%) remained to have REM-OSA.
Our study shared a similar but slightly lower prevalence of REM-predominant OSA in childhood than previous studies.6–8 The difference in the prevalence is not surprising given the significant heterogeneity in defining REM-OSA. Nonetheless, our study echoed previous studies that REM-OSA is a predominant subtype of childhood OSA. Overall the natural history of OSA did not vary significantly among different subtypes. REM-OSA remained to be more common when our participants reached late adolescence and early adulthood. We also documented that REM-OSA was a rather stable phenotype as most participants with persistent OSA in young adulthood remained to have REM-predominant disease. However, REM-OSA was relatively less prevalent among young adult-onset OSA when compared to childhood-onset OSA. Our findings are consistent with other studies that REM-OSA is more common in children than in adults.7,37
Individual variations in endotypes that predispose to airway obstruction in different sleep stages may explain distinctive polysomnographic phenotypes.9 A recent study demonstrated that individuals with REM-OSA displayed a significantly more collapsible airway in REM compared with NREM sleep, while individuals with NREM-OSA had a higher loop gain and lower ventilatory drive during obstructed breathing at arousal threshold during NREM sleep.9 The increase in airway collapsibility during REM sleep is likely related to the characteristic muscle atonia during this period of sleep.9 Pharyngeal dilators are important to maintain pharyngeal patency against factors that tend to collapse the airway. During sleep, the neuromuscular tone of the pharyngeal dilators is diminished making them less able to compensate. The muscle activity further decreases during REM sleep when compared to NREM sleep and there is decreased genioglossus muscle tone secondary to the cholinergic mediated inhibition of the hypoglossal nerve, therefore enhancing the likelihood of upper airway collapse.38–40 How the arousal threshold differs between REM and NREM sleep remains inconclusive.41 Some studies demonstrated reduced arousal threshold during REM sleep while others reported equal or increased arousal threshold during REM sleep when compared to NREM sleep.38,41 In fact, a recent study reported a higher arousal threshold in REM than NREM sleep in individuals with REM-OSA.9 In our study, we observed a lower arousal index in those with REM-OSA at both baseline and follow-up. The arousal indexes were also generally higher at 10-year follow-up than baseline in all OSA subtypes, which was consistent with previous findings of decreasing arousal threshold with increasing age.42 Similarly, the changes in ventilatory control during sleep with aging have not been fully elucidated. As there is an increasing prevalence of SDB and central apneas in the elderly, it is postulated that the stability in ventilatory control decreases with aging. However, studies have demonstrated contrasting results in this aspect that some reported an increase in loop gain in the elderly42 while others did not.43 Further studies are needed to evaluate how the ontogenetic variations in the endotypes, including airway collapsibility, arousal threshold, and ventilatory control, influence the patterns of occurrence of disordered breathing in different sleep stages across age groups.
REM-OSA was associated with higher nocturnal BP and lower degree of nocturnal SBP dipping when compared with those without OSA. Those with REM-OSA also demonstrated worse BP outcomes when compared with non REM-OSA when we narrowed the analysis to those with BP outcomes from both baseline and follow-up visits. However, such differences were not observed between those with non REM-OSA and those without OSA. Similar results and cardiovascular risks were previously reported in adult REM-OSA.4,15–17,44 Sympathetic activation is believed to be one of the major mechanisms by which OSA increases cardiovascular risk. REM sleep is associated with greater sympathetic activity and cardiovascular instability than NREM sleep in both healthy subjects and patients with OSA.45–47 Such acute changes in hemodynamics and oxygen saturation caused by respiratory events during REM sleep in REM-OSA could play an important role in conferring a higher cardiovascular risk.4 Interestingly, with subgroup analysis, the significant association between REM-OSA and lower nocturnal SBP dipping was observed at the baseline visit only. Although the exact reason is not well understood, it is important to note that at follow-up, subjects with REM-OSA had less severe OSA than those with non REM-OSA as reflected by the lower total OAHI and arousal index while the two OSA subtypes shared similar disease severity at baseline. This finding is consistent with a recent adult study.9 The differences in the overall OSA severity between the two subtypes at follow-up likely have significantly modulated the effect of stage-dependent OSA on the BP outcomes. Moreover, the evaluation between REM-OSA and BP outcomes at follow-up was likely limited by the sample size. The complex relationship between stage-dependent OSA and OSA severity on cardiovascular outcomes remains to be explored.
A unique community-based longitudinal cohort that allowed the observation of the natural history of stage-dependent OSA was a strength in our study. This is one of few studies which evaluated the prevalence and clinical significance of REM-OSA in children. However, our study had certain limitations. In our previous publication, we found moderate-to-severe OSA in childhood was associated with a higher risk of having hypertension at 10-year follow-up when compared to individuals without OSA.25 However, we did not find any significant associations between REM-OSA and risks of hypertension in the current analysis. Subtyping of the disease limited the sample size in each OSA subtype and therefore restricted the study power. The associations between OSA subtypes and BP outcomes could not be fully evaluated with the current sample size. Studies with a large sample size would be needed to further explore the associations. Moreover, even after statistical adjustment for NREM OAHI as a covariate in some of our models, residual confounding effects of OSA events not occurring during REM sleep could not be fully eliminated. In our cohort, many declined treatment primarily because they were recruited from the community and in their parents’ perspective were relatively asymptomatic. Only 20% of children with OSA underwent surgery, while 14% received intranasal corticosteroids. Moreover, they received treatments at variable time points. Therefore, the effects of OSA treatment on the associations between stage-dependent OSA and BP outcomes could not be assessed systematically. Finally, the mechanisms of the development of stage-dependent OSA and how it is associated with BP outcomes have not been explored in our study. Future studies assessing the pathophysiologic endotypes, endotype differences between age groups, and mechanistic pathways linking to cardiovascular complications would be essential to guide individualized management of stage-dependent OSA.
Conclusion
REM-OSA was a common subtype in childhood OSA and remained to be prevalent in adolescent/young adult OSA. It was associated with higher nocturnal SBP and less nocturnal SBP dipping. Future research is needed to further evaluate the clinical significance and mechanisms of predisposition to stage-dependent OSA in children to redefine treatment strategies for stage-dependent OSA.
Abbreviations
ABP, ambulatory blood pressure; ArI, arousal Index; BMI, body Mass Index; BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; MAP, mean arterial pressure; NREM, non rapid-eye-movement; OAHI, obstructive apnea hypopnea index; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PS, primary snoring; PSG, polysomnography; REM, rapid-eye-movement; SaO2, arterial oxyhemoglobin saturation; SBP, systolic blood pressure; SDB, sleep disordered breathing.
Acknowledgments
This research project was supported by funding from the Research Grants Council of the Hong Kong Special Administrative Region, China (CUHK 470913). The authors thank all the participants and their parents for their contribution in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research project was supported by the Research Grants Council of the Hong Kong Special Administrative Region, China (CUHK 470913). The funder had no role in study design, data collection and analysis, preparation of the manuscript or in the decision to submit the paper for publication.
Disclosure
Professor Yun K Wing report grants from General Research Fund of University Grants Committee, grants from Health and Medical Research Fund, personal fees from Eisai Co., Ltd, personal fees from Lundbeck HK Limited, outside the submitted work. All authors report no other conflicts of interest in this work.
References
1. Li AM, So HK, Au CT, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010;65(11):991–997. doi:10.1136/thx.2010.134858
2. Dehlink E, Tan HL. Update on paediatric obstructive sleep apnoea. J Thorac Dis. 2016;8(2):224–235. doi:10.3978/j.issn.2072-1439.2015.12.04
3. Horner RL, Hughes SW, Malhotra A. State-dependent and reflex drives to the upper airway: basic physiology with clinical implications. J Appl Physiol. 2014;116(3):325–336. doi:10.1152/japplphysiol.00531.2013
4. Alzoubaidi M, Mokhlesi B. Obstructive sleep apnea during rapid eye movement sleep: clinical relevance and therapeutic implications. Curr Opin Pulm Med. 2016;22(6):545–554. doi:10.1097/MCP.0000000000000319
5. Vyazovskiy VV, Delogu A. NREM and REM sleep: complementary roles in recovery after wakefulness. Neuroscientist. 2014;20(3):203–219. doi:10.1177/1073858413518152
6. Verginis N, Jolley D, Horne RSC, Davey MJ, Nixon GM. Sleep state distribution of obstructive events in children: is obstructive sleep apnoea really a rapid eye movement sleep-related condition? J Sleep Res. 2009;18(4):411–414. doi:10.1111/j.1365-2869.2009.00760.x
7. Spruyt K, Gozal D. REM and NREM sleep-state distribution of respiratory events in habitually snoring school-aged community children. Sleep Med. 2012;13(2):178–184. doi:10.1016/j.sleep.2011.10.025
8. El-Kersh K, Cavallazzi R, Patel PM, Senthilvel E. Effect of sleep state and position on obstructive respiratory events distribution in adolescent children. J Clin Sleep Med. 2016;12(4):513–517. doi:10.5664/jcsm.5678
9. Joosten SA, Landry SA, Wong A-M, et al. Assessing the physiologic endotypes responsible for REM- and NREM-based OSA. Chest. 2021;159(5):1998–2007. doi:10.1016/j.chest.2020.10.080
10. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. PMID: 23721582; PMCID: PMC3826282. doi:10.1164/rccm.201303-0448OC
11. Kass JE, Akers SM, Bartter TC, Pratter MR. Rapid-eye-movement-specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med. 1996;154(1):167–169. doi:10.1164/ajrccm.154.1.8680674
12. Lee S-A, Paek J-H, Han S-H. REM-related sleep-disordered breathing is associated with depressive symptoms in men but not in women. Sleep Breath. 2016;20(3):995–1002. doi:10.1007/s11325-016-1323-2
13. Varga AW, Kishi A, Mantua J, et al. Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. J Neurosci. 2014;34(44):14571–14577. doi:10.1523/JNEUROSCI.3220-14.2014
14. Hoshino T, Sasanabe R, Tanigawa T, et al. Effect of rapid eye movement-related obstructive sleep apnea on adherence to continuous positive airway pressure. J Int Med Res. 2018;46(6):2238–2248. doi:10.1177/0300060518758583
15. Mokhlesi B, Hagen EW, Finn LA, Hla KM, Carter JR, Peppard PE. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin sleep cohort. Thorax. 2015;70(11):1062–1069. doi:10.1136/thoraxjnl-2015-207231
16. Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin sleep cohort. Am J Respir Crit Care Med. 2014;190(10):1158–1167. doi:10.1164/rccm.201406-1136OC
17. Appleton SL, Vakulin A, Martin SA, et al. Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest. 2016;150(3):495–505. doi:10.1016/j.chest.2016.03.010
18. Liu Y, Su C, Liu R, et al. NREM-AHI greater than REM-AHI versus REM-AHI greater than NREM-AHI in patients with obstructive sleep apnea: clinical and polysomnographic features. Sleep Breath. 2011;15(3):463–470. doi:10.1007/s11325-010-0358-z
19. Chami HA, Baldwin CM, Silverman A, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med. 2010;181(9):997–1002. doi:10.1164/rccm.200908-1304OC
20. Oh SM, Choi SH, Kim HJ, Park KS, Lee YJ. The association between obstructive sleep apnea during REM sleep and autonomic dysfunction as measured by heart rate variability. Sleep Breath. 2019;23(3):865–871. doi:10.1007/s11325-018-01779-y
21. Nisbet LC, Yiallourou SR, Biggs SN, et al. Preschool children with obstructive sleep apnea: the beginnings of elevated blood pressure? Sleep. 2013;36(8):1219–1226. doi:10.5665/sleep.2890
22. Geng X, Wu Y, Ge W, et al. Ambulatory blood pressure monitoring in children with obstructive sleep apnea syndrome. Pediatr Investig. 2019;3(4):217–222. doi:10.1002/ped4.12163
23. Au CT, Ho CKW, Wing YK, Li AM. The effect of childhood obstructive sleep apnea on ambulatory blood pressure is modulated by the distribution of respiratory events during rapid eye movement and nonrapid eye movement sleep. Sleep Med. 2013;14(12):1317–1322. doi:10.1016/j.sleep.2013.09.017
24. Chan KC, Au CT, Hui LL, Ng S-K, Wing YK, Li AM. How OSA evolves from childhood to young adulthood: natural history from a 10-year follow-up study. Chest. 2019;156(1):120–130. doi:10.1016/j.chest.2019.03.007
25. Chan KCC, Au CT, Hui LL, Wing YK, Li AM. Childhood OSA is an independent determinant of blood pressure in adulthood: longitudinal follow-up study. Thorax. 2020;75(5):422–431. doi:10.1136/thoraxjnl-2019-213692
26. Li AM, Cheung A, Chan D, et al. Validation of a questionnaire instrument for prediction of obstructive sleep apnea in Hong Kong Chinese children. Pediatr Pulmonol. 2006;41(12):1153–1160. doi:10.1002/ppul.20505
27. Leung SS, Cole TJ, Tse LY, Lau JT. Body mass index reference curves for Chinese children. Ann Hum Biol. 1998;25(2):169–174. doi:10.1080/03014469800005542
28. Berry RB, Brooks R, Gamaldo CE, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.0. Darien, Illinois: American Academy of Sleep Medicine; 2012.
29. American Academy of Sleep Medicine. International Classification of Sleep Disorders.
30. Li AM, Au CT, Sung RY, et al. Ambulatory blood pressure in children with obstructive sleep apnoea: a community based study. Thorax. 2008;63(9):803–809. PMID: 18388205. doi:10.1136/thx.2007.091132
31. Yip GWK, Li AM, So H-K, et al. Oscillometric 24-h ambulatory blood pressure reference values in Hong Kong Chinese children and adolescents. J Hypertens. 2014;32(3):606–619. doi:10.1097/HJH.0000000000000062
32. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557–576. doi:10.1016/j.cjca.2017.03.005
33. Kazuomi K. Nocturnal hypertension. Hypertension. 2018;71(6):997–1009. doi:10.1161/HYPERTENSIONAHA.118.10971
34. Li AM, Au CT, Ng C, Lam HS, Ho CKW, Wing YK. A 4-year prospective follow-up study of childhood OSA and its association with BP. Chest. 2014;145(6):1255–1263. doi:10.1378/chest.13-1333
35. Crinion SJ, Ryan S, McNicholas WT. Obstructive sleep apnoea as a cause of nocturnal nondipping blood pressure: recent evidence regarding clinical importance and underlying mechanisms. Eur Respir J. 2017;49(1):1601818. doi:10.1183/13993003.01818-2016
36. Boggia J, Li Y, Thijs L, et al.; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) investigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370(9594):1219–1229. PMID: 17920917. doi:10.1016/S0140-6736(07)61538-4
37. Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162(2 Pt 1):682–686. doi:10.1164/ajrccm.162.2.9908058
38. Varga AW, Mokhlesi B. REM obstructive sleep apnea: risk for adverse health outcomes and novel treatments. Sleep Breath. 2019;23(2):413–423. doi:10.1007/s11325-018-1727-2
39. Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187(3):311–319. doi:10.1164/rccm.201209-1654OC
40. McSharry DG, Saboisky JP, DeYoung P, et al. Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep. 2014;37(3):561–569. doi:10.5665/sleep.3498
41. Ermis U, Krakow K, Voss U. Arousal thresholds during human tonic and phasic REM sleep. J Sleep Res. 2010;19(3):400–406. doi:10.1111/j.1365-2869.2010.00831.x
42. Chowdhuri S, Pranathiageswaran S, Loomis-King H, Salloum A, Badr MS. Aging is associated with increased propensity for central apnea during NREM sleep. J Appl Physiol. 2017;124(1):83–90. doi:10.1152/japplphysiol.00125.2017
43. Wellman A, Malhotra A, Jordan AS, Schory K, Gautam S, White DP. Chemical control stability in the elderly. J Physiol. 2007;581(Pt 1):291–298. doi:10.1113/jphysiol.2006.126409
44. Peter JH, Grote L, Fus E, Ploch T, Stammnitz A. REM-sleep-hypertension in obstructive sleep apnea. Eur J Med Res. 1995;1(3):132–136.
45. Kohler M, Stradling JR. CrossTalk proposal: most of the cardiovascular consequences of OSA are due to increased sympathetic activity. J Physiol. 2012;590(12):
46. Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328(5):303–307. doi:10.1056/NEJM199302043280502
47. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi:10.1172/JCI118235
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
