Back to Journals » OncoTargets and Therapy » Volume 12
Nanosomal docetaxel lipid suspension based chemotherapy in a pregnant MBC patient – a case report
Authors Ramaswamy R , Joshi N, Khan MA , Siddhara S
Received 23 February 2019
Accepted for publication 15 June 2019
Published 15 July 2019 Volume 2019:12 Pages 5679—5685
DOI https://doi.org/10.2147/OTT.S206573
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Rajkumar Ramaswamy,1 Nisarg Joshi,2 Mujtaba A Khan,2 Seerin Siddhara1
1Department of Medical Oncology, Velammal Medical College and Research Institute, Madurai, Tamil Nadu 625009, India; 2Department of Medical Affairs & Clinical Development, Intas Pharmaceuticals Ltd., Ahmedabad, Gujarat 380054, India
Abstract: The current report presents a case of a pregnant woman with breast cancer metastasized to liver and lungs. The standard of care for breast cancer in pregnancy is anthracycline/taxane-based chemotherapy regimens. Docetaxel has shown a favorable toxicity profile during the second and third trimesters of pregnancy. A novel nanosomal docetaxel lipid suspension (NDLS) (DoceAqualip), with a proven efficacy and tolerability profile, has been approved in India for the treatment of advanced solid tumors since 2013. We present here a case of a pregnant woman with metastatic breast cancer managed with NDLS based TAC regimen showing a partial response after six cycles. The patient delivered a healthy male child with normal Apgar score and weight at the 32nd week of gestation.
Keywords: NDLS, PABC, DoceAqualip, pregnancy, breast cancer, metastatic
Introduction
Pregnancy-associated cancer ranges from ~25 to 190/100,000 pregnancies,1 and has become a leading cause of maternal death.2 Breast, cervical, lymphoma, ovarian, and melanoma cancers are the most common types reported during pregnancy.3 Breast cancer is one of the most common malignancy associated with pregnancy, arising in 1/10,000–1/3000 pregnancies.4 Breast cancer during pregnancy generally occurs in women with advanced age, with only 10% reported under 40 years of age.5,6 Pregnancy-associated breast cancer (PABC) is defined as breast cancer diagnosed during pregnancy or within one year of delivery.6,7 The average age of diagnosis of PABC is 33 years with a median gestational age of 21 weeks.6 The incidence of PABC is ~15–35/100,000 deliveries.8 During pregnancy, breast cancer is generally diagnosed after it has metastasized due to advanced maternal age and difficulty in diagnosis arising from the pregnancy-related changes in the breast.9 The average reported delay for diagnosis of breast cancer during pregnancy is 5–15 months from the onset of symptoms.10
The optimal treatment of cancer during pregnancy remains elusive because there are limited data from retrospective studies with small samples.11 The management of PABC is dependent on the trimester of pregnancy, type, and stage of the cancer. Surgery is recommended in all trimesters, chemotherapy in the second and third trimesters, and radiotherapy only in the postpartum period.6 The National Comprehensive Cancer Network (NCCN) Clinical Practice Guideline suggests chemotherapy followed by endocrine therapy and palliative radiotherapy for the management of breast cancer during the second and third trimester of pregnancy.12,13 In literature, several agents have been evaluated for the treatment of PABC including anthracyclines and taxanes.
We report here a case of a pregnant woman with breast cancer metastasized to liver and lungs. The patient was treated with nanosomal docetaxel lipid suspension (NDLS, DoceAqualip) based TAC (NDLS, doxorubicin, cyclophosphamide) regimen for six cycles.
Case report
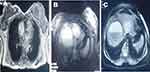
A 44-year-old pregnant woman presented with a lump in the left breast and pedal edema. The patient was married from the past 2 years and it was a non-consanguineous marriage. She was a second gravida woman with intracytoplasmic sperm injection conception after 1 year of a previous abortion (conception by in vitro fertilization) a single live uterine pregnancy of 26 weeks (second trimester). She was a known case of hypertension and hypothyroidism, and was receiving nifedipine 10 mg OD and thyroxine 75 µg OD. Medical history showed the patient was anemic with low hemoglobin levels (10.7 gm%). Genotyping for BRCA1, BRCA2, or FANCJ was not performed. Magnetic resonance imaging (MRI) of chest and abdomen (Jun 2018) showed a large soft tissue intensity irregular mass lesion with central necrosis with restricted diffusion measuring 6.1×5.5×5.0 cm in the inner quadrant of the left breast parenchyma with adjusted lobulated prominent ducts suggestive of left breast malignant mass. Multiple enlarged lymphnodes were seen in the left axillary region. Multiple tiny randomly distributed pulmonary nodules with restricted diffusion were observed suggestive of lung metastasis. Multiple mixed hyperintense necrotic, non-necrotic lesions with restricted diffusion of varying size (1.9–8 cm) were seen in both lobes of liver with the largest measuring 8×7 cm. Staging was done as T3N2M1 – Stage IV.
Biopsy from the left breast lump showed a tumor occupying 60% of the biopsy specimen. Tumor cells were arranged in a diffuse pattern; few glands and pseudo papillae with surrounding desmoplastic stroma were noted. Tumor cells showed moderate pleomorphic nuclei, coarse chromatin, and moderate cytoplasm. In addition, few benign ductules were noted. Frequent mitotic figures were reported (12/10 hpf). The impression was made as invasive carcinoma, no special type (NOS) (Figure 1).
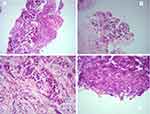 |
Figure 1 Biopsy of left breast: (A) Diffuse pattern (100x); (B) Pseudo papilla; (C) Occasional tubular pattern with desmoplastic stroma (400x); (D) Frequent mitosis (400x). |
Immunohistochemical examination showed negative estrogen receptor, weak positive progesterone receptor (10%), and negative HER2 neu status. The final diagnosis was PABC with lung and liver metastases. 2D ECHO with Color Doppler showed normal cardiac functions with an ejection fraction of 63%.
The patient was planned for palliative chemotherapy with 3-weekly NDLS based TAC (NDLS, doxorubicin, cyclophosphamide) regimen for six cycles. The first chemotherapy cycle was started in Jul 2018 with intravenous (IV) NDLS 100 mg (75 mg/m2), IV doxorubicin 80 mg, and IV cyclophosphamide 800 mg on Day 1 of the cycle along with IV ranitidine 50 mg BD, IV dexamethasone 8 mg BD, and IV ondansetron 8 mg BD. Filgrastim 300 µg was given subcutaneously on Days 2–4 of the cycle.
Ultrasound antenatal scan with Doppler done a day before the second chemotherapy cycle demonstrated a single live intrauterine fetus in cephalic presentation with fetal spine to the maternal right side. Active fetal movements and fetal cardiac pulsations were present with fetal heart rate measuring 132/min. Placenta was seen in fundal posterior and left lateral wall with grade II maturity. The average gestational age was 31 weeks and 5 days with normal Doppler parameters. After 3 weeks following the first chemotherapy cycle, a second cycle with NDLS based TAC regimen was administered similar to the first cycle.
At the 32nd week of gestation, the patient presented with the complaints of diminished fetal movements. There was no bleeding or prevaginal draining. Her amniotic fluid index was 7.2 cm. Medical oncologists’ opinion was sought for emergency low segment cesarean section (LSCS). She was advised 6 units of fresh frozen plasma and 6 units of platelets transfusion. She was administered 4 doses of IV dexamethasone 6 mg at 6 hourly intervals. Emergency LSCS was performed, and intraoperative findings were normal. The patient delivered an alive male child. The child’s birth weight was 1.76 kg which was normal as per the gestational age, and Apgar score was also normal. The child was kept for observation in the neonatal intensive care unit for 2 days as a precautionary measure. Tablet cabergoline 0.25 mg (two tablets) was given to the patient to suppress breast milk secretion. Her Hb level was 7.7 g%, and platelets were 182,000/mm3; 2 units of packed cell volume was administered, and the patient was asked to check and inform in case of any bleeding. The patient was advised to take plenty of oral fluids, salt-restricted diet, postnatal exercises, to avoid lifting heavy weight, and use of temporary contraception.
Her 3rd, 4th, and 5th chemotherapy cycles with NDLS based TAC regimen were given similar to previous cycles in Aug, Sep, and Oct 2018, respectively. The sixth chemotherapy cycle was given in Nov 2018 similar to the previous cycles except inj. filgrastim 300 µg OD for 3 days was replaced with inj. pegfilgrastim 6 mg OD on Day 2 of the cycle.
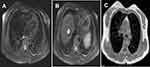
After completion of six chemotherapy cycles, MRI done in Nov 2018 showed a small focus of restricted diffusion measuring 8×7 mm noted in the inner quadrant of the left breast. There were no significantly enlarged mediastinal or axillary lymphnodes. Lung parenchyma showed normal densities with no evidence of alveolar densities, intrinsic thickening, fibrosis, or emphysematous changes – suggestive of no lung metastasis. Trachea and main bronchi were within normal limits. A few heterogeneous lesions with restricted diffusion were noted in the liver measuring 2.5×2 cm in segment VIII, suggestive of residual liver metastasis. A cystic lesion measuring 1.2×1.5 cm was noted in segment VIII of the liver. Overall, in comparison to the baseline MRI (Jun 2018; Figure 2) as per RECIST 1.1 criteria a “partial response” was reported with the NDLS based TAC chemotherapy regimen (Figure 3). Table 1 highlights pre- and post-NDLS based chemotherapy MRI findings. The patient is currently stable and receiving hormonal therapy with tamoxifen 20 mg PO. Consent was obtained from the patient for publication of this case report.
 |
Table 1 Pre- and post-NDLS based chemotherapy MRI findings |
Discussion
Pregnancy can delay breast cancer diagnosis, evaluation, and treatment.7 PABC is not so common and requires thorough workup of breast symptoms to diagnose at early stages so that the treatment can be started as soon as the diagnosis is made.7 The risk of axillary lymph node metastasis is increased by ~0.9% to 1.8% in case of delayed diagnosis by one month.14 The incidence of PABC is lower (0.7% in India) in developing countries as the age of the mother at delivery is younger.15 Pregnancy should not be considered as an obstacle for cancer treatment and majority of PABC woman are candidates for chemotherapy.16 A multidisciplinary approach is required in the diagnosis and treatment of PABC in order to treat the cancer and at the same time to protect the fetus.17
We report here a case of a pregnant woman with advanced stage (Stage IV) breast cancer metastasized to liver and lungs who was successfully managed with NDLS based TAC regimen.
The gold standard for diagnosis of PABC remains the histopathological diagnosis based on core biopsy.17 This patient underwent MRI and core biopsy to confirm the diagnosis as invasive carcinoma of left breast – NOS. Breast cancer is usually diagnosed with metastasis in pregnant women with advanced age,9 and similar was the case with this 44-year-old patient in whom the cancer had spread to the lungs and liver. Estrogen and progesterone receptors are frequently negative in PABC,18 as evidenced in this patient who was estrogen receptor negative and weak progesterone positive.
PABC is associated with an increased risk of preterm delivery but it does not increase the risk of growth restriction, stillbirths, or congenital malformations.19 Similarly, this patient also delivered a healthy child at 32nd week, whose birth weight was normal as per the gestational age and had normal psychomotor development.
Anemia is common in pregnant women and in India ~50% of the pregnant women in are anemic as per National Family Health Survey-4.20 Few studies have correlated the biallelic mutation in BRCA1/BRCA2 with Fanconi’s anemia.21 Bona fide Fanconi anemia proteins, BRCA2 (FANCD1), PALB2 (FANCN), and BRIP1 (FANCJ) genes along with BRCA1 have a role in DNA interstrand crosslink (ICL) repair, and deficiency in BRCA-dependent ICL repair is associated with breast cancer susceptibility.22 However, genotyping was not performed in our patient.
The management of breast cancer during pregnancy includes surgery during all three trimesters and chemotherapy in the second and third trimesters without increasing the risk of fetal malformations.17 However, lower birth weight has been reported in infants exposed to chemotherapy in utero. According to the NCCN guidelines, surgery should not be considered for advanced stage (Stage IV) patients, and the treatment should involve chemotherapy. Several chemotherapeutic agents belonging to pregnancy category D including 5-fluorouracil, doxorubicin, epirubicin, cyclophosphamide, docetaxel, paclitaxel, and trastuzumab have been evaluated for the treatment of breast cancer in pregnancy.16 During pregnancy, systemic therapy with taxanes and platinum agents can be safely used after careful risk/benefit assessment for mother and child.23
The standard of care for PABC is anthracycline/taxane-based chemotherapy regimens.23,24 As per the published data, doxorubicin in combination with cyclophosphamide and 5-fluorouracil after the first trimester of pregnancy was not associated with antepartum complications,25 and the anthracycline‐based chemotherapy can be used with minimal risk to the fetus in the second and third trimesters.26 Similarly, taxanes such as docetaxel and paclitaxel when used after the first trimester of gestation showed no increase in the occurrence of fetal malformations and maternal complications.16,27 A systematic review of 16 studies (50 pregnancies) demonstrated that exposure to docetaxel or paclitaxel was well-tolerated during pregnancy with manageable toxicities and can be considered as an optimal treatment option for patients with PABC.28
Preclinical evidence shows low levels of docetaxel/paclitaxel presence in the fetal tissue.29 Mir et al, in a systematic review mention that an increase (50–100%) in the activity of cytochrome P-450 (CYP) 3A4 during the third trimester of pregnancy may increase the metabolism of docetaxel, a substrate of CYP-450 3A4, that may result in a shorter half-life and a higher clearance.30,31 Thus, the favorable toxicity profile of taxanes during the second and third trimesters of pregnancy makes it a potential choice toward management of cancers in such cases.
Docetaxel has demonstrated efficacy and tolerability for the treatment of breast cancer as a part of the TAC regimen in several studies in neoadjuvant, adjuvant, and metastatic settings.32–36 Furthermore, docetaxel has been successfully evaluated for the treatment of PABC. Santis and colleagues published the first case report on the use of docetaxel in PABC in a 34-year-old woman with 15 weeks of gestation and skeletal metastasis. The patient was successfully treated with docetaxel monotherapy for three cycles and the patient delivered a female infant during the 32nd week of pregnancy with normal birth weight and Apgar score without any abnormalities.37 Potluri et al,27 reported that adjuvant treatment with docetaxel (four cycles) after doxorubicin and cyclophosphamide-based neoadjuvant treatment (four cycles) resulted in a delivery of a normal child at the 34th week of gestation.27 Neoadjuvant therapy with doxorubicin/docetaxel at 14 weeks of gestation did not show any fetal malformations after completion of six cycles and resulted in delivery of a normal child at the 35th week of gestation. Potluri et al, concluded that pregnant patients with cancer can be treated with chemotherapy including taxanes during the second and third trimesters without significant risks to the fetus.27 Several other reports have established the use of docetaxel alone or in combination with other chemotherapeutic agents for the treatment of PABC.38
Conventional docetaxel formulation uses polysorbate-80 and ethanol as excipients, which may cause acute hypersensitivity reactions necessitating corticosteroid use as a premedication.39 Published evidence suggests that solvent-free nanoparticle drug formulations may alter the pharmacokinetic and pharmacodynamics properties of docetaxel resulting in improved efficacy and decreased incidence of adverse effects associated with wide and nonspecific body distribution (eg, neurotoxicity, musculo-skeletal toxicity, neutropenia).40 A novel NDLS formulation of docetaxel was developed based on “Aqualip” technology (patented [WO2008127358] in Europe, Japan, and Canada) with lipids Generally Regarded As Safe (GRAS) by the US Food and Drug Administration. NDLS is devoid of polysorbate-80 and ethanol, thus, not expected to cause adverse effects such as acute hypersensitivity reaction and cumulative fluid retention.40–45 NDLS has shown efficacy and safety in the treatment of breast cancer without corticosteroid premedication.46 NDLS is also effective and safe for gastric, ovarian, cervical, penile, hormone refractory prostate, and non-small cell lung cancers.39,47–50 NDLS has been approved by the Drug Controller General of India. In this patient, NDLS was preferred over conventional docetaxel as it does not contain polysorbate-80 as a carrier, which can potentially trigger acute hypersensitivity reactions and cumulative fluid retention.41–43
This report presents the evidence for efficacy and safety of NDLS in the treatment of breast cancer with liver and lung metastasis in a pregnant woman. NDLS, when used in combination with cyclophosphamide and doxorubicin, was effective in controlling the symptoms and have reduced the disease burden in this patient. The patient delivered a healthy child with normal body weight and Apgar score without any malformations, consistent with published literature of conventional docetaxel.37 Overall, the patient was clinically asymptomatic with NDLS based chemotherapy and showed a partial response as per RECIST 1.1. Furthermore, the patient completed six cycles of TAC and is on treatment with tamoxifen. On follow-up, both the mother and infant were doing well. The patient tolerated NDLS treatment well without any clinically significant adverse events.
Conclusion
The current report highlights that NDLS with cyclophosphamide and doxorubicin as first-line therapy was effective in a pregnant woman with MBC. The treatment demonstrated a partial response with complete resolution of lung metastasis. The treatment was well-tolerated. Due to its favorable tolerability profile, NDLS can potentially be used in pregnant women.
Ethics approval and consent to participate
The patient provided written informed consent for the publication of this report and the accompanying images. Institutional approval was not required to publish this manuscript.
Acknowledgments
The development of this case report was supported by Intas Pharmaceuticals Ltd. We would like to thank Mr. Shreekant Sharma (Lambda Therapeutic Research) for his support in developing the concept/medical writing, and follow-up with the journal/publisher, Dr. Venugopal Madhusudhana (Lambda Therapeutic Research) for additional editorial assistance, and Mr. Vinju Eswaran Hariharan (Intas Pharmaceuticals Ltd) for assisting in data collection.
Author contributions
RR, NJ, MK, SS contributed to the concept and design, acquisition, analysis, and interpretation of data. RR and SS provided medical care for the patients and collected the data. All authors contributed to data analysis, drafting, or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Drs. Nisarg Joshi and Mujtaba A Khan are employees of Intas Pharmaceuticals Ltd. The authors report no other conflicts of interest in this work.
References
1. Froehlich K, Stensheim H, Markert UR, Turowski G. Breast carcinoma in pregnancy with spheroid-like placental metastases – a case report. APMIS. 2018;5:448–452. doi:10.1111/apm.2018.126.issue-5
2. Sekine M, Kobayashi Y, Tabata T, et al. Malignancy during pregnancy in Japan: an exceptional opportunity for early diagnosis. BMC Pregnancy Childbirth. 2018;1:50. doi:10.1186/s12884-018-1678-4
3. Pavlidis NA. Coexistence of pregnancy and malignancy. Oncologist. 2002;4:279–287.
4. Balaya V, Bonsang-Kitzis H, Ngo C, et al. What about sentinel lymph node biopsy for early breast cancer during pregnancy? J Gynecol Obstet Hum Reprod. 2018;5:205–207. doi:10.1016/j.jogoh.2018.03.003
5. Durrani S, Akbar S, Heena H. Breast cancer during pregnancy. Cureus. 2018;7:e2941.
6. Martinez MT, Bermejo B, Hernando C. et al. Breast cancer in pregnant patients: a review of the literature. Eur J Obstet Gynecol Reprod Biol;2018. 222–227. doi:10.1016/j.ejogrb.2018.04.029
7. Szekely B, Langmar Z, Somlai K, et al. [Treatment of pregnancy associated breast cancer]. Orv Hetil. 2010;32:1299–1303.
8. Antonelli NM, Dotters DJ, Katz VL, et al. Cancer in pregnancy: a review of the literature. Part I. Obstet Gynecol Surv.1996;51:125–134.
9. Aktoz F, Yalcin AC, Yuzdemir HS, et al. Treatment of massive liver metastasis of breast cancer during pregnancy: first report of a complete remission with trastuzumab and review of literature. J Matern Fetal Neonatal Med. 2018;1–6.
10. Shlensky V, Hallmeyer S, Juarez L, et al. Management of breast cancer during pregnancy: are we compliant with current guidelines? AJP Rep. 2017;1:e39–e43.
11. Iavazzo C, Minis EE, Gkegkes ID. Current management of gynecologic cancer in pregnancy. J Turk Ger Gynecol Assoc. 2018;2:104–110.
12. Kuo K, Caughey AB. Management strategy for breast cancer in pregnancy. Obstet Gynecol. 2018;1:122–125. doi:10.1097/AOG.0000000000002647
13. Wist E, Mjaaland I, Løkkevik E, Sommer HH. Weekly paclitaxel plus capecitabine versus docetaxel every 3 weeks plus capecitabine in metastatic breast cancer. J Oncol. 2012. doi:10.1155/2012/862921
14. Nettleton J, Long J, Kuban D, et al. Breast cancer during pregnancy: quantifying the risk of treatment delay. Obstet Gynecol. 1996;3:414–418. doi:10.1016/0029-7844(95)00470-X
15. Gogia A, Deo S, Shukla N, et al. Pregnancy associated breast cancer: an institutional experience. Indian J Cancer. 2014;2:167.
16. Monteiro DLM, Trajano AJB, Menezes DCS, et al. Câncer de mama na gravidez e quimioterapia: revisão sistemática. Revista Da Associação Médica Brasileira. 2013;2:174–180.
17. Lund PS, Saltvig I, Oldenburg MH, et al. [Diagnostics, treatment and prognosis in breast cancer during pregnancy]. Ugeskr Laeger. 2018;180:pii: V09170665.
18. Sasidharan R, Harvey V. Pregnancy and breast cancer. Obstetric Med. 2010;2:54–58. doi:10.1258/om.2010.090066
19. Shechter Maor G, Czuzoj-Shulman N, Spence AR, et al. Neonatal outcomes of pregnancy-associated breast cancer: population-based study on 11 million births. Breast J. 2018;25:86–90. doi:10.1111/tbj.13156
20. National Health Portal, Anaemia during Pregnancy (maternal Anemia). New Delhi (India): National Institute of Health and Family Welfare; 2019. Available from: https://www.nhp.gov.in/disease/gynaecology-and-obstetrics/anaemia-during-pregnancy-maternal-anemia.
21. Howlett NG, Taniguchi T, Olson S, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;5581:606–609. doi:10.1126/science.1073834
22. Sawyer SL, Tian L, Kahkonen M, et al. Biallelic mutations in BRCA1 cause a new Fanconi anemia subtype. Cancer Discov. 2015;2:135–142. doi:10.1158/2159-8290.CD-14-1156
23. Loibl S, Schmidt A, Gentilini O, et al. Breast cancer diagnosed during pregnancy: adapting recent advances in breast cancer care for pregnant patients. JAMA Oncol. 2015;8:1145–1153. doi:10.1001/jamaoncol.2015.2413
24. Peccatori FA, Lambertini M, Scarfone G, et al. Biology, staging, and treatment of breast cancer during pregnancy: reassessing the evidences. Cancer Biol Med. 2018;1:6–13.
25. Berry DL, Theriault RL, Holmes FA, et al. Management of breast cancer during pregnancy using a standardized protocol. J Clin Oncol. 1999;3:855–861. doi:10.1200/JCO.1999.17.3.855
26. Loibl S, von Minckwitz G, Gwyn K, et al. Breast carcinoma during pregnancy. Cancer. 2006;2:237–246. doi:10.1002/cncr.21610
27. Potluri V, Lewis D, Burton GV. Chemotherapy with taxanes in breast cancer during pregnancy: case report and review of the literature. Clin Breast Cancer. 2006;2:167–170. doi:10.3816/CBC.2006.n.029
28. Zagouri F, Sergentanis TN, Chrysikos D, et al. Taxanes for breast cancer during pregnancy: a systematic review. Clin Breast Cancer. 2013;1:16–23. doi:10.1016/j.clbc.2012.09.014
29. Calsteren KV, Verbesselt R, Devlieger R, et al. Transplacental transfer of paclitaxel, docetaxel, carboplatin, and trastuzumab in a baboon model. Int J Gynecol Cancer. 2010;9:1456–1464.
30. Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;10:989–1008. doi:10.2165/00003088-200544100-00001
31. Mir O, Goldwasser F, Treluyer J-M, et al. Taxanes for breast cancer during pregnancy: a systematic review. Ann Oncol. 2009;2:425–426.
32. O’Regan RM, Von Roenn JH, Carlson RW, et al. Final results of a phase II trial of preoperative TAC (docetaxel/doxorubicin/cyclophosphamide) in stage III breast cancer. Clin Breast Cancer. 2005;2:163–168. doi:10.3816/CBC.2005.n.019
33. Martin M. Docetaxel, doxorubicin and cyclophosphamide (the TAC regimen): an effective adjuvant treatment for operable breast cancer. Womens Health (Lond). 2006;4:527–537. doi:10.2217/17455057.2.4.527
34. Eiermann W, Pienkowski T, Crown J, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol. 2011;29:3877–3884. doi:10.1200/JCO.2010.28.5437
35. Mackey JR, Martin M, Pienkowski T, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013;1:72–80. doi:10.1016/S1470-2045(12)70525-9
36. Martin M, Lluch A, Seguí MÁ, et al. Toxicity and health-related quality of life (HRQoL) in node-negative breast cancer (BC) patients (pts) receiving adjuvant treatment with TAC (docetaxel, doxorubicin, cyclophosphamide) or FAC (5-fluorouracil, doxorubicin, cyclophosphamide): impact of adding prophylactic growth factors (GF) to TAC. GEICAM study 9805. J ClinOncol. 2005;16_suppl:604.
37. De Santis M, Lucchese A, De Carolis S, Ferrazzani S, Caruso A. Metastatic breast cancer in pregnancy: first case of chemotherapy with docetaxel. Eur J Cancer Care (Engl). 2000;4:235–237. doi:10.1046/j.1365-2354.2000.00231.x
38. Nakagomi H, Miyauchi Y, Okuda J, et al. [A patient with recurrent breast cancer whose liver metastasis regressed following combined use of weekly docetaxel and MPA.5ʹ-DFUR]. Gan To Kagaku Ryoho. 2001;10:1431–1435.
39. Naik R, Khan MA. Doceaqualip in a patient with prostate cancer who had an allergic reaction to conventional docetaxel: a case report. Mol ClinOncol. 2017;3:341–343. doi:10.3892/mco.2017.1147
40. Engels FK, Mathot RA, Verweij J. Alternative drug formulations of docetaxel: a review. Anticancer Drugs. 2007;2:95–103. doi:10.1097/CAD.0b013e3280113338
41. Weiszhar Z, Czucz J, Revesz C, et al. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur J Pharm Sci. 2012;4:492–498. doi:10.1016/j.ejps.2011.09.016
42. Fumoleau P, Tubiana-Hulin M, Soulie P, et al. A dose finding and pharmacokinetic (PK) phase I study of a new formulation of docetaxel (D) in advanced solid tumors. [abstract]. Ann Oncol. 1998;Suppl. 2:101.
43. Ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;7:665–685. doi:10.2165/00003088-200342070-00005
44. Mei L, Zhang Y, Zheng Y, et al. A novel docetaxel-loaded poly (epsilon-caprolactone)/pluronic F68 nanoparticle overcoming multidrug resistance for breast cancer treatment. Nanoscale Res Lett. 2009;12:1530–1539. doi:10.1007/s11671-009-9431-6
45. Zheng D, Li D, Lu X, et al. Enhanced antitumor efficiency of docetaxel-loaded nanoparticles in a human ovarian xenograft model with lower systemic toxicities by intratumoral delivery. Oncol Rep. 2010;3:717–724.
46. Ahmad A, Sheikh S, Taran R, et al. Therapeutic efficacy of a novel nanosomal docetaxel lipid suspension compared with taxotere in locally advanced or metastatic breast cancer patients. Clin Breast Cancer. 2014;3:177–181. doi:10.1016/j.clbc.2013.09.011
47. Ashraf M, Sajjad R, Khan M, et al. 156P Efficacy and safety of a novel nanosomal docetaxel lipid suspension (NDLS) as an anti cancer agent – a retrospective study. Ann Oncol. 2016;suppl_9:ix46–ix51.
48. Prasanna R, Bunger D, Khan MA. Efficacy and safety of DoceAqualip in a patient with locally advanced cervical cancer: a case report. Mol Clin Oncol. 2018;2:296–299.
49. Vyas V, Joshi N, Khan M. Novel Docetaxel Formulation (NDLS) in low cardiac reserve ovarian cancer. Open Access J Cancer Oncol. 2018;2:000122.
50. Gupta S, Pawar SS, Bunger D. Successful downstaging of locally recurrent penile squamous cell carcinoma with neoadjuvant nanosomal docetaxel lipid suspension (NDLS) based regimen followed by curative surgery. BMJ Case Rep. 2017;bcr2017220686. doi:10.1136/bcr-2017-220686
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.