Back to Journals » Journal of Inflammation Research » Volume 15
Naples Prognostic Score is an Independent Prognostic Factor in Patients with Small Cell Lung Cancer and Nomogram Predictive Model Established
Authors Chen S, Liu S, Xu S, Cao S, Han Z, Kong L, Ren D , Duan G
Received 29 April 2022
Accepted for publication 18 June 2022
Published 28 June 2022 Volume 2022:15 Pages 3719—3731
DOI https://doi.org/10.2147/JIR.S371545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Shuangqing Chen,1,2 Shicheng Liu,1 Siwei Xu,3 Shumin Cao,4,5 Zhaohui Han,1,2 Lingxin Kong,1,5 Dahu Ren,1,5 Guochen Duan6
1Department of Thoracic Surgery, Hebei General Hospital, Shijiazhuang, 050057, People’s Republic of China; 2Graduate School, Hebei North University, Zhangjiakou, 075000, People’s Republic of China; 3Department of Thoracic Surgery, The First Hospital of Hebei Medical University, Shijiazhuang, 050057, People’s Republic of China; 4Department of Oncology, Hebei General Hospital, Shijiazhuang, 050057, People’s Republic of China; 5Graduate School, Hebei Medical University, Shijiazhuang, 050011, People’s Republic of China; 6Department of Thoracic Surgery, Children’s Hospital of Hebei Province, Shijiazhuang, 050000, People’s Republic of China
Correspondence: Guochen Duan, Children’s Hospital of Hebei Province, No. 133 Jianhua South Street, Yuhua District, Shijiazhuang, 050000, People’s Republic of China, Tel +8613513380703, Email [email protected] Shicheng Liu, Hebei General Hospital, No. 348, Heping West Road, Xinhua District, Shijiazhuang, 050000, People’s Republic of China, Tel +861309388955, Email [email protected]
Background: The routine clinical nutritional and inflammatory indicators such as serum albumin, total cholesterol and lymphocytes have been widely investigated in the prognosis of small cell lung cancer (SCLC). The Naples prognostic score (NPS), based on nutritional and inflammatory status, has been identified as a prognostic impactor in several malignancies. However, the prognostic role of NPS in SCLC has not been elucidated. This study aims to evaluate the prognostic effect of NPS in SCLC patients.
Patients and Methods: Patients with SCLC were recruited at Hebei General Hospital between April 2015 and August 2021. Pretreatment clinical and laboratory data were obtained. Participants were assigned into three groups according to NPS (group 0: NPS=0, group 1: NPS=1 or 2, group 2: NPS=3 or 4). Kaplan-Meier and Cox regression analysis were performed to assess the prognostic significance of NPS. The RMS package in R software was used to draw the nomogram predictive model.
Results: A total of 128 patients were enrolled. The median progression-free survival (PFS) and overall survival (OS) was 7.2 and 12.3 months, respectively. The median PFS and OS was 12.3 vs 19.8 months, 7.6 vs 14.1 months and 6.0 vs 8.45 months for the three groups respectively. There were significant differences in both OS and FPS among the three groups. Survival analysis showed that NPS was significantly correlated with both OS and PFS (P< 0.05). Lower NPS is associated with longer OS and PFS. Multivariate analysis showed that NPS has an independent prognostic impact on OS (P< 0.05). The nomogram predictive model showed that NPS has good predictive power for survival rates.
Conclusion: NPS is an independent prognostic factor for OS in SCLC patients. Low NPS may predict longer OS. Therefore NPS plays a vital role in the nomogram predictive model of survival rates in SCLC patients.
Keywords: small cell lung cancer, Naples prognostic score, prognostic factor, nomogram predictive model
Introduction
Lung cancer is one of the most common types of cancer, accounting for approximately 11.6% of all cancer types, and is one of the leading causes of death from solid tumors worldwide.1 SCLC, as a subtype, accounts for 15% of lung cancer.2,3 SCLC is a rapidly progressing and highly aggressive neuroendocrine cancer, and it is sensitive to initial chemotherapy and radiotherapy.4,5 Most patients with SCLC relapse within one year after initial treatment; therefore, SCLC patients suffer a poor prognosis, with a 5-year survival rate of only 7%. Some clinical indicators such as performance status (PS), age, smoking status, and staging are prognostic factors in patients with SCLC.6,7 Recent evidences demonstrated that factors associated with immunity, inflammation, nutritional status, and liver function have been found to play essential roles in tumor prognosis, such as the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), systemic immune‐inflammation index (SII), Prognostic Nutrition Index (PNI), and Albumin-bilirubin (ALBI) grade.7–10
The Naples prognostic score (NPS) is a scoring system that includes serum albumin, total cholesterol (TC) level, NLR, and lymphocyte/monocyte ratio (LMR), which can comprehensively reflect the immune and nutritional status of patients. NPS was used to evaluate the prognosis of colorectal cancer, and its predictive value was proved to be superior to other immune and nutritional indicators.11 NPS has been reported to be an independent prognostic factor for osteosarcoma,12 non-small cell lung cancer,1 endometrial cancer,13 and pancreatic cancer.14 However, there is no study exploring the significance of NPS in SCLC prognosis. Therefore, this study aimed to understand the relationship between NPS and SCLC patients’ prognosis.
Patients and Methods
We retrospectively analyzed inpatients with SCLC treated at the Department of Thoracic Surgery of Hebei General Hospital admitted from April 2015 to August 2021. Patients with incomplete test results, inaccurate clinical data, or failure to follow up, diagnosed with other malignant tumors or immune-related diseases were excluded. No patient was combined with hematological diseases, liver diseases, kidney diseases, and other adverse factors affecting blood routine or biochemical indicators before treatment. The clinical data of all patients were obtained before treatment. All patients received sequential treatment in accordance with National Comprehensive Cancer Network (NCCN) guidelines. Follow-up time was no less than four months, and the deadline for data collection was December 31, 2021. The primary endpoint was overall survival (OS), which referred to the time from the pathological diagnosis of SCLC to the death of the patients. The secondary endpoint was progression-free survival (PFS), which is from the pathological diagnosis of SCLC to when the disease progressed. The follow-up interval was one month. Two consecutive failures to follow-up were regarded as death as the nature of advanced SCLC, and the date of death was regarded as the last loss to follow-up.
Demographic and clinical information was extracted from the electronic medical record system at Hebei General Hospital, including gender, age, smoking status, body mass index (BMI), PS, Charlson comorbidity index (CCI), treatment methods, and disease progression. Laboratory parameters, including lactate dehydrogenase (LDH), serum albumin (ALB), TC, LMR, NLR, SII, PLR, PNI, carcinoembryonic antigen (CEA), and neuron-specific enolase (NSE) were collected. According to Galizia et al11 the definition and grading standard of NPS is shown in Table 1.
 |
Table 1 The Grading Standard of NPS |
Patients were divided into three groups according to the NPS: group 0, patients with a score of 0; group 1, patients with a score of 1 or 2; and group 2, patients with a score of 3 or 4. The Ethical Committee of Hebei General Hospital approved this study. We confirm the confidentiality of the data maintained and compliance with the “Declaration of Helsinki”. Informed consent was waived due to the retrospective nature of the study.
Statistical Analyses
The IBM SPSS statistics software program, version 22.0 (IBM Corporation, Armonk, NY, USA), was used for statistical analysis. P<0.05 indicated statistical significance. Shapiro-wilk test was performed to test whether the data is normally distributed. Normally distributed data were expressed as 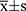 , and data distributed non-normally were expressed as M (P25~P75). Using receiver operating characteristic (ROC) curve analysis, we determined cutoff values for LDH, SII, PLR, CEA, and NSE using receiver operating characteristic (ROC) curve analysis. The Chi-square test was used to examine the differences of various factors among groups. Kaplan-Meier analysis was performed to plot survival curves, and differences among the curves were tested by the Log Rank test. The COX proportional hazard regression model was used for univariate and multivariate analysis. Hazard ratio (HR) 95% confidence interval (CI) was used as a common indicator for assessing relative risk.
, and data distributed non-normally were expressed as M (P25~P75). Using receiver operating characteristic (ROC) curve analysis, we determined cutoff values for LDH, SII, PLR, CEA, and NSE using receiver operating characteristic (ROC) curve analysis. The Chi-square test was used to examine the differences of various factors among groups. Kaplan-Meier analysis was performed to plot survival curves, and differences among the curves were tested by the Log Rank test. The COX proportional hazard regression model was used for univariate and multivariate analysis. Hazard ratio (HR) 95% confidence interval (CI) was used as a common indicator for assessing relative risk.
Results
Patient Characteristics
A total of 128 patients with SCLC were enrolled with a median age of 65 years (range 14–82 years). Ninety-seven patients (75.8%) were male, and 78 (60.9%) had a smoking history. In the PS scoring system, 47 patients (36.7%) scored as two. Only three cases (2.3%) scored CCI of three and above. According to the clinical and pathological findings, 51 patients (39.8%) were in the limited stage. As for treatment methods, 35 patients (27.3%) underwent surgery. The demographic and clinical information and baseline laboratory parameters collected are shown in Table 2. According to the ROC curves in Figure 1, the optimal cutoff points of LDH, SII, PLR, CEA, and NSE after binary screening were 208.35 U/L, 874.43, 281.90*109/L, 10.29μg/L, and 7.91, respectively (Figure 1).
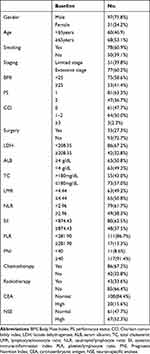 |
Table 2 Clinical Characteristics of Patients |
 |
Figure 1 ROC curves of LDH, SII, PLR, CEA, and NSE cutoff values were determined. |
Relationship Between Clinicopathological Features and NPS Grade
We analyzed the relationship between NPS and clinicopathological characteristics. The results are shown in Table 3. Gender (P=0.033), age (P=0.03), staging (P<0.01), BMI (P=0.023), PS (P=0.004), Surgery (P<0.01), LDH (P=0.026), SII (P=0.002), PNI (P<0.01), CEA (P=0.012), NSE (P=0.026), and undergoing radiotherapy (P=0.043) had significant correlation with NPS groups 0, 1, and 2 were significantly different.
 |
Table 3 Relationship Between Patient Characteristics and NPS Grade |
In addition, the high NPS group had lower serum ALB levels (<4 g/dL), TC levels (≤180mg/dL), and LMR (≤4.44) than the other groups (all P<0.01), and the NLR (≥2.96) was higher than other groups (P<0.01).
Univariate and Multivariate Analysis in Terms of PFS
The median PFS of all 128 participants was 7.2 months. For the three groups, the median PFS is 12.3 months in group 0, 7.6 months in group 1, and 6 months in group 2. The survival curves in terms of PFS plotted by Kaplan-Meier analysis are shown in Figure 2A. PFS of patients in group 0 was significantly longer than in other groups. The Log rank test indicated that lower NPS scores were significantly associated with longer PFS (P<0.01). Results of univariate and multivariate analysis are shown in Table 4. Univariate analysis showed that age≧65 years, smoking, extensive stage, BMI<25, high PS score, no surgery was performed, LDH (≥208.35), SII (≥874.43), PLR (≥281.90), no chemotherapy was performed, CEA (High), NSE (high) and high NPS groups may predict shorter FPS in SCLC patients. It is suggested that the above factors may be potential independent risk factors for PFS (all P<0.05). In multivariate analysis, a score of 1 or 2 in the NPS score is an independent prognostic factor for PFS (P=0.048).
 |
Table 4 Univariate and Multivariate Analysis for PFS |
 |
Figure 2 (A) Maier curves of PFS for each NPS group. (B) Maier curves of OS for each NPS group. |
Univariate and Multivariate Analysis in Terms of OS
The median OS is 12.3 months. For each group, the median OS is 19.8 months in group 0, 14.1 months in group 1, and 8.45 months in group 2. The survival curves are shown in Figure 2B. The results showed significant differences in OS among groups (P<0.01). SCLC patients in group 2 had a significantly shorter OS. Results of univariate and multivariate analysis based on OS are shown in Table 5. Univariate analysis showed that age<65 years, no smoking, limited stage, BMI≥25, low PS score, undergone surgery, LDH (<208.35), SII (<874.43), PLR (<281.90), undergone chemotherapy, CEA (normal), NSE (normal)) and PNI (≥40) may predict longer OS in SCLC patients. It is suggested that the above factors are potential independent risk factors for OS (all P<0.05). Multivariate analysis showed that smoking, NSE (high), and high NPS group might predict shorter OS in SCLC patients (all P<0.05).
 |
Table 5 Univariate and Multivariate Analysis for OS |
Nomogram Predictive Model
To further confirm the clinical significance of NPS in SCLC patients, we built the nomogram predictive model (Figure 3). A total of four factors were incorporated into the nomogram predictive model. It includes multivariate COX regression analysis of meaningful indicators: Smoking, NSE and NPS (all P<0.05). In addition, the stage is generally considered an independent factor of OS in clinical practice. Therefore, staging was also included. The model indicated that NPS has the most significant influence on survival rates, followed by staging, NSE and smoking. According to this model, the survival rates of 1 and 2 years in SCLC patients was reliably predicted simply and intuitively.
 |
Figure 3 The 1-and 2-year nomogram predictive model for OS in SCLC patients. |
We used the ROC curve to evaluate the predictive performance of the model. The result showed that the area under curve (AUC) of nanogram for the main queue was 0.817 (1-year) and 0.865 (2-year), respectively (Figure 4). In addition, the calibration curve was used as a calibration tool to verify the predicted results. 1000 internal tests were performed using Bootstrap. There is a good agreement between the nomogram’s prediction and the actual situation in the main and validation queues (Figure 5).
 |
Figure 4 ROC curves of the nomogram predictive model for the main queue. |
Discussion
NPS comprises four biomarkers: ALB, TC, NLR and LMR. As well-known inflammatory indicators, NLR and LMR contain three types of cells involved in the regulation of inflammation: neutrophils, lymphocytes, and monocytes. Neutrophils can promote the development and growth of blood vessels in tumors and create a microenvironment suitable for tumor survival by inhibiting lymphocyte-mediated cytolysis, which led the promotion of growth, proliferation and differentiation of cancer cells.13,15
Therefore, neutrophil count is associated with tumor prognosis.13,15,16 The role of lymphocytes is to have growth-regulating effects on tumor cells in the tumor immune microenvironment.17 Lymphocytosis is thought to be associated with a good prognosis, and lymphopenia is thought to be associated with a poor prognosis, whereas increased NLR and decreased LMR are usually accompanied by a substantial decrease in peripheral blood lymphocytes. Increased NLR and decreased LMR are generally associated with higher mortality and poorer prognosis in solid tumors.18,19 In addition, after tumor cell invasion, monocytes can gradually differentiate into macrophages, and tumor-associated macrophages (TAM) can affect the intrinsic properties of tumor cells, tumor microenvironment, development and distant metastasis of tumor cells. Therefore, an increase in LMR often indicates a poor prognosis for the patient.20–22 At the same time, NPS also contains serum albumin and total cholesterol. Studies have shown that certain pro-inflammatory factors, such as interleukin-6 and tumor necrosis factor-ɑ, can reduce serum albumin levels by reducing serum albumin production by liver cells.23 Studies have shown that serum albumin levels are associated with postoperative prognosis in patients with non-small cell lung cancer and gastric cancer.24,25 As an essential component of cell membranes, cholesterol plays an important role in membrane fluidity and protein activity, both of which are potentially related to the occurrence and development of cancer.11 Low cholesterol levels largely impair the fluidity of cell membranes and the ability of cell surface receptors to transmit transmembrane signals, so a decrease in cholesterol levels may indicate a poor prognosis for the patient.26 In addition, nutritional status is related to the stage of cancer patients, and the later the stage, the more necessary the intervention of nutritional therapy. It has been reported that early nutritional support for cancer patients with pre cachexia (a potential early stage of cachexia) can improve patient function, increase tolerance to antitumor therapy, and improve patient outcomes.27,28
Although the levels of inflammation-related factors, serum albumin levels, and total cholesterol can be used as prognostic markers for cancer patients, the level of only one inflammatory cell or one nutritional biomarker may be insufficient to assess the prognosis among SCLC patients. Moreover, due to the heterogeneity of patients, the cutoff values of individual indicators are often different, so it is difficult to establish a unified and widespread standard for use. NPS integrates a variety of biomarkers, including comprehensive nutritional and inflammatory immune indicators to show better prognostic value.
In this study, we first investigated the value of the NPS scoring system, which can reflect patients’ immune-inflammatory and nutritional status and predict the prognosis of SCLC patients. We found that OS in univariate was consistent with PFS. In multivariate analysis, only group 1 had statistical significance in PFS, whereas insignificance in group 0 and group 2, which may be due to insufficient sample size. We concluded that NPS is an independent prognostic factor for OS and potentially an independent prognostic factor for PFS in SCLC patients.
Our study also showed that age, SII, chemotherapy, BMI, and PS were all prognostic factors for PFS and OS in SCLC patients in univariate analysis. The increasing life expectancy of people, the continuous increase of the elderly population, and the long-term accumulation of carcinogens caused by unhealthy lifestyles (such as smoking) may explain why age is related to PFS and OS. Guo et al reported that elevated SII was associated with poorer prognosis in patients with non-small cell lung cancer.29 SCLC is sensitive to chemotherapy, which may be why chemotherapy can be used as a prognostic factor for PFS and OS.BMI and PS can be used as independent prognostic factors for PFS and OS in univariate analysis of SCLC patients, which potentially because both of which are related to patients’ nutritional status. Evidence has shown that weight loss in advanced cancer patients may increase the patient’s risk of death.30
The NPS combines nutritional parameters and immune parameters to establish a relatively comprehensive prognostic indicator of nutritional inflammation, which is largely superior to the other prognostic indicators in terms of prediction and prognosis.
Innovation and Limitations
We applied NPS for the first time to predict the prognosis of patients with SCLC. In addition, we built the nomogram predictive model of survival rates in SCLC patients based on prognostic factors including NPS. All biomarkers were objective laboratory indicators with widely available tests and no additional costs.
However, there are several limitations in this study. Firstly, this is a single-center retrospective study, and there may be bias in patient selection and data collection. Secondly, the small number of samples may lead to poor credibility of the argument. Large-scale prospective studies and experiments are needed to consolidate our conclusion and further explore the mechanism.
Conclusion
In conclusion, as a simple and practical prognostic scoring system, NPS is an independent prognostic factor for OS in SCLC patients and potentially an independent prognostic factor for PFS in SCLC patients. Low NPS may predict longer OS. In addition, NPS plays a vital role in the nomogram predictive model of survival rates in SCLC patients.
Data Sharing Statement
The data sets analyzed during the current study are available from the corresponding author (Shicheng Liu) on reasonable request.
Ethical Approval and Consent to Participate
The Ethical Committee of Hebei General Hospital approved this study (Ethics approval number:2022079). We confirm the confidentiality of the data maintained and compliance with the “Declaration of Helsinki”. Informed consent was waived due to the retrospective nature of the study.
Acknowledgments
This study would like to thank all the participants and their families as we as medical staff at the Department of Thoracic Surgery, Hebei General Hospital, especially Dr. Wenbo Wu and Dr. Zhonghui Hu, for their contribution to this study.
Disclosure
Dr. Shuangqing Chen, Shicheng Liu, Siwei Xu, Shumin Cao, Zhaohui Han, Lingxin Kong, Dahu Ren, and Guochen Duan have no conflicts of interest or financial ties to disclose.
References
1. Li S, Wang H, Yang Z, et al. Naples Prognostic Score as a novel prognostic prediction tool in video-assisted thoracoscopic surgery for early-stage lung cancer: a propensity score matching study. Surg Endosc. 2021;35(7):3679–3697. doi:10.1007/s00464-020-07851-7
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Wang D, Guo D, Shi F, et al. The predictive effect of the systemic immune-inflammation index for patients with small-cell lung cancer. Future Oncol. 2019;15(29):3367–3379. doi:10.2217/fon-2019-0288
4. Frese KK, Simpson KL, Dive C. Small cell lung cancer enters the era of precision medicine. Cancer Cell. 2021;39(3):297–299. doi:10.1016/j.ccell.2021.02.002
5. van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–1755. doi:10.1016/S0140-6736(11)60165-7
6. Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Determinants of improved outcome in small-cell lung cancer: an analysis of the 2580-patient. Southwest Oncol Group Database. 1990;8:1563–1574.
7. Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236(4):297–304. doi:10.1620/tjem.236.297
8. Wang C, Jin S, Xu S, Cao S. High Systemic Immune-Inflammation Index (SII) represents an unfavorable prognostic factor for small cell lung cancer treated with etoposide and platinum-based chemotherapy. Lung. 2020;198(2):405–414. doi:10.1007/s00408-020-00333-6
9. Jin S, Cao S, Xu S, Wang C, Meng Q, Yu Y. Clinical impact of pretreatment prognostic nutritional index (PNI) in small cell lung cancer patients treated with platinum-based chemotherapy. Clin Respir J. 2018;12(9):2433–2440. doi:10.1111/crj.12925
10. Kinoshita F, Yamashita T, Oku Y, et al. Prognostic impact of Albumin-bilirubin (ALBI) grade on non-small lung cell carcinoma: a propensity-score matched analysis. Anticancer Res. 2021;41(3):1621–1628. doi:10.21873/anticanres.14924
11. Galizia G, Lieto E, Auricchio A, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis Colon Rectum. 2017;60(12):1273–1284. doi:10.1097/DCR.0000000000000961
12. Yang Q, Chen T, Yao Z, Zhang X. Prognostic value of pretreatment Naples prognostic score (NPS) in patients with osteosarcoma. World J Surg Oncol. 2020;18(1):24. doi:10.1186/s12957-020-1789-z
13. Li Q, Cong R, Wang Y, et al. Naples prognostic score is an independent prognostic factor in patients with operable endometrial cancer: results from a retrospective cohort study. Gynecol Oncol. 2021;160(1):91–98. doi:10.1016/j.ygyno.2020.10.013
14. Nakagawa N, Yamada S, Sonohara F, et al. Clinical implications of Naples prognostic score in patients with resected pancreatic cancer. Ann Surg Oncol. 2020;27(3):887–895. doi:10.1245/s10434-019-08047-7
15. Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4(2):83–91. doi:10.1158/2326-6066.CIR-15-0313
16. Grieshober L, Graw S, Barnett MJ, et al. Pre-diagnosis neutrophil-to-lymphocyte ratio and mortality in individuals who develop lung cancer. Cancer Causes Control. 2021;32(11):1227–1236. doi:10.1007/s10552-021-01469-3
17. Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno oncology biomarkers working group: Part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24(5):235–251. doi:10.1097/PAP.0000000000000162
18. Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110(6):1595–1605. doi:10.1038/bjc.2014.46
19. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi:10.1016/j.critrevonc.2013.03.010
20. Chan JC, Chan DL, Diakos CI, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265(3):539–546. doi:10.1097/SLA.0000000000001743
21. Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402–1410. doi:10.1016/j.imbio.2013.06.003
22. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi:10.1038/nrclinonc.2016.217
23. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr. 2009;12(3):223–226. doi:10.1097/MCO.0b013e32832a7902
24. Lien YC, Hsieh CC, Wu YC, et al. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8(8):1041–1048. doi:10.1016/j.gassur.2004.09.033
25. Li SJ, Lv WY, Du H, et al. Albumin-to-alkaline phosphatase ratio as a novel prognostic indicator for patients undergoing minimally invasive lung cancer surgery: propensity score matching analysis using a prospective database. Int J Surg. 2019;69:32–42. doi:10.1016/j.ijsu.2019.07.008
26. Zhang G, Zhang D, Wu J, et al. Low serum levels of pre-surgical total cholesterol are associated with unfavorable overall survival in patients with operable non-small cell lung cancer. Clin Lab. 2018;64(3):321–327. doi:10.7754/Clin.Lab.2017.170823
27. Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83(6):1345–1350. doi:10.1093/ajcn/83.6.1345
28. Huang Z, Wang X, Zou Q, et al. High platelet-to-lymphocyte ratio predicts improved survival outcome for perioperative NSAID use in patients with rectal cancer. Int J Colorectal Dis. 2020;35(4):695–704. doi:10.1007/s00384-020-03528-8
29. Guo W, Cai S, Zhang F, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer. Thorac Cancer. 2019;10(4):761–768. doi:10.1111/1759-7714.12995
30. Shepshelovich D, Xu W, Lu L, et al. Body Mass Index (BMI), BMI change, and overall survival in patients with SCLC and NSCLC: a pooled analysis of the international lung cancer consortium. J Thorac Oncol. 2019;14(9):1594–1607. doi:10.1016/j.jtho.2019.05.031
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

