Back to Journals » Journal of Asthma and Allergy » Volume 11
Nasopharyngeal isolates and their clinical impact on young children with asthma: a pilot study
Authors Alsuwaidi AR , Alkalbani AM , Alblooshi A , George J, Albadi G, Kamal SM, Narchi H , Souid AK
Received 2 April 2018
Accepted for publication 5 June 2018
Published 12 September 2018 Volume 2018:11 Pages 233—243
DOI https://doi.org/10.2147/JAA.S169966
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Ahmed R Alsuwaidi,1 Alia M Alkalbani,2 Afaf Alblooshi,1 Junu George,1 Ghaya Albadi,1 Salwa M Kamal,3 Hassib Narchi,1 Abdul-Kader Souid1
1Department of Pediatrics, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates; 2Tawam Hospital, Abu Dhabi Health Services Company (SEHA), Al Ain, United Arab Emirates; 3Ambulatory Healthcare Services, SEHA, Abu Dhabi, United Arab Emirates
Introduction: Respiratory infections have significant effects on childhood asthma. Viral respiratory infections, such as rhinovirus and respiratory syncytial virus are likely to be important in the development and exacerbation of asthma. In this study, we investigated the nasopharyngeal colonization in children with asthma to determine the prevalence of pathogens and their contribution to respiratory symptoms and airway resistance during winter.
Methods: From December 2016 to March 2017, 50 nasopharyngeal specimens were collected from 18 patients (age, 5.0±1.1 years) with asthma and 9 specimens from 9 control children (age, 4.9±1.0 years). Samples were tested for 19 viruses and 7 bacteria, using multiplex real-time PCR. Respiratory disease markers included the Global Asthma Network Questionnaire, the Common-Cold Questionnaire, the Global Initiative for Asthma assessment of asthma control, and the airway resistance at 5 Hz by forced-oscillation technique.
Results: The most commonly isolated organisms in both groups (patients and controls) were Streptococcus pneumoniae, Haemophilus influenzae, and rhinovirus. Most patients had multiple isolates (median, 3.5; range, 1–5), which changed during the study period. Types of isolates were 4 bacteria (S. pneumoniae, H. influenzae, Bordetella pertussis, and Bordetella parapertussis) and 6 viruses (rhinovirus, enterovirus, metapneumovirus, adenovirus, coronaviruses, and parainfluenza viruses). Similar isolates, including influenza A-H3 virus and bocavirus, were detected in the controls. Of the 9 patients with “wheezing disturbing sleep ≥1 per week”, 6 had rhinovirus, 2 coronaviruses, and 1 no detectable viruses. Patients with mild common cold symptoms had significantly higher airway resistance at 5 Hz z-score (P=0.025).
Conclusion: Multiple respiratory pathogens were isolated from many patients with asthma, which appeared to contribute to disease symptoms and airway resistance. Minimizing children’s exposure to respiratory pathogens might be beneficial, especially during winter.
Keywords: asthma, inhaled corticosteroids, respiratory pathogens, nasopharyngeal colonization, communicable diseases
Introduction
Viruses and bacteria contribute to the pathogenesis and natural history of childhood asthma.1 Respiratory syncytial virus (RSV) predominates in infants and toddlers, while human rhinovirus (hRV), influenza (Flu) viruses, parainfluenza virus (PIV), adenovirus (AdV), coronavirus (CoV), and human enterovirus (hEV) are more prevalent in older children.2
It is common for children with asthma to develop respiratory symptoms, especially during winter. Many of their clinical findings, however, result from respiratory infections that require supportive care and minimizing exposure to respiratory pathogens.3,4 The prevailing practice, nevertheless, is escalating the use of short-acting β-agonists (SABAs), long-acting β-agonists (LABAs), inhaled corticosteroids (ICSs), and leukotriene receptor antagonist. These medications are expensive and may impose serious adverse events, especially in young children.5 Therefore, identifying and controlling triggers of asthma deserve further studies.
There are no studies from the United Arab Emirates (UAE) or surrounding countries that have evaluated respiratory infections or nasopharyngeal isolates in patients with asthma. However, few studies have addressed respiratory viral infections at community level. For example, in Saudi Arabia, most cases of RSV occur from November through March and some cases have been reported at other months of the year.6 In Kuwait, a study that has investigated the causative agents in >1,000 patients with lower respiratory tract infections using PCR revealed that RSV and hRV are the major isolates among hospitalized children from October to March. PIV-2 and human CoV were not detected in any of the patients’ samples.7
Regular surveillance, especially during the winter is necessary if pathogens are to be identified with a view to possible prevention. This prospective, case–control pilot study aimed to estimate the prevalence of nasopharyngeal isolates in children with asthma during winter season and identify their clinical impact on respiratory symptoms and function. Its main objective was to address the importance of controlling respiratory pathogens in the treatment of childhood asthma.
Materials and methods
This study was conducted between December 2016 and March 2017 in the Pediatric Pulmonary Clinic at Tawam Hospital (Al Ain, UAE). It was approved by the Research Ethics Committee of the UAE University – College of Medicine and Health Sciences (ERH 2015 3235 15 111). Written informed consent was obtained from the parents of all participants.
Children 3–6 years of age, with a diagnosis of asthma, were eligible to participate in the study. The clinical diagnosis of asthma was made by the pediatric pulmonologist, based on the Global Initiative for Asthma (GINA) criteria.8 Asthma staging ranged from mild to moderate, according to severity level and if the children required either ICS alone or in association with either LABA or montelukast. The study was explained to the parents of all eligible patients, and, if they agreed to the procedures, their children were enrolled in the study. The control group consisted of age-matched children without asthma, mainly relatives of our co-workers.
Immunization was up-to-date in all the studied participants. Children were excluded from the study if they had significant illnesses, such as chronic lung disease of prematurity, cystic fibrosis, congenital heart disease, immune deficiency, or upper airway anomaly. Demographic, medical, and vaccination data were reviewed. History of asthma symptoms was obtained using a modified global asthma network questionnaire.9 For the assessment of symptoms of viral infection, we used a validated Common Cold Questionnaire (CCQ).10 This questionnaire inquired about the presence of fever, chills, muscle pain, watery eyes, runny nose, sneezing, sore throat, cough, and chest pain during 2 days prior to specimen collection; each complaint was scored as none (0), mild (1), moderate (2), or severe (3). The GINA assessment of asthma control was used to assess the level of asthma control over the past 4 weeks.8 This instrument scored the presence (1) or absence (0) of each of the 4 clinical variables, day symptoms, night symptoms, reliever use, and exercise limitation. A score of zero indicated well-controlled asthma, 1–2 partly-controlled asthma, and 3–4 uncontrolled asthma.
Forced oscillation technique (FOT) was used to assess airway resistance using a commercial device (tremoFlo™ C-100, tremoFlo software, version 1.0.34 build 32; Thorasys Medical Systems, Montreal, QC, Canada), as previously described.11 Briefly, measurements were performed with the child sitting upright with the head in a neutral position, the cheeks supported, and the nose clipped. The child was instructed to breathe tidally through a mouthpiece. Data were collected over several seconds and the average of 3 acceptable measurements (coefficient of variation ≤15%) was taken. Airway resistance at 5 Hz (R5, in cmH2O.s.L−1) was expressed as R5 z-score.11
Sample collection and processing
Multiple (1–3 and 4–6 weeks apart) nasopharyngeal specimens were collected from each patient between December 2016 and March 2017 (Tables 1, 2, S1, and S2). For controls, each participant had only 1 sample collected between January and March 2017 (Table 3). The specimens were stored at −70°C until analysis, which was performed using the Allplex™ Respiratory Full Panel Assay (Seegene Biotechnology Inc., Seoul, Korea) as instructed by the manufacturer.12 The assay was composed of four panels and utilized a multiplex one-step real-time PCR to identify 16 viruses (with three influenza A subtypes) and seven bacteria. The viruses were influenza (Flu A, B, A-H1, A-H1pdm09, and A-H3), PIV (1–4), RSV (A and B), AdV, hEV, human metapneumovirus (MPV), human bocavirus (hBoV), hRV, and CoV NL63, CoV 229E, and CoV OC43. The bacteria were Streptococcus pneumoniae (SP), Haemophilus influenzae (HI), Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Bordetella pertussis (BP), and Bordetella parapertussis (BPP).
Statistical analysis
The data were analyzed with the SPSS statistical package (version 20). Descriptive statistics included the number and percentage, or the measured value of the observed variables, as appropriate. ANOVA was used to compare the R5 z-score on one hand, with the number of isolated pathogens, the GINA asthma control score, and the CCQ score on the other hand. The unpaired t-test was used to compare the R5 z-score between the children with asthma and the controls. For all tests, statistical significance was defined by a 2-sided P-value <0.05.
Results
Fifty nasopharyngeal specimens were collected between December 2016 and March 2017 from 18 patients with asthma (age, 5.0±1.1 years). As patient 18 was recruited into the study only in February 2017 (Table 1), no data are available on him prior to that time, and his later results are included in Table S1. The results of studies performed between December 2016 and January 2017 are shown in Table 1, between January 2017 and February 2017 in Tables S1, and in March 2017 in Tables S2. Patients’ responses to the Global Asthma Network Questionnaire are shown in Table S3. Only 6 patients used daily asthma prophylaxis (Table S3). In addition, 9 nasopharyngeal specimens were also collected from 9 control children who had no history of asthma (age, 4.9±1.0 years); their results are shown in Table 3.
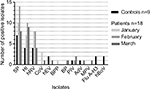
The prevalence of each isolate in patients and controls are shown in Figures 1 and S1. The most common organisms were SP, HI, and hRV for both groups.
Patients (December 2016–January 2017): Thirty-three pathogens (18 bacteria and 15 viruses) of 10 different types were isolated from 16 (94%) of 17 patients with asthma; Patient 7 had no detectable isolate (Table 1). These organisms were SP (10 patients), HI (7 patients), BPP (1 patient), rhinovirus (7 patients), CoV (2 patients), hEV (2 patients), AdV (1 patient), and PIV-3 (1 patient). Four (23%) patients had 4 pathogens, 1 (6%) had 3 pathogens, 3 (18%) had 2 pathogens, 8 (47%) had 1 pathogen, and 1 (6%) had none. Six (75%) of 8 patients with rhinovirus had a treatment modification, such as starting ICS (3 patients), increased ICS dosing (2 patients), or adding montelukast or salmeterol to the ICS (3 patients), Table 1.
Nine (52%) patients had mild common cold symptoms (CCQ scores ranged from 1 to 7 of a maximum score of 27), 4 (24%) had moderate symptoms (CCQ scores ranged from 8 to 16), and 4 (24%) had no symptoms (CCQ score =0), Table 1. Isolates from patients with CCQ score ≥7 included SP (Patients 4, 9, and 12), HI (Patients 4, 12, and 17), BPP (Patient 4), and hRV (Patients 4, 12, and 13).
Twelve (80%) of 15 patients had GINA assessment of asthma control score of ≥2 (maximum score, 4; Table 1). The R5 z-score (airway resistance) was not significantly different when compared among children with different number of pathogens isolated (P=0.2, ANOVA). Similarly, the R5 z-score was not significantly different among children with different CCQ scores (P=0.1, ANOVA) or GINA control scores (P=0.5, ANOVA).
Patients (February–March 2017): Isolate profiles changed in the majority of patients (Tables S1 and S2). For example, Patient 3 acquired BP and Patient 6 rhinovirus (Table S1). Many of previously detected viruses were not present on the subsequent testing; eg, Patients 6 and 8 lost their colonizations with CoV (Table S1). Different strains of PIV also appeared in March 2017 (PIV1 in Patient 11, and PIV4 in Patient 12; Table S2); while the strain detected in December–January was PIV3 (Patient 14, Table 1).
It is worth noting that BP and BPP were detected during the entire study period. The 2 patients with BP (Patients 2 and 3, Tables S1 and S2) had increased ICS dosing.
Table 2 shows the pathogens isolated from patients sorted by varying degrees of CCQ and GINA assessment of asthma control scores. The 4 patients with CCQ scores =0 and GINA assessment of asthma control scores ≥2 had increased airway resistance (high mean R5 z-scores), suggesting poor asthma control. However, only 2 of these patients had treatment escalation.
The impact of common cold symptoms on airway resistance is shown in Figure 2A. Patients with a CCQ score of 1–7 had significantly higher R5 z-score (P=0.025).
Nine of the 18 patients had “wheezing disturbing sleep ≥1 per week” (Table S3). Six of these patients had rhinovirus, 2 had CoV, and 1 had severe atopy (Patient 18), Table 1.
Treatment modifications: The treatment modifications, based on clinical findings, were made at the discretion of their pediatric pulmonologist and are detailed in Table 1. Many patients were already on controller medications prior to the study as shown in Table S3.
Controls (December 2016–March 2017): Twenty-three pathogens isolated from the control children: SP (7 children), HI (4 children), rhinovirus (4 children), Flu A-H3 (2 children), enterovirus (2 children), hBoV (2 children), PIV4 (1 child) and AdV (1 child), Table 3 and Figure 1. Two (22%) children had 4 pathogens, 2 (22%) had 3 pathogens, 4 (45%) had 2 pathogens, and 1 (11%) had 1 pathogen. The results of their airway resistance and CCQ scores are detailed in Table 3. The R5 z-score (airway resistance) was not significantly different between children with asthma and controls (P=0.8, unpaired t-test).
Discussion
The nasopharynx is a reservoir for pathogens associated with respiratory diseases, such as asthma. Airway microbiome studies have shown that bacteria may play a substantial role in the onset, evolution, and severity of asthma.13
This study was conducted to test the hypothesis that nasopharyngeal colonizations with community-acquired pathogens have an adverse impact on the natural history of asthma in young children. The results show that the majority of patients with asthma had several viral pathogens, which contributed to symptoms and airway resistance (Tables 1 and 2). The clinical impact of the respiratory pathogens on asthma control is shown in Table 1. Patients 2 and 3 had increased CCQ scores in association with colonizations with HI and BP on the second sampling. The increased airway resistance in Patient 5 on the second sample collection could be due to colonization with HI or adverse events of prior AdV. Similarly, the increased airway resistance in Patient 8 could be due to prior CoV OC43. The high airway resistance in Patient 14 could be due to PIV-3. As the course and severity of asthma are also related to the role of many environmental factors (such as air pollution, humidity, and smoking), medication adherence, and poor inhaler technique, it is not totally unexpected that the role of respiratory pathogens cannot be considered in isolation and cannot be solely responsible for asthma control. With respect to the controls (Table 3), Children 1 and 6 both had high airway resistance associated with multiple colonizations. Child 1 was found subsequently to have symptoms of allergic rhinitis and mild night cough.
The 2 patients who were found to have pertussis had increased symptoms, possibly falsely attributed to worsening asthma control, leading to inappropriate escalation of their medications before the infection was diagnosed (Tables S1 and S2). In addition, patients with asthma are at increased risk for pertussis infection. This was highlighted during the 2004 pertussis outbreak in MN, USA where the population attributable risk percentage of asthma for risk of pertussis was calculated to be 17%.14 Consistently, humoral immunoreaction to BP could be suppressed in patients with asthma.15 Therefore, targeting patients with asthma for pertussis surveillance and vaccination as a selective high-risk group might be an appropriate strategy.
Children with asthma may have an increased risk of pneumonia.16 This might be facilitated by their use of ICS, as these medications also inhibit mucosal immune responses, thus encouraging colonization with organisms.5
In children with no common cold symptoms, 33% had asthma prophylaxis therapy escalated in association with an elevated GINA assessment of asthma control score and increased airway resistance (probably justifiably; Table 2). In those with mild common cold symptoms, 20% had asthma prophylaxis therapy increased in association with increased airway resistance, although 50% had low GINA assessment of asthma control score. In those with more severe common cold symptoms, 50% had asthma prophylaxis therapy escalated, regardless of the GINA assessment of asthma control score and without increase in airway resistance (probably intensification triggered by the severity of cold symptoms instead of asthma score or airway resistance; probably not justified here), Table 2.
In the northern hemisphere, most asthma-related emergency department visits are higher in September than other months. In late fall, there is often a “second wave” with fluctuations throughout winter probably coinciding with rhinovirus episodes.17,18 Symptomatic rhinovirus infections are found to be an important contributor to asthma exacerbations in children.19 In this study, rhinovirus (hRV) was detected in the majority of patients (Figure 1). Future studies are needed to address important variables relevant to this organism, such as serotypes (especially HRV-16), upper vs lower respiratory colonization, and host susceptibility (eg, variability in expression of intercellular adhesion molecule-1).2
Airway inflammation has been reported in infections with Flu viruses, which are well known to induce severe exacerbation in adults.4 M. pneumoniae and C. pneumoniae, on the other hand, seem to be involved in asthma persistence.1,4 The atypical bacteria C. pneumoniae and M. pneumoniae were not detected in our studied population. These pathogens may be more important in adults with chronic asthma. Similarly, RSV and MPV were not detected in the patients. This finding could be due to the small sample size of the study or to the regional epidemic pattern of these pathogens. Thus, surveillances that cover the entire year are necessary.
In 1 study, children (3 months to 16 years of age) with asthma exacerbation had a high prevalence of respiratory pathogens that included RSV, hRV, M. pneumoniae, and C. pneumoniae. Most hospitalizations were associated with seasonal hRV and RSV.4 MPV and hBoV were previously reported in children with asthma exacerbation.12,13,20,21 In this current study, hBoV was detected in 1 healthy child (Table 3) and MPV in 1 patient (Table S2).
It is worth noting that R5 z-score in patients with a CCQ score ≥8 was similar to those with CCQ score =0 (Figure 2A). In addition, R5 z-scores correlated best with GINA assessment of asthma control scores in patients with CCQ score =0 (R>0.695) (Figure 2B–D). These findings reflect a limitation in using FOT in patients with significant upper respiratory symptoms, which have been shown to influence FOT resistance measurements.
The study finding supports the need for strategies that limit children’s exposure to respiratory viruses and bacteria, especially those with poorly controlled disease. Specific measures that have been suggested to minimize recurrent infections in children with asthma include hand hygiene, a healthy balanced diet, active probiotic supplements, and the immunostimulant OM-85.22 Further studies, however, are needed to investigate whether reducing exposure to pathogens would improve asthma control. In addition, the role of other environmental factors (such as air pollution, humidity, and smoking), medication adherence, and poor inhaler technique has to be taken into consideration in future studies.
Conclusion
The majority of patients with asthma had viral and bacterial pathogens contributing to their disease. Regular surveillance could play a role in asthma care, especially in those with poorly controlled disease. Effective strategies to minimize exposure to respiratory pathogens, such as hand hygiene should be incorporated in childhood asthma guidelines. Such approach would emphasize the importance of environmental control measures rather than relying solely on escalating asthma drug therapy with its potential toxicity. Further studies are needed to evaluate whether strategies that minimize children’s exposure to pathogens would improve asthma control.
Acknowledgments
The authors are grateful to all participating children and their parents. The contribution of Mrs Sania M Al-Hamad toward data collection is greatly appreciated. The study was funded by a grant from the College of Medicine and Health Sciences, UAE University (31M252). The funding body had no role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
Author contributions
ARA conceived the study, participated in its design and coordination, and drafted the manuscript. AMA, AA, and GA recruited participants and collected clinical data. JG and SMK coordinated and performed PCR experiments. HN and AKS participated in the data analysis and manuscript preparation. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Papadopoulos NG, Christodoulou I, Rohde G, et al. Viruses and bacteria in acute asthma exacerbations--a GA2 LEN-DARE systematic review. Allergy. 2011;66(4):458–468. | ||
Tovey ER, Stelzer-Braid S, Toelle BG, et al. Rhinoviruses significantly affect day-to-day respiratory symptoms of children with asthma. J Allergy Clin Immunol. 2015;135(3):663–669. | ||
Halmø Hürdum S, Zhang G, Khoo SK, et al. Recurrent rhinovirus detections in children following a rhinovirus-induced wheezing exacerbation: A retrospective study. Int J Pediatr Child Health. 2015;3(1):10–18. | ||
Maffey AF, Barrero PR, Venialgo C, et al. Viruses and atypical bacteria associated with asthma exacerbations in hospitalized children. Pediatr Pulmonol. 2010;45(6):619–625. | ||
Zhang L, Prietsch SO, Mendes AP, et al. Inhaled corticosteroids increase the risk of oropharyngeal colonization by Streptococcus pneumoniae in children with asthma. Respirology. 2013;18(2):272–277. | ||
Al-Alaiyan S, Pollack P, Notario GF. Safety and pharmacokinetics of extended use of palivizumab in Saudi Arabian infants and children. Drugs Context. 2015;4: pii: 212270. | ||
Khadadah M, Essa S, Higazi Z, Behbehani N, Al-Nakib W. Respiratory syncytial virus and human rhinoviruses are the major causes of severe lower respiratory tract infections in Kuwait. J Med Virol. 2010;82(8):1462–1467. | ||
GINA. Global Initiative for Asthma, Global Strategy for Asthma Management and Prevention; 2015. Available from: https://www.ginasthma.org. Accessed on October 24, 2016. | ||
Global Asthma Network. Available from: http://www.globalasthmanetwork.org/surveillance/manual/study6.php. Accessed October 24, 2016. | ||
Powell H, Smart J, Wood LG. Validity of the common cold questionnaire (CCQ) in asthma exacerbations. PLoS One. 2008; 19(3):e1802. | ||
Alblooshi A, Alkalbani A, Narchi H, et al. Respiratory function in healthy Emirati children using forced oscillations. Pediatr Pulmonol. 2018;53(7):936–941. | ||
Park S, Oh KC, Kim KS, et al. Role of Atypical Pathogens and the Antibiotic Prescription Pattern in Acute Bronchitis: A Multicenter Study in Korea. J Korean Med Sci. 2015;30(10):1446–1452. | ||
Pérez-Losada M, Alamri L, Crandall KA, Freishtat RJ. Nasopharyngeal Microbiome Diversity Changes over Time in Children with Asthma. PLoS One. 2017;12(1):e0170543. | ||
Capili CR, Hettinger A, Rigelman-Hedberg N, et al. Increased risk of pertussis in patients with asthma. J Allergy Clin Immunol. 2012;129(4):957–963. | ||
Nakamura A, Iwashima Y, Takakuwa O, Sato S. Sensitivity to bordetella pertussis in asthmatic patients. Eur Respir J. 2011;38:p2515. | ||
Martin M, Shaw D. Effect of inhaled corticosteroids on the microbiology of the respiratory tract. Respirology. 2013;18(2):201–202. | ||
Larsen K, Zhu J, Feldman LY, et al. The Annual September Peak in Asthma Exacerbation Rates. Still a Reality? Ann Am Thorac Soc. 2016;13(2):231–239. | ||
Cohen HA, Blau H, Hoshen M, Batat E, Balicer RD. Seasonality of asthma: a retrospective population study. Pediatrics. 2014;133(4):e923–e932. | ||
Khetsuriani N, Kazerouni NN, Erdman DD, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119(2):314–321. | ||
Jartti T, van den Hoogen B, Garofalo RP, Osterhaus AD, Ruuskanen O. Metapneumovirus and acute wheezing in children. Lancet. 2002; 360(9343):1393–1394. | ||
Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44(7):904–910. | ||
Ahanchian H, Jones CM, Chen Y-Sheng, Sly PD. Respiratory viral infections in children with asthma: do they matter and can we prevent them? BMC Pediatr. 2012;12:147. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.