Back to Journals » International Journal of Nanomedicine » Volume 12
Nanoparticles for the treatment of liver fibrosis
Authors Poilil Surendran S, George Thomas R, Moon MJ, Jeong YY
Received 10 July 2017
Accepted for publication 19 August 2017
Published 20 September 2017 Volume 2017:12 Pages 6997—7006
DOI https://doi.org/10.2147/IJN.S145951
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Suchithra Poilil Surendran, Reju George Thomas, Myeong Ju Moon, Yong Yeon Jeong
Department of Radiology, BioMolecular Theranostics (BiT) Lab, Chonnam National University Medical School, Chonnam National University Hwasun Hospital (CNUHH), South Korea
Abstract: Chronic liver diseases represent a global health problem due to their high prevalence worldwide and the limited available curative treatment options. They can result from various causes, both infectious and noninfectious diseases. The application of nanoparticle (NP) systems has emerged as a rapidly evolving area of interest for the safe delivery of various drugs and nucleic acids for chronic liver diseases. This review presents the pathogenesis, diagnosis and the emerging nanoparticulate systems used in the treatment of chronic liver diseases caused by liver fibrosis. Activated hepatic stellate cell (HSC) is considered to be the main mechanism for liver fibrosis. Ultrasonography and magnetic resonance imaging techniques are widely used noninvasive diagnostic methods for hepatic fibrosis. A variety of nanoparticulate systems are mainly focused on targeting HSC in the treatment of hepatic fibrosis. As early liver fibrosis is reversible by current NP therapy, it is being studied in preclinical as well as clinical trials. Among various nanoparticulate systems, inorganic NPs, liposomes and nanomicelles have been widely studied due to their distinct properties to deliver drugs as well as other therapeutic moieties. Liposomal NPs in clinical trials is considered to be a milestone in the treatment of hepatic fibrosis. Currently, NP therapy for liver fibrosis is updating fast, and hopefully, it can be the future remedy for liver fibrosis.
Keywords: liver fibrosis, inorganic nanoparticles, liposomes, micelles
Introduction
Liver fibrosis results from chronic damage to the liver in conjunction with the accumulation of extracellular cell matrix (ECM) proteins, which is a characteristic of most types of chronic liver diseases. Alcohol abuse, hepatitis viral infections, genetic abnormalities, steatohepatitis, autoimmunity and other noninfectious diseases like fatty liver contribute to liver fibrosis. The major causes of chronic liver diseases are given in Figure 1. The accumulation of ECM proteins distorts the hepatic architecture by forming a fibrous scar, and the subsequent development of nodules of regenerating hepatocytes defines cirrhosis, ie, the so-called advanced liver fibrosis. Cirrhosis produces hepatocellular dysfunction, hepatocellular carcinoma (HCC) and hepatic failure.
  | Figure 1 Major causes of chronic liver diseases. |
Fibrosis is a result of excessive accumulation of scar tissue resulting from the inflammation of liver cells. Abnormal spherical areas of cells called nodules form dying liver cells, which will be replaced by regenerating cells. As a result of a series of events resulting in hepatocyte damage, the retainment of inflammatory cells in the injured liver and the activation of collagen producing cells contribute to the liver in becoming hard, finally leading to liver fibrosis. It is characterized by the excessive deposition of ECM proteins, especially collagen type 1, and it is mainly contributed by hepatic stellate cells (HSCs).1–3
Conventional therapy is not effective for the treatment of liver diseases due to the inability to deliver adequate concentration of therapeutic agents into the liver. Recently, treatments using nanotechnology have attracted more attention owing to the targeted delivery of therapeutic agents into the liver.4–6 Using a large variety of materials, a number of nanoparticle (NP) systems have been developed for the effective treatment of liver fibrosis. The composition, architecture, shape, diverse size and surface properties of the NP systems contribute to their unique properties for the successful delivery of therapeutic precursor.7,8 This review summarizes the NP systems for the treatment of liver fibrosis and discusses the future prospects.
Pathogenesis and therapeutic target of liver fibrosis
The pathogenesis of liver fibrosis mainly includes the deposition of fibrillar collagen as well as ECM proteins as a result of the wound healing response. The main mechanism behind this is the activation of quiescent HSC in a myofibroblast-like cell with subsequent upregulation of several proteins like interstitial collagen, α-smooth muscle actin (α-SMA), proteoglycans and matrix metalloproteinase.9,10 The progression and reversal of liver fibrosis and the formation of myofibroblast are given subsequently (Figure 2).
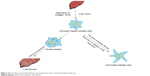  | Figure 2 Formation of myofibroblasts and progression and reversal of hepatic fibrosis. |
Several etiological factors are involved in the pathogenesis of liver fibrosis, such as alcohol consumption, viral infection, metabolic disorders, toxins, obesity, steatosis and cholestasis. Alcohol consumption is considered to be the major factor. The metabolism of alcohol results in the production of acetaldehyde and reactive oxygen species (ROS). Acetaldehyde increases the production of transforming growth factor β1 (TGFβ1) in HSC and upregulates the collagen 1 protein expression, which in turn leads to hepatic fibrosis. TGFβ1 is considered to be the major factor in the progression of alcoholic liver diseases (ALDs). At the same time, the generation of ROS will lead to cell death and damage via hepatocyte necrosis or apoptosis.11,12 Other factors, like viral infection and nonalcoholic steatohepatitis (NASH), also greatly contribute to the progression of liver fibrosis. NASH is also considered to be the predominant etiological factor in the pathogenesis of liver fibrosis, and it is characterized by the elevated expression of latent cytokine TGFβ1 as well as elevated levels of serum alanine and aspartate aminotransferase (ALT and AST, respectively). It will also result in the deposition of collagen and increase the chance for the degeneration of hepatocytes.13 NASH will also lead to increased levels of free fatty acids (FFAs) followed by the activation of peroxisome proliferator-activated receptor alpha (PPAR-α), which in turn results in ROS generation and cell damage.14
As a general rule, the currently available antifibrotic therapies have been directed against suppressing hepatic inflammation rather than subduing fibrosis. Therapeutic intervention may include efforts to remove the injurious stimuli, suppress hepatic inflammation, downregulate HSC activation and promote matrix degradation.15 Advanced cirrhosis with nodule formation, portal hypertension and early liver failure are generally considered irreversible, but less advanced lesions can show remarkable reversibility when the underlying cause of the liver injury is controlled, possibly by other therapeutic interventions. In studies of patients with hepatitis B16 and hepatitis C,17 at least 70% of the patients showed reversal of cirrhosis following successful antiviral therapies.
Activated HSC is considered to be the main reason for liver fibrosis, and all of the current NP therapies are mainly focused on targeting HSC in different manners with a variety of nanoparticulate systems. When liver injury happens, profibrogenic factors will be released by macrophages, which will activate HSC. Therefore, targeting macrophages can be useful for the therapeutic approach toward liver fibrosis, and hepatocytes are the main contributors of the accumulation of fibroblast in the injured liver. Targeting both macrophages and hepatocytes can be a good approach for therapy. There are a number of drugs targeting different pathways of liver fibrosis progression by macrophages and hepatocytes; however, few are being studied using the NP form.18–20
Liver fibrosis results from changes in four major liver cells such as hepatocytes, HSC, macrophages and liver sinusoidal endothelial cells (LSECs).21 It is evident that macrophages or Kupffer cells and HSCs are mainly responsible for both fibrogenesis and fibrolysis of the liver among different innate immune liver cells. When a liver injury occurs, macrophages initiate a fibrotic response by recruiting additional immune cells, and the activated Kupffer cells will destroy the hepatocytes and trigger the activation of HSC.22–25 The fibrolytic character of macrophages and HSC is considered to be the major reason for the reversal of fibrosis and can be utilized for therapy.26,27 Targeting mainly profibrogenic macrophages and HSC could be useful for the immunotherapy against liver fibrosis.28,29
Diagnosis of liver fibrosis
Early fibrosis can be difficult to diagnose because it is often asymptomatic. If a blood test indicates fibrosis of the liver, a liver biopsy will typically be performed. A liver biopsy requires a needle to remove a small sample of liver tissue so that doctors can assess the extent of liver damage and the degree of fibrosis. Considered as the “gold standard” for determining the extent of liver disease, several points of interest regarding liver biopsy should be considered. Liver biopsy is not always accurate and has several shortcomings. Several scales are used to determine the stage of fibrosis. One common classification is a scale from 0 to 4.30–32 The degree of fibrosis can be assessed as none, minimal, mild, moderate or severe. Ultrasonography is widely used in the diagnosis of liver fibrosis because it is an inexpensive and accurate method. However, this approach is operator dependent and has limitations for detecting early liver fibrosis in obese patients and in patients with ascites.33 Magnetic resonance (MR) imaging is a more “challenging” method for radiologists and especially for patients. MR elastography has high diagnostic accuracy for the detection of fibrosis.34
NP systems for fibrosis
Nanomedicines for liver diseases are mainly formulated using liposomes, polymers and special moieties. The delivery of drug molecules, small interfering nucleic acids, antibodies and moieties for targeting and imaging can be successfully framed using the help of liposomes as well as an enormous number of polymers. Nanoparticulate systems with stimuli sensitive polymers and liposomes have gained much attention for the treatment of liver fibrosis as well as a number of other diseases, especially cancer.35–37 Pharmacotherapy, gene therapy and immunotherapy are being studied and considered to be promising research fields in the future, despite the fact that current therapies are not that effective in completely curing hepatic fibrosis. Other than NPs, a number of small molecule drugs and monoclonal antibodies are in clinical trials now (Table 1).
  | Table 1 Clinical trials of liver fibrosis |
There are many kinds of nanoparticulates for the treatment of liver fibrosis. Our review article classifies the nanoparticulate systems based on their chemical structure and components. All the NP systems currently used for the therapy of liver fibrogenesis are characterized by their unique properties. Inorganic NPs are considered a good therapeutic option with special structures to carry drugs for the treatment. These NPs are characterized by a metal oxide or metal core, which is covered with an organic layer. These metal cores give them unique optical, electrical and magnetic properties according to their size and shape. Moreover, they have many advantages in terms of incorporating different drugs. These NPs are still in the preclinical stage of studies due to their lack of biocompatible characteristics.38–42
Liposome for cancer therapy is an emerging field of research interest currently, in both preclinical and clinical stages. Liposome can entrap both hydrophilic and hydrophobic drugs and can release them in the proper target sites. Biocompatibility, biodegradability and low toxicity are the main advantages of liposomal delivery systems. However, the low solubility, the high cost of production and the probability of leakage of drugs are challenging for researchers as well as clinicians.43,44 Liposomal drug delivery or gene delivery for the treatment of liver fibrosis is currently in the clinical stages of studies, which indicates the efficiency of these NPs compared with other NPs in practice.
Nanomicelles with a core–shell architecture composed of a semisolid hydrophobic core can trap water-insoluble drugs and can be used for a number of anticancer treatments as the majority of anticancer drugs are water insoluble. Drugs entrapped in the core will be more stable, and the smaller size contributes to the effective active targeting of the NP. One of the main advantages of polymeric micelles is that stimuli-responsive drug release is possible. In contrast to the many advantages, these NPs have a number of challenges. The small size of the polymeric micelles limits the loading of drugs inside, and the long-term stability of these NPs is also being questioned currently.45
Compared to other NPs, the development and research of solid lipid NPs (SLNs) have evolved fast due to their distinctive properties over other NPs. The controlled drug release and enhanced drug content compared to other NPs differentiate them from other carriers. SLNs are characterized by excellent biocompatibility and have the possibilities of incorporating both hydrophilic and hydrophobic drugs as well as genes. As SLNs are made up of lipids, the more complex lipids will be more sensible for the encapsulation of drugs.46 Other than these NPs, a number of normal nanoparticulate systems are used for the therapy of liver fibrosis and other liver-related diseases. However, liposomal-based nanoparticulates are the only nanocarriers that are currently being studied at the clinical level. The structures of different NPs currently used for liver fibrosis treatment are given in Figure 3.
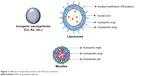  | Figure 3 Different nanoparticles used for liver fibrosis treatment. |
Inorganic NPs
Inorganic NPs are being widely used for the therapy of liver fibrosis. Cerium oxide NPs (CeO2NPs), gold NPs, as well as silver NPs are among the different inorganic NPs47 commonly used for liver fibrosis therapy. The structures of these inorganic NPs enable them for modification with particular drugs for the therapy of liver fibrosis, for example, doxorubicin (DOX), cisplatin and capecitabine. Moreover, most of the inorganic NPs are proven to be nontoxic.48 In one study, the systemic and hepatic effects of CeO2 NPs on CCL4-induced liver fibrosis were checked in rats. The hepatic and renal functions were checked after treatment with NPs. A decreased level of hepatic fibrosis was confirmed by checking the reduction in the mRNA expression of inflammatory cytokines and messengers for oxidative stress, etc. Furthermore, histology examinations of liver-like α-SMA expression, macrophage infiltration and apoptotic studies exhibited a reduction in hepatic fibrosis to a great extent after the treatment.49 CCl4-induced hepatic injury can be reduced by downregulating HSCs and Kupffer cells using silymarin-coated gold NPs. All the in vivo studies in male Wistar rats exhibited a decrease in different fibrosis markers. After treatment, reduction in the α-SMA expression was observed indicating the decreased fibrosis level.50
Immunotherapy and gene therapy of liver fibrosis using inorganic NP systems is a less explored area of research. Inorganic NPs have been used for immunotherapy and gene therapy of different diseases. However, for liver fibrosis treatment, no studies have been reported.
Liposomes
Liposomes are being used as one of the potent carriers for delivering drugs to different pathological sites, and drug delivery through liposome-based drug nanocarriers is considered the most powerful tool for the treatment of liver fibrosis.51 The therapeutic potential of dexamethasone-loaded liposomes has been proven by different researchers, and it is evident that the treatment reduced both liver inflammation and liver fibrosis. These NPs are proven to target hepatic macrophages by reducing T cells in the liver through an immune reaction, which results in the reduction of liver inflammation and fibrosis.52 Another group reported cationic liposomes bearing microbubbles for the effective delivery of artificial microRNA, which was used to target connective tissue growth factor (CTGF) and can be useful for the inhibition of hepatic fibrosis. In their study, the ultrasound-mediated bubble destruction gene delivery of artificial microRNA in a dimethyl nitrosamine-induced fibrotic mouse model resulted in a decrease in fibrotic marker collagen, as well as α-SMA, by targeting CTGF.53
There are a number of drugs being studied in clinical trials for the treatment of liver diseases like liver fibrosis, HCC, HBV and HCV, but in the NP form, only liposomes are available. Most of the NP systems for liver fibrosis therapy are in the preclinical stage of study; however, the only type of NPs in the clinical stage of study is liposomal nucleic acid carrier. The gene delivery system of vitamin A-conjugated siRNA lipid NPs is now under clinical Phase I trials for the treatment of hepatic fibrosis. siRNA delivery through PLK-1 targeting lipid particles, as well as double-stranded RNA-encapsulated liposomes, is also now being studied in Phase II and Phase I trials, respectively, for the treatment of HCC. In that study, the successful delivery of siRNA to HSC against gp46 using vitamin A-coupled liposomes resulted in the suppression of collagen secretion and therefore reduced liver fibrosis in a ccl4- and bile duct-ligated fibrosis mouse model.54
PPAR-γ ligand-loaded mannose-6-phosphate (M6P)–human serum albumin (HSA)-conjugated liposomes have been effectively used to target HSC, thereby opening a new path for the treatment of hepatic fibrosis. NPs with a size of 130 nm showed targeting of the M6P receptor and therefore reduced the symptoms of liver fibrosis both in vitro and in vivo in a carbon tetrachloride-induced fibrosis mouse model.55 In another study, the modification of liposomes using cyclic peptides to deliver drugs to fibrotic liver cells was analyzed. It was proposed that platelet-derived growth factor (PDGF) played a crucial role in the HSC proliferation. Therefore, targeting the PDGF receptor by sterically stabilized liposome and cyclic peptide loaded with interferon (INF-γ) can be utilized for the treatment of liver fibrosis. The antifibrotic effect of INF-γ was shown to be improved in the NP form in a thioacetamide (TAA)-induced fibrosis mouse model.56 Various kinds of modifications can be done on liposomes to target different liver cells (Figure 4).
  | Figure 4 Various modifications on liposomes and other nanoparticles to target different liver cells. |
Other than liposomes, polymersomes and lentiviral particles have also been used to target different liver cells. Another category of NPs that can be useful for the treatment strategy is lipid NPs. Lipid NPs are comparable with liposomes. Studies have shown that lipid NPs loaded with siRNA remarkably downregulated procollagen α I(I) gene expression and therefore reduced the total hepatic collagen content, which in turn reduced hepatic fibrosis in carbon tetrachloride-induced liver fibrosis in Balb/c mice.57 Another group studied a CXCR4-targeted lipid-based NP formulation to specifically deliver VEGF siRNA, which can also be used for the therapy of liver fibrosis and hepatic cellular carcinoma. The downregulation of VEGF expression in vitro and in vivo can be done using AMD-modified NPs (AMD-NPs) by the effective delivery of VEGF siRNAs into HSC.58
Nanomicelles
Hyaluronic acid (HA) micelles can be utilized to target LSEC and HSC by targeting the HA receptor. HA micelles carrying losartan are an effective NP system for the therapy of advanced liver fibrosis in a C3H/HeN mouse model. The overexpression of CD44 receptors during liver injury is confirmed to be suitable for HA receptor-mediated drug delivery to HSC. Angiotensin receptor type 1 receptor blocker losartan delivery showed a decreased level of α-SMA both in vitro and in vivo.59 The antioxidant and anti-inflammatory effects of natural products are also utilized currently for liver fibrosis treatments. The use of curcumin NPs is considered to be a very effective treatment for hepatic fibrosis. Curcumin-encapsulated HA–polylactic acid micelles can deliver curcumin to the HSCs and are capable of eliciting the cytotoxic effect of TAA to the HSCs in a TAA-induced liver fibrosis mouse model.60
Other NPs
Protein aggregates like HSA and bovine serum albumin (BSA) NPs can be used to target the liver for the treatment of liver fibrosis as well as HCC. Berberine/BSA NPs are a potent candidate for liver fibrosis therapy and have been shown to reduce liver fibrosis in a CCl4-induced liver fibrosis mice model in vitro and in vivo mediated by antiproliferative activity against activated HSC. These kinds of NPs are considered to be safe and effective.61 Another group studied the efficiency of dexamethasone-coupled mannosylated albumin to selectively deliver the anti-inflammatory drug to Kupffer cells. Both in vitro and in vivo studies in bile duct-ligated mice showed that the NP effectively inhibited tumor necrosis factor (TNF-α) in vitro and reduced intrahepatic ROS in vivo.62 HSAs modified with M6P NPs with a particle size of 280 nm have been used for targeting TGFβ fibrogenic cytokine through the M6P/IG II receptor to inhibit collagen production and therefore inflammation. Both in vitro and in vivo studies in male Wistar rats showed that these NPs were a potent nanocarrier for targeting HSC and can utilize the immune response against fibrosis.63–65 A similar kind of study was done by another group using M6P to modify HSA, which showed successful delivery of DOX, cisplatin and chlorambucil for the pharmacotherapy of liver fibrosis. The reduction in liver fibrosis markers was confirmed using in vitro studies as well as in vivo studies in BDL rats. HAS-M6P NPs containing DOX inhibited liver fibrosis in BDL rats, which showed the ability of antiproliferative drugs against antifibrotic action.66
Polymeric NPs for the delivery of drugs, nucleic acid and other therapeutic moieties are considered emerging field of interest among researchers. Different polymeric-based NPs have also been proven to deliver drugs and other therapeutic moieties to different liver cells for the treatment of liver fibrosis and HCC. For example, sorafenib is a tyrosine kinase inhibitor that has recently been shown to be a potential antifibrotic agent. Poly(ethylene glycol)-b-poly(lactic-co-glycolic acid) (PEG-PLGA) copolymers with PLGA were developed recently for the systemic delivery of sorafenib into the fibrotic livers of CCl4-induced fibrosis mouse models. The treatment group showed decreased α-SMA content and collagen production in the liver with significantly shrunken abnormal blood vessels and decreased microvascular density, leading to vessel normalization in fibrotic livers.67 Another polymeric NP system for the treatment of liver fibrosis is characterized by the presence of nitric oxide, which is a new treatment option. The di-block copolymer poly(oligo ethylene glycol)-methyl ether-methacrylate-block- 2-vinyl-4,4-dimethyl-5-oxazolone coated with vitamin A NPs is considered a potential carrier of nitric oxide to the HSC. It has been proven that the release of nitric oxide will decrease the rate of collagen I and α-SMA level in the liver.68 A summarization of different NP systems for liver fibrosis treatment is given in Table 2.
Prospective and conclusion
Currently, treatment using NP systems is a promising tool for the therapy of both acute and chronic liver diseases in animal models. As inorganic NPs are commonly used to load therapeutic drugs, they have a slight toxic effect to different cells unless they are modified with some biocompatible moiety.18 Liposomes and micelles are considered to be first-generation NPs with less toxicity and a wide range of advantages. SLNs are now in the stage of development for the treatment of liver fibrosis. The extremely stable structure with a lipid core for this type of particle enables them for prolonged drug release and reduces unwanted cellular uptake.69 Liposomal-based NP gene delivery for liver fibrosis, HCC and hepatitis is now in different phases of clinical trials, which indicates their promising future.
Tailoring of NPs can be utilized for specific targeting to liver cells and delivering potent therapeutic precursor with low systemic toxicity for pharmaco-, gene- and immune therapy. In the current scenario, pharmacotherapy and immunotherapy are being studied in the preclinical stage but are not yet in clinical trials. However, more advanced systems have been developed with improved delivery and efficacy, which can be next-generation solutions for the treatment of different kinds of liver diseases. Compared to other therapeutic options, gene delivery is more effective and is now in clinical trials.
Nanomedicine offers great prospects for progress in the prevention and treatment of chronic liver diseases like hepatitis and liver fibrosis. There are a number of NPs in preclinical trials; however, only a few are approved and tested in clinical trials for liver fibrosis and HCC.
Acknowledgments
This research was supported by Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and Future Planning (2015R1A2A2A01007798).
Disclosure
The authors report no conflicts of interest in this work.
References
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. | ||
Giannitrapani L, Soresi M, Bondi ML, Montalto G, Cervello M. Nanotechnology applications for the therapy of liver fibrosis. World J Gastroenterol. 2014;20(23):7242–7251. | ||
Mona H, Ismail MP. Reversal of liver fibrosis. Saudi J Gastroenterol. 2009;15(1):72–79. | ||
Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–194. | ||
Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20(10):2515–2532. | ||
Sekhon BS, Kamboj SR. Inorganic nanomedicine – part 2. Nanomedicine. 2010;6(5):612–618. | ||
Chithrani DB. Nanoparticles for improved therapeutics and imaging in cancer therapy. Recent Pat Nanotechnol. 2010;4:171–180. | ||
Heneweer C, Gendy SE, Peñate-Medina O. Liposomes and inorganic nanoparticles for drug delivery and cancer imaging. Ther Deliv. 2012;3(5):645–656. | ||
Svegliati-Baroni G, Inagaki Y, Rincon-Sanchez AR, et al. Early response of α2 (I) collagen to acetaldehyde in human hepatic stellate cells is TGF-β independent. Hepatology. 2005;42(2):343–352. | ||
Xu T, Ni MM, Xing L, et al. NLRC5 regulates TGF-beta1-induced proliferation and activation of hepatic stellate cells during hepatic fibrosis. Int J Biochem Cell Biol. 2016;70:92–104. | ||
Bian Z, Ma X. Liver fibrogenesis in non-alcoholic steatohepatitis. Front Physiol. 2012;3:248. | ||
Carpino G, Morini S, Ginanni Corradini S, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37(5):349–356. | ||
David A, Brenner TK, Scholten D, et al. Origin of myofibroblasts in liver fibrosis. Fibrogenesis Tissue Repair. 2012;5(1):1–4. | ||
Giby VG, Ajith TA. Role of adipokines and peroxisome proliferator-activated receptors in nonalcoholic fatty liver disease. World J Hepatol. 2014;6(8):570–579. | ||
Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64(5):830–841. | ||
Zhou L, Ding L, Yin P, et al. Serum metabolic profiling study of hepatocellular carcinoma infected with hepatitis B or hepatitis C virus by using liquid chromatography-mass spectrometry. J Proteome Res. 2012;11(11):5433–5442. | ||
Wong GL, Chan HL, Yu Z, Chan HY, Tse CH, Wong VW. Liver fibrosis progression in chronic hepatitis B patients positive for hepatitis B e antigen: a prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol. 2013;28(11):1762–1769. | ||
Soenen SJ, Rivera-Gil P, Montenegro J-M, Parak WJ, De Smedt SC, Braeckmans K. Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today. 2011;6(5):446–465. | ||
Vollmar B, Siegmund S, Richter S, Menger MD. Microvascular consequences of Kupffer cell modulation in rat liver fibrogenesis. J Pathol. 1999;189(1):85–89. | ||
Zeisberg M, Yang C, Martino M, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282(32):23337–23347. | ||
Bartneck M, Warzecha KT, Tacke F. Therapeutic targeting of liver inflammation and fibrosis by nanomedicine. Hepatobiliary Surg Nutr. 2014;3(6):364–376. | ||
Tanaka M, Miyajima A. Liver regeneration and fibrosis after inflammation. Inflamm Regener. 2016;36(1):1–6. | ||
Schuppan D. Liver fibrosis: common mechanisms and antifibrotic therapies. Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S51–S59. | ||
Baeck C, Wei X, Bartneck M, et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C(+) macrophage infiltration in mice. Hepatology. 2014;59(3):1060–1072. | ||
Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20(23):7312–7324. | ||
Li H, You H, Fan X, Jia J. Hepatic macrophages in liver fibrosis: pathogenesis and potential therapeutic targets. BMJ Open Gastroenterol. 2016;3(1):e000079. | ||
Karsdal MA, Manon-Jensen T, Genovese F, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2015;308(10):G807–G830. | ||
Aravalli RN, Steer CJ. Immune-mediated therapies for liver cancer. Genes (Basel). 2017;8(2):1–20. | ||
Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol. 1999;14(7):618–633. | ||
Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21(41):11567–11583. | ||
Fallatah HI. Noninvasive biomarkers of liver fibrosis: an overview. Adv Hepatol. 2014;2014:1–15. | ||
Tannapfel A, Dienes HP, Lohse AW. The indications for liver biopsy. Dtsch Arztebl Int. 2012;109(27–28):477–483. | ||
Frulio N, Trillaud H. Ultrasound elastography in liver. Diagn Interv Imaging. 2013;94(5):515–534. | ||
Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26(5):1431–1440. | ||
Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13(11):813–827. | ||
Kateb B, Chiu K, Black KL, et al. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: what should be the policy? Neuroimage. 2011;54(Suppl 1):S106–S124. | ||
Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116(5):2826–2885. | ||
Mudshinge SR, Deore AB, Patil S, Bhalgat CM. Nanoparticles: emerging carriers for drug delivery. Saudi Pharm J. 2011;19(3):129–141. | ||
Anselmo AC, Mitragotri S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015;17(5):1041–1054. | ||
Paul W, Sharma CP. Inorganic nanoparticles for targeted drug delivery. Biointegration of Medical Implant Materials. 2010;1(8):204–236. | ||
Giner-Casares JJ, Henriksen-Lacey M, Coronado-Puchau M, Liz-Marzán LM. Inorganic nanoparticles for biomedicine: where materials scientists meet medical research. Mater Today. 2016;19(1):19–28. | ||
Sipai Altaf Bhai M, Vandana Y, Mamatha Y, Prasanth VV. Liposomes: an overview. J Pharm Sci Innov. 2012;1(1):13–21. | ||
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):1–9. | ||
Toh M-R, Chiu GNC. Liposomes as sterile preparations and limitations of sterilisation techniques in liposomal manufacturing. Asian J Pharm Sci. 2013;8(2):88–95. | ||
Movassaghian S, Merkel OM, Torchilin VP. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):691–707. | ||
Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2(3):289–300. | ||
Hendi A. Silver nanoparticles mediate differential responses in some of liver and kidney functions during skin wound healing. J King Saud Univ Sci. 2011;23(1):47–52. | ||
Tomuleasa C, Soritau O, Orza A, et al. Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cells. J Gastrointestin Liver Dis. 2012;21(2):1–10. | ||
Oro D, Yudina T, Fernandez-Varo G, et al. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J Hepatol. 2016;64(3):691–698. | ||
Kabir N, Ali H, Ateeq M, Bertino MF, Shah MR, Franzel L. Silymarin coated gold nanoparticles ameliorates CCl4-induced hepatic injury and cirrhosis through down regulation of hepatic stellate cells and attenuation of Kupffer cells. RSC Adv. 2014;4(18):9012–9020. | ||
Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine (Lond). 2013;8(9):1509–1528. | ||
Bartneck M, Scheyda KM, Warzecha KT, et al. Fluorescent cell-traceable dexamethasone-loaded liposomes for the treatment of inflammatory liver diseases. Biomaterials. 2015;37:367–382. | ||
Yang D, Gao YH, Tan KB, et al. Inhibition of hepatic fibrosis with artificial microRNA using ultrasound and cationic liposome-bearing microbubbles. Gene Ther. 2013;20(12):1140–1148. | ||
Bansal R, Nagorniewicz B, Prakash J. Clinical advancements in the targeted therapies against liver fibrosis. Mediators Inflamm. 2016;2016:7629724. | ||
Zhang F, Kong D, Lu Y, Zheng S. Peroxisome proliferator-activated receptor-gamma as a therapeutic target for hepatic fibrosis: from bench to bedside. Cell Mol Life Sci. 2013;70(2):259–276. | ||
Du SL, Pan H, Lu WY, Wang J, Wu J, Wang JY. Cyclic Arg-Gly-Asp peptide-labeled liposomes for targeting drug therapy of hepatic fibrosis in rats. J Pharmacol Exp Ther. 2007;322(2):560–568. | ||
Jimenez Calvente C, Sehgal A, Popov Y, et al. Specific hepatic delivery of procollagen alpha1(I) small interfering RNA in lipid-like nanoparticles resolves liver fibrosis. Hepatology. 2015;62(4):1285–1297. | ||
Liu J-Y, Chiang T, Liu C-H, et al. Delivery of siRNA Using CXCR4-targeted nanoparticles modulates tumor microenvironment and achieves a potent antitumor response in liver cancer. Mol Ther. 2015;23(11):1772–1782. | ||
Thomas RG, Moon MJ, Kim JH, Lee JH, Jeong YY. Effectiveness of losartan-loaded hyaluronic acid (HA) micelles for the reduction of advanced hepatic fibrosis in C3H/HeN mice model. PLoS One. 2015;10(12):e0145512. | ||
Chen YN, Hsu SL, Liao MY, et al. Ameliorative effect of curcumin-encapsulated hyaluronic acid-PLA nanoparticles on thioacetamide-induced murine hepatic fibrosis. Int J Environ Res Public Health. 2016;14(1):E11. | ||
Lam PL, Kok SHL, Gambari R, et al. Evaluation of berberine/bovine serum albumin nanoparticles for liver fibrosis therapy. Green Chem. 2015;17(3):1640–1646. | ||
Melgert BN, Olinga P, Van Der Laan JM, et al. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology. 2001;34(4 Pt 1):719–728. | ||
Rachmawati H, Reker-Smit C, Lub-de Hooge MN, van Loenen-Weemaes A, Poelstra K, Beljaars L. Chemical modification of interleukin-10 with mannose 6-phosphate groups yields a liver-selective cytokine. Drug Metab Dispos. 2007;35(5):814–821. | ||
Trim N, Morgan S, Evans M, et al. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am J Pathol. 2000;156(4):1235–1243. | ||
Beljaars L, Olinga P, Molema G, et al. Characteristics of the hepatic stellate cell-selective carrier mannose 6-phosphate modified albumin (M6P28-HSA). Liver. 2001;21(5):320–328. | ||
Greupink R, Bakker HI, Bouma W, et al. The antiproliferative drug doxorubicin inhibits liver fibrosis in bile duct-ligated rats and can be selectively delivered to hepatic stellate cells in vivo. J Pharmacol Exp Ther. 2006;317(2):514–521. | ||
Lin Ts T, Gao DY, Liu YC, et al. Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J Control Release. 2016;221:62–70. | ||
Duong HT, Dong Z, Su L, et al. The use of nanoparticles to deliver nitric oxide to hepatic stellate cells for treating liver fibrosis and portal hypertension. Small. 2015;11(19):2291–2304. | ||
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

