Back to Journals » International Medical Case Reports Journal » Volume 13
Natural History of a Large Bubble of Migratory Submacular Perfluorocarbon
Authors Okonkwo ON , Sibanda D , Akanbi T, Hassan AO
Received 27 June 2020
Accepted for publication 25 August 2020
Published 7 October 2020 Volume 2020:13 Pages 477—486
DOI https://doi.org/10.2147/IMCRJ.S266528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ogugua Ndubuisi Okonkwo,1,2 Dennis Sibanda,3 Toyin Akanbi,2 Adekunle Olubola Hassan1,2
1Department of Ophthalmology, Eye Foundation Retina Institute, Lagos, Lagos State, Nigeria; 2Ophthalmology Clinics, Eye Foundation Hospital, Apo, Abuja, Nigeria; 3Department of Ophthalmology, The Eye Zone, Harare, Zimbabwe
Correspondence: Ogugua Ndubuisi Okonkwo
Eye Foundation Retina Institute, 27 Isaac John Street, GRA, Ikeja, Lagos, Nigeria
Tel +234 8035027308
Email [email protected]
Purpose: To report the natural history of a large bubble of perfluorocarbon liquid (PFCL) in a parafoveal subretinal position which was monitored using optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). The bubble of PFCL was removed when it migrated to the subfoveal position; outcome after removal is also reported.
Observation: A 62-year-old male, after repair of a giant retinal tear (GRT) detachment, regained Snellen acuity of 6/18 from preoperative vision of CF at 1 meter. A large bubble of subretinal PFCL was in the superior parafoveal area. For 18 months, the PFCL bubble was monitored using OCT and OCTA until it migrated into the subfoveal position after 22 months, coinciding with a decrease in vision to 6/36. Surgical removal of subfoveal PFCL was performed. This involved detachment of the foveomacular, epiretinal membrane (ERM) peel (complicated by iatrogenic macular hole (MH) formation), intravitreal injection of PFCL to displace subretinal PFCL to the periphery, creation of an inverted internal limiting membrane (ILM) flap to repair the MH and air exchange. Postoperative vision remained 6/36. His postoperative OCT showed significant loss of subfoveal outer retina. On OCTA, the superficial and deep vascular plexi and choriocapillaris appeared to be intact after the removal of the subfoveal PFCL.
Conclusion and Importance: This report suggests that a large superior parafoveal bubble of PFCL may take as much as 18 months to migrate to the subfoveal position. Before this time, vision does not appear to be affected, though macular edema is present. Therefore, removal of the PFCL can be delayed until a convenient time for surgeon and patient. A large bubble of parafoveal PFCL ought to be removed before migrating to a subfoveal position and vision loss, since its removal will reduce the risk of outer retina loss.
Keywords: perfluorocarbon liquid, sub-retinal, complications, vitreoretinal surgery, retinal detachment, giant retinal tear
Introduction
Perfluorocarbon liquid (PFCL) is a useful adjunct in complex vitreoretinal surgery.1 Submacular migration of PFCL is an uncommon complication of its use and occurs when the PFCL bubble migrates through the retina break into the subretinal space. This may occur when there are large retinal breaks (such as giant retinal tear (GRT) or large retinectomy), unreleased vitreoretinal traction and increased PFCL turbulence within the vitreous cavity.2 There are several reports of surgical removal of subfoveal PFCL, but there is no agreed time or technique for its removal. Some reports describe a direct aspiration of the subretinal PFCL using a small gauge cannula,3–6 while others have opted for a displacement of the PFCL bubble.7 Also, epiretinal membrane (ERM) has been described with submacular PFCL and may require removal.
We present a case of a large parafoveal submacular PFCL bubble in the left eye of a male patient which was monitored using optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) until it migrated into the subfoveal position, after which it was removed. From this case report, we derive information about the natural history of such large parafoveal PFCL bubble.
Case Report
A 62-year-old male presented to the retina clinic with a GRT detachment in his left eye (his right eye had been blind for several years), reducing vision to counting fingers at 1 meter. Following the repair of the retinal detachment (using a combined vitrectomy and encirclement band) in December 2017, his vision improved to 6/36. In the early postoperative period, a large bubble of sub-macular PFCL was noted supero-temporal to the fovea. He was reluctant to having this PFCL bubble removed, since he did not wish to risk a reduction in his gained vision.
After 4 months, he had an uneventful phacoemulsification of cataract, with implantation of a posterior chamber intraocular lens and silicone oil removal (SOR). His left eye vision improved to 6/18, N10 (aided). His vision was stable for over 18 months despite the presence of the parafoveal PFCL. His macula status and the PFCL bubble were monitored using OCT and OCTA, and he was quite satisfied with his vision. At his clinic visit in October 2019 (22 months post-primary retinal surgery), the parafoveal PFCL had migrated to the subfoveal position and this was associated with a reduction in vision to 6/36.
As a result of the decrease in vision, he consented to the removal of the subfoveal PFCL. He also had an ERM at this time and the surgical plan was to remove both subfoveal PFCL and ERM.
Surgical steps for the intended surgery was, first detach the foveo-macula, permitting the displacement (using intravitreal injection of PFCL) of the subfoveal PFCL away from the fovea to a peripheral retina position where it can be safely and easily removed via a retinotomy. Peel the ERM and perform air tamponade. It was the thinking that the injection of subretinal fluid would guarantee a more complete removal of all subfoveal PFCL compared to a trans foveal drainage approach.
Surgery was performed in October 2019. As in previous surgeries, he gave written informed consent. During surgery, a 39-gauge flexible cannula was used to inject subretinal balanced salt solution (BSS) superior to the fovea (Figure 1), detaching the entire foveo-macula area (Figure 2). A thick ERM was peeled from the detached macula (Figures 3 and 4). This was complicated by the formation of an iatrogenic macular hole (MH). Intra-vitreal injection of PFCL displaced the subretinal fluid with the bubble of PFCL to the retina periphery. An inverted internal limiting membrane (ILM) flap was created and used to close the MH. A superior drainage retinotomy enabled drainage of all subretinal fluid, which included the displaced PFCL bubble. Air fluid exchange was performed, and the vitreous cavity was filled with air. He was advised to have 3 days of face-down position.
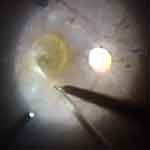 |
Figure 1 Introduction of the 39G flexible cannula into the subretinal space in the superior macula area and injection of balanced salt solution into the subretinal space. |
 |
Figure 2 Detachment of the foveomacular retina using the slow injection of balanced salt solution into the subretinal space. |
 |
Figure 3 Initiating the epiretinal membrane peel from the macula area. |
 |
Figure 4 Peeled off epiretinal membrane. |
Since this was a single case report, the institutional review board (IRB) waived the need for a formal review process. Institutional approval was required to publish this case report and a permission to report the case was given by the IRB. A verbal informed consent for publication of his case report and all accompanying images was given by the patient. He was in a remote locality, with limited access to electronic media and was unable to give a written informed consent. This verbal informed consent was approved by the IRB.
OCT and OCTA During the Period of Monitoring and After PFCL Removal
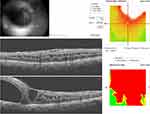
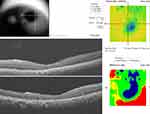
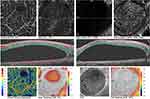
Conventional OCT was more valuable than the OCTA in assessing the effect of the PFCL on the outer retina. Pre- and post-operative OCT images as seen in Figures 5–7, show the subretinal location of the PFCL bubble, and intraretinal cystic spaces. Figure 5 represents the parafoveal location of the PFCL, while Figure 6 is the subfoveal migration. In Figure 7 the post-operative microstructure of the retina is imaged. While there is subfoveal ellipsoid zone (EZ) and external limiting membrane (ELM) in Figure 5, these layers are disrupted in Figures 6 and 7. In Figure 7, there is the presence of subfoveal RPE; the photoreceptors in the subfoveal area are replaced by drusen like material which is most likely degenerated photoreceptors. Also, a layer of ERM is present in the preoperative Figures 5 and 6, but absent in Figure 7.
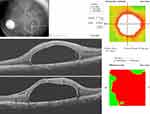 |
Figure 6 Line scans showing interrupted subfoveal ellipsoid zone (EZ) and external limiting membrane (ELM) over the large bubble of subfoveal PFCL. ERM is also present. |
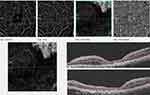
There were significant projection artifacts on OCTA due to the large size of the PFCL bubble. The presence of significant areas of hypo autofluorescence in the OCTA image was as a result of this projection artifacts and imaging within the large PFCL bubble. This demonstrates the difficulty of OCTA imaging of such a large bubble of subretinal PFCL.
Preoperative OCTA of the parafoveal PFCL bubble in Figure 8 showed the deep capillary plexus was intact. The foveal avascular zone (FAZ) was oval-shaped, with a wider horizontal diameter. The FAZ appeared to be wider in the deep plexus compared to the superficial plexus. In the choriocapillaris layer, there was a well-circumscribed hyperreflective area within a circular boundary of hypo reflectivity corresponding to the boundary of the PFCL bubble, due to reflectivity patterns from the PFCL. Similar hyperreflectivity was also present in the foveal area.
In the preoperative subfoveal OCTA scans in Figure 9 the superficial capillary plexus could be imaged with some difficulty and showed intact vasculature. The choriocapillaris still showed similar diffuse hyperreflectivity within a circular hyporeflective boundary corresponding to the area of the subfoveal PFCL bubble.
Postoperative OCTA image in Figure 10, suggests there was an improvement in the overall ability to image the vasculature of the retina as shown by improved imaging of the deep and superficial capillary plexus. Imaging of the FAZ in the superficial plexus was defective because of persistent retinal swelling from intraretinal edema. In the deep plexus, the FAZ could be better imaged. In general, there was a fairly intact superficial and deep capillary plexi.
The most significant finding was in the avascular outer retina. There was a wedge-shaped patch of hyperreflectivity in the superior parafoveal area corresponding to the area of initial PFCL bubble localization. The choriocapillaris layer no longer had the previous hyper- and hypo-reflective areas described in the preoperative image’s Figures 8 and 9.
Discussion
PFCL is an invaluable tool in vitreoretinal surgery; for complex vitreoretinal presentations such as GRT, trauma-related cases, proliferative vitreoretinopathy (PVR) and submacular hemorrhage.1,8 Subfoveal retention of PFCL is a recognized complication and several authors have described the management of this complication. Prevention of subretinal PFCL includes injection of the PFCL into a single bubble, reduction of infusion flow to decrease turbulence and keeping the PFCL level below the infusion to prevent the formation of bubbles. Use of valve cannula which create less turbulence compared to the non-valve cannula is also helpful in preventing this complication. There are reports on the toxic effect of PFCL on the RPE and photoreceptors.9 Research on experimental animal models have suggested inflammatory, chemical or mechanical compression as mechanism of this damage.10,11 When in a subfoveal position, PFCL removal is often performed; however, there is no consensus on the timing and technique, and this is open to debate.
Suk and Flynn reported on eight eyes diagnosed to have subretinal PFCL. Seven of these eyes were managed by observation.12 In their series one of the eyes with a subfoveal PFCL bubble maintained 20/25 vision during the 4 years of observation. Therefore, immediate removal of subfoveal PFCL is not indicated in all cases and useful vision can be maintained despite the presence of subfoveal PFCL. Also, there are reports of improvement in vision after PFCL removal, even following prolonged retention of subfoveal PFCL.13 These reports question if PFCL is indeed toxic to the surrounding retina, since its removal after chronic contact with surrounding retina does not prevent visual improvement. It may suggest that the PFCL mostly has a mechanical-compressive effect on the surrounding retina, which is reversible or in some cases tolerable. The case we have reported had a large bubble of PFCL whose natural history (until removal) seems to support the observation that parafoveal PFCL, even with significant edema may be associated with stable vision for several months. PFCL migration into the subfoveal position coincided with a reduction in vision; suggesting that the subfoveal migration of PFCL was responsible for the decrease in vision.
The migratory nature of subretinal PFCL is well known. The intraoperative peripheral location and subsequent post-operative migration of peripherally located subretinal PFCL to the posterior pole may be the reason why subretinal PFCL is missed at primary surgery. In this case, the migration of PFCL from the superior parafoveal to the subfoveal position occurred over an 18-month period. Serial OCT was used to observe this migratory movement as an elongation; changing from a circular bubble to a more ellipsoid shape. Despite the long duration of sub-retinal PFCL in this case (and in several published case reports), there was no sub-retinal emulsification of PFCL seen. Large bubbles of intravitreal and anterior chamber PFCL does easily emulsify. The physical tightness within the subretinal space, on the PFCL bubble, may exert a restraining effect on the PFCL. While in some OCT images of subretinal PFCL, there is only a thin (presumably ILM) roof over the PFCL bubble, in some other images there is a full-thickness neurosensory retina roof over the PFCL bubble, as in our case. The physical restraining grip on the bubble of PFCL by this stretching roof may be different in these two scenarios and influence the likelihood to migrate.
The size of the subretinal PFCL bubble and location is important in decision making. Migration is more likely to occur if the PFCL bubble is large. If a large bubble of PFCL is seen in the posterior pole, removal should be undertaken, and can be done at the time of silicone oil removal; direct aspiration of the bubble is an option. On the other hand, very, small bubbles are less likely to migrate. Bubbles in the inferior periphery tend to remain stable. Another option if the PFCL bubble is not in the posterior pole is to demarcate them with laser to reduce the chance of migration. If delay in the removal of the bubble is anticipated, the patient can be positioned face down (as for sleep) so that the bubble by gravity migrates to the periphery.
Monitoring: OCT and OCTA Features
Submacular PFCL is best evaluated using OCT imaging. OCTA provides additional information about the vasculature of the surrounding layers of the retina. So, we performed serial OCT and OCTA to determine microstructural characteristics of the surrounding retina and the state of the macular and choroidal vasculature. While OCT features of subfoveal PFCL have been described previously,14,15 only Wang et al reported on OCTA imaging of an eye before and after submacular PFCL removal.16 The size of the PFCL bubble in our case was much larger than that reported by Wang et al, and this resulted in considerable imaging difficulty and image artifacts. Wang et al suggest retinal capillary plexi seem to improve after removal of the PFCL.
We observed that removal of the PFCL bubble improved the quality of the image acquired. There was better overall superficial and deep capillary vascular network imaging postoperatively. Also, we found that the retinal vasculature appeared to be intact after such prolonged presence of submacular PFCL. The large bubble of PFCL to our surprise did not appear to have profound toxic effect on the retinal vasculature (as shown by OCTA). This further increases the suspicion of a mechanical effect on the outer retina by the PFCL as the major mechanism of damage. Wang et al reported recovery and reduction in the size of the FAZ months after surgery. The enlarged post-operative FAZ noted in our case may be related to the iatrogenic MH and surgery to repair the MH. It was our view, to avoid comparing between pre- and post-operative OCTA images as significant retinal distortion and image artifacts may yield the conclusion of “improvement of retinal capillary plexi” inaccurate. It is possible that the apparent changes are due to better postoperative image quality rather than real improvement.
ERM has been reported to occur with submacular PFCL and following its removal.17 The exact mechanism for this is unclear but is likely inflammatory in origin. Chandra et al have suggested that this may arise due to RPE cells escaping from the retinotomy created.17 In our case, a significant ERM was present. This ERM may be associated with the primary GRT. More proliferating RPE cells have access to the epiretinal surface following a GRT. Also, the vitrectomy procedure can be a cause of the ERM. The peeling of this ERM resulted in the iatrogenic MH, due to the tangential force exerted during the peel. Repair of the MH was achieved without difficulty. It is worthwhile mentioning that the ERM could have been peeled prior to inducing a detachment of the posterior pole. An attached retina during the ERM peel will provide the counter (opposing) force to the traction generated during the peel. This may have ultimately made the ERM peel easier, and since there is reduced traction on the retina there is reduced chance of creating an iatrogenic MH.
MHs have been reported to occur spontaneously following a submacular bubble of PFCL.18 Therapeutic MHs have also been created for the removal of the PFCL.19 According to previous reports, it does not appear that MH formation is associated with non-improvement in vision since most reported cases in literature recorded gains in vision. However, it is worth mentioning that the iatrogenic MH is a possible cause of non-improvement of visual acuity and outer retinal layer damage in our case.
Conclusion
The decision for surgical intervention in this patient was primarily because of the decrease in his vision. Postoperative vision did not return to the pre-operative 6/18 acuity. Based on this case report and several other reports considering the subject of submacular PFCL, it seems prudent not to wait until subfoveal migration occurs and vision deteriorates before parafoveal PFCL is removed in such large parafoveal PFCL. Subretinal PFCL can result in an absolute scotoma and if subfoveal can cause permanent and irreversible visual deterioration. PFCL removal is likely to be beneficial to the retinal capillary network and choriocapillaris, help resolution of macular edema, preserve the retinal microstructure and prevent outer retina damage. Removal of the parafoveal PFCL can be undertaken at a time convenient for surgeon and patient as a routine surgery.
Patient Consent
The patient gave written informed consent before each surgery and gave verbal consent to the publication of this case report. All efforts have been made towards anonymity of the patient’s identity.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding or grant support.
Disclosure
All of the authors have no financial interest to declare.
References
1. Chang S, Lincoff H, Zimmerman NJ, Fuchs W. Giant retinal tears. Surgical techniques and results using perfluorocarbon liquids. Arch Ophthalmol. 1989;107:761–766.
2. Garcia-Valenzuela E, Ito Y, Abrams GW. Risk factors for retention of subretinal perfluorocarbon liquid in vitreoretinal surgery. Retina. 2004;24(5):746–752. doi:10.1097/00006982-200410000-00010
3. Liu W, Gao M, Liang X. Management of subfoveal perfluorocarbon liquid: a review. Ophthalmologica. 2018;240(1):1–7. doi:10.1159/000488118
4. Shulman M, Sepah YJ, Chang S, Abrams GW, Do DV, Nguyen QD. Management of retained subretinal perfluorocarbon liquid. Ophthalmic Surg Lasers Imaging Retina. 2013;44:577–583. doi:10.3928/23258160-20131105-07
5. Lin Z, Chen Y, Gao S, Zhong Y, Shen X. Surgical removal of submacular perfluorocarbon liquid with a 38-gauge flexible cannula combined with internal limiting membrane peeling and intravitreal air tamponade: a case series. BMC Ophthalmol. 2018;18(1):132. doi:10.1186/s12886-018-0798-y
6. De Cillà S, Alkabes M, Radice P, Carini E, Mateo C. Direct transretinal removal of subfoveal perfluorocarbon liquid: the role and timing of internal limiting membrane peeling. European J Ophthalmol. 2017;27(2):249–252. doi:10.5301/ejo.5000934
7. Sakurai E, Ogura Y. Removal of residual subfoveal perfluoro-n-octane liquid. Graefes Arch Clin Exp Ophthalmol. 2007;245:1063–1064. doi:10.1007/s00417-007-0535-3
8. Chang S, Ozmert E, Zimmerman NJ. Intraoperative perfluorocarbon liquids in the management of proliferative vitreoretinopathy. Am J Ophthalmol. 1988;106:668–674. doi:10.1016/0002-9394(88)90698-8
9. Tewari A, Eliott D, Singh CN, Garcia-Valenzuela E, Ito Y, Abrams GW. Changes in retinal sensitivity from retained subretinal perfluorocarbon liquid. Retina. 2009;29(2):248–250. doi:10.1097/IAE.0b013e318188c7ea
10. de Queiroz JM
11. Berglin L, Ren J, Algvere PV. Retinal detachment and degeneration in response to subretinal perfluorodecalin in rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 1993;231:233–237. doi:10.1007/BF00918847
12. Suk KK, Flynn HW. Management options for submacular perfluorocarbon liquid. Ophthalmic Surg Lasers Imaging. 2011;42(4):284–291. doi:10.3928/15428877-20110603-07
13. Lai JC, Postel EA, Mccuen BW. Recovery of visual function after removal of chronic subfoveal perfluorocarbon liquid. Retina. 2003;23(6):868–870. doi:10.1097/00006982-200312000-00022
14. Soheilian M, Nourinia R, Shoeibi N, Peyman GA. Three-Dimensional OCT features of perfluorocarbon liquid trapped under the fovea. Ophthalmic Surg Lasers Imaging. 2010;1–4.
15. Sridhar J, Shahlaee A, Vander JF. En face optical coherence tomography of subfoveal perfluorocarbonliquid.Ophthalmology.2015;122:1853. doi:10.1016/j.ophtha.2015.06.034
16. Wang H, Chen F, Cao H. Optical coherence tomography angiography characteristics of fovea in residual subfoveal perfluorocarbon liquid eye. Ophthalmic Surgery, Lasers and Imaging Retina. 2016;47(11):1062–1066.
17. Chandra P, Kumar V, Takkar B, Kumar A. Epiretinal membrane development after submacular perfluorocarbon liquid removal. Oman J Ophthalmol. 2017;10(3):253–254. doi:10.4103/ojo.OJO_39_2015
18. Oellers P, Charkoudian LD, Hahn P. Spontaneous resolution of subfoveal perfluorocarbon. Clin Ophthalmol. 2015;9:517–519. doi:10.2147/OPTH.S82019
19. Kim JM, Woo SJ, Park KH, Chung H. Surgical removal of retained subfoveal perfluorocarbon liquid through a therapeutic macular hole with intravitreal PFCL injection and gas tamponade. Korean J Ophthalmol. 2013;27(5):392.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.