Back to Journals » International Journal of Nanomedicine » Volume 14
Nanoparticle-based model of anti-inflammatory drug releasing LbL coatings for uncemented prosthesis aseptic loosening prevention
Authors Alotaibi HF, Perni S, Prokopovich P
Received 25 May 2019
Accepted for publication 27 July 2019
Published 9 September 2019 Volume 2019:14 Pages 7309—7322
DOI https://doi.org/10.2147/IJN.S217112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Hadil Faris Alotaibi, Stefano Perni, Polina Prokopovich
School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, UK
Correspondence: Polina Prokopovich
School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Redwood Building, King Edward VII Avenue, Cardiff CF10 3NB, UK
Tel +44 29 208 75820
Fax +44 29 208 74149
Email [email protected]
Introduction: The only treatment for aseptic loosening is the replacement of the prosthesis through revision surgery. A preventive approach, achieved through anti-inflammatory drugs released from the device, has shown to be a viable strategy; however, the performance of these devices is not yet satisfactory thus further improvements are necessary.
Methods: We used titanium nanoparticles as a model for implant surfaces and developed a coating containing dexamethasone (DEX) using layer-by-layer deposition.
Results: The amount of deposited drug depended on the number of layers and the release was sustained for months. The efficiency of the released DEX in reducing inflammation markers (tumor necrosis factor alpha and IL-6) produced by human monocytes and macrophages was similar to the pure drug at the same concentration without negative impacts on the viability and morphology of these cells.
Conclusion: These coatings were not inferior to medical grade titanium (the standard material used in uncemented devices) regarding their ability to sustain osteoblasts and fibroblasts growth.
Keywords: titanium, cementless prosthesis, aseptic loosening, dexamethasone
Introduction
Sixty-eight thousand primary hip and 71 thousand knee replacement procedures were performed by the National Health Service in England and Wales in 2017.1 It is expected that these operations will be performed on an increasing number of patients because of the improvement in life quality they provide and the constantly aging population. The joint replacement device can be fixed to the adjacent bone tissue through two main methods; either using bone cement or cementless fixation which aims to the direct integration of the implant with the host tissue.
Cementless fixation requires a shorter time in the operating room, allows greater preservation of bone stock and provides easer revision;2 additionally, uncemented fixation is not affected by any of the possible complications associated with cemented fixation like third body wear and bone cement implantation syndrome.2,3 Despite these benefits, instability of uncemented joint replacements was a major drawback that prevented their diffusion.4 Modern designs have solved this problem and now these two fixation methods are used in a similar proportion of cases worldwide (although their relative popularity varies in different countries) and have similarly good clinical outcomes.4
Aseptic loosening is a severe bone loss (osteolysis) in the tissue surrounding the implant and it is a consequence of a prolonged inflammation;5 it is the most likely cause of revision surgery for both hip6 and knee7 and it is responsible for about 40% of all revision procedures.8 Hence, the development of anti-inflammatory drugs releasing devices has been seen as tool in the fight toward the reduction of arthroplasty failure9,10 with the objective of improving on the current NICE guidelines that have set a maximum rate of revision after 10 years for the failure of 10%.11
The direct release of anti-inflammatory drugs from the surface of cementless devices has been suggested as a strategy to reduce the risk of aseptic loosening.12,13 The administration of anti-inflammatory drugs does not treat osteolysis but aims at reducing the periprosthetic inflammation that leads to bone loss and consequent aseptic loosing of the device.14
Dexamethasone (DEX) is a well known anti-inflammatory steroidal drug widely used in clinical practice15,16 to reduce inflammation.17 It is not water soluble, but the esterification with phosphate groups results in dexamethasone phosphate (DEX-P) that is highly water soluble and is the main component of the medical formulations containing DEX.
Layer-by-layer (LbL) is a self-assembly technique employed to produce a coating and it relies on the alternated deposition of positively and negatively charged polymers (polyelectrolytes) through electrostatic attraction.18–22 LbL has found medical applications when biocompatible polyelectrolytes are employed. LbL can be also used to release a drug from the coating, replacing one of the polyelectrolytes with the target molecule, whose only requirement is the presence of electrostatic charges. Additionally, to prolong the release of the target molecule, simply more layers are required. Scalability and ease of fabrication of LbL coating are further advantageous characteristics of such technique.18–22 The deposition of antibiotics on surfaces using LbL enabled prolonged release of these drugs.23,24 Controlled release of DEX from titanium surfaces has not been satisfactorily achieved as the direct conjugation did not sustain release for more than a few days.25 In this work, such steroidal drug was embedded into a dissolvable coating made using alginate and a polymer belonging to the class of poly-beta-amino-esters (PBAEs).26 Titanium nanoparticles were used as a model material for titanium surfaces in light of their high surface/volume ratio and identical chemical structure. The developed material was characterized and the released drug anti-inflammatory activity monitored against human monocytes and macrophages; cytocompatibility was assessed against human osteoblast and fibroblasts. Our research was driven by the following hypothesis: DEX could be embedding in coatings through the use of negatively charged, and water soluble, phosphate form of the drug (DEX-P), taking advantage of the negative charge exhibited by DEX-P; controlled and prolonged release of DEX would be achieved through LbL; anti-inflammatory activity of DEX would be retained once released from the coatings and no detrimental effects on osteoblast and fibroblast cells would be caused by the coatings activity.
Experimental
Chemicals
Titanium (IV) oxide (Anatase, <25 nm, 99.7%), (3-Aminopropyl) triethoxy-silane (APTS, 99%), PBS tablets, sodium acetate trihydrate (≥99%), DEX, DEX-P, 1,6 hexanediol diacrylate and piperazine were purchased from Sigma-Aldrich, UK. HPLC grade acetonitrile, glacial acetic acid and toluene were purchased from Fisher, UK. All other chemicals were reagent grade, stored according to manufacturer’s guidelines and used as received.
Polymer synthesis
Amino terminated poly-beta-amino esters, denoted as POLY in the text, were synthesized by mixing 1,6 hexanediol diacrylate and piperazine in DCM with piperazine excess (molar ratio acrylate:amine 1:1.1). The reaction was carried out at 50°C for 48 hrs and the polymers recovered by pouring the reaction mixture in about ten times volumes of diethyl-ether followed by vacuum drying.27
Nanoparticles preparation
Surface functionalization of titanium oxide nanoparticles
Titanium oxide nanoparticles were functionalized with amino groups (Ti-O-NH2) via silanation in toluene. Briefly, the particles were dispersed in toluene containing APTS for 24 hrs followed by repeated centrifugation/washing.25
LbL coating technique
The amino fictionalized titanium nanoparticles were coated with polyelectrolyte multilayers. The layers consisted of different numbers of a repeating sequence of alginate, POLY, DEX-P and POLY. One sequence containing these four layers was termed quadruple layers, up to ten quadruple layers were coated on the titanium nanoparticles, named as Qn where n represents the number of quadruple layers. The concentrations used were: 2 mg/mL for sodium alginate and POLY, and 10 mg/mL for DEX-P.27
Nanoparticles surface and material characterization
Transmission electron microscopy – particle size determination
Images of particles were obtained using a Zeiss 902 transmission electron microscope (TEM) operating at a voltage of 80 kV. The aqueous dispersion of the particles was drop-cast onto a carbon-coated copper grid, and grid was dried at room temperature (20ºC) before loading into the microscope (direct deposition). The average particle size, size-distribution and morphology analysis of the samples were carried out from at least ten non-overlapping fields of view transmission electron micrographs using ImageJ for Windows (Version 1.50i).
Thermogravimetric assay (TGA)
TGA was performed using a Perkin-Elmer TGA 4000 instrument. Coated nanoparticles were initially weighed and heated from 50°C to 800°C with a heating rate of 10°C/min. Weight loss percentage of each sample at 100°C and 800°C was determined relative to initial weight of the sample. Organic and inorganic content was determined by subtracting the point at initial weight loss (%) up to when the line plateaus (approximately around 800°C).
DEX release quantification
DEX release was measured by dispersing the DEX-coated nanoparticles (10 mg) into 1 mL of a buffer media (acetate buffer pH=5, and phosphate buffer pH=7.3) and incubated at 37°C in Eppendorf.
Daily, the suspension was centrifuged at 4000 g for 10 mins at room temperature; the supernatant was withdrawn for quantification and the medium was replaced with an equal volume of fresh buffer and the nanoparticles resuspended.
The amount of DEX/DEX-P released from the coating layer was determined using reverse-phase High Performance Liquid using an Agilent Technologies® HPLC (1100 series) equipped with a Waters-Spherisorb ODS2 column (Pore size – 80 Å, 5 µm, and packing dimension of 4.6 mm×150 mm). The injection volume was 10 µL. The mobile phase was a mixture of PBS-acetonitrile-glacial acetic acid 70:26:4 at a flow rate of 1 mL/min; a UV detector at 244 nm was employed.
Stock solutions of DEX-P and DEX with a concentration of 1 mg/mL were prepared separately and a series of standards ranging from 0.4 to 250 µg/mL were prepared for calibration.
Cells growth
Human monocytic leukemia cells (THP-1) and human osteoblast cells (Saos-2) were obtained from ATCC and grown in RPMI-1640 medium supplemented with 10% heat-inactivated FBS and 1% penicillin – streptomycin (PS) (Gibco by Life Technologies, USA). Human dermal fibroblast cells were kindly supplied by Prof. Stephens28 from Cardiff University and grown in Minimum Essential Medium Eagle supplemented with 10% heat-inactivated FBS and 1% PS (Gibco by Life Technologies). All types of cells were incubated at 37°C in a humidified 5% CO2-95% air atmosphere. Media were changed twice per week.
Differentiation of THP-1 monocytes to macrophages
To initiate the differentiation of monocyte-derived macrophages, 30 ng/mL of phorbol-12-myristate-13-acetate (PMA, Sigma-Aldrich) was added to the RPMI-1640 medium (10% FCS, 1% PS) for 3 days, where human monocytic leukemia cells (THP-1) were grown in suspension. Then, adhered monocyte-derived macrophages were grown in RPMI-1640 medium supplemented with 10% heat-inactivated FBS and 1% PS (Gibco by Life Technologies) at 37°C in a humidified 5% CO2 atmosphere. Cell medium was changed twice per week.
Exposure of cells to release media containing drug
Media containing DEX released from the LbL-coated nanoparticles was prepared from fresh nanoparticles suspended in buffer pH=7.3 as described in the release experiment protocol; after incubation for 24 hrs at 37°C, the suspension was centrifuged at 4000 g for 10 mins at room temperature. The supernatant was withdrawn and passed through 0.22-µm syringe filter for sterilization; DEX concentration in this solution was 3 mg/L. A solution of DEX-P containing an equivalent concentration of DEX to that in the release buffer from LbL-coated nanoparticles was prepared in buffer pH=7.3 and passed through 0.22 µm syringe filter. These two solutions were added to fresh medium (1:9) to prepare solutions containing 0.3 mg/L of DEX.
Cells were seeded in 96-well plates (Thermo Fisher Scientific, Denmark) at a density of 5×104 cells per well and incubated for 24 hrs at 37°C in a humidified 5% CO2 atmosphere to allow cell attachment. After removing the media and washing the cells with sterile PBS, cells were incubated for 3 hrs at 37°C in a humidified 5% CO2 atmosphere with release media from nanoparticles or media containing DEX-P to an equivalent amount of DEX. The cells incubated in cell medium only with neither 24 hrs solute of nanoparticles nor lipopolysaccharides (LPS) were used as blank control.
1 mg/L of LPS (Sigma-Aldrich) was added to the cells in each well when required. The experiments were performed in triplicate wells to evaluate the cytokines produced by cells and cell mitochondrial activity at two time points, 6 and 24 hrs.
Biological test
MTT
MTT assay was conducted to measure human macrophages (THP-1), human osteoblasts (Saos-2) or fibroblasts mitochondrial activity post-exposure to DEX-coated titanium nanoparticles after 1, 2 and 3 days exposure.24
IL-6 and tumor necrosis factor alpha (TNFα)
The amount of IL-6 and TNFα released by activated and nonactivated THP-1 cells in the media after exposure to LPS and DEX for 6 and 24 hrs was determined using appropriate ELISA kit (Sigma, UK) according to manufacturer’s recommendations. The concentration of the inflammation markers was performed by absorbance at 450 nm with (TNFα) or without reference wavelength at 650 nm (IL-6) in a multiwell microplate reader (LT-5000MS ELISA reader, Labtech). Calibration curves were obtained at each measurement to reduce variability between experiments.
Epifluorescent imaging
Cells were fixed with 3.7% (w/v) formaldehyde in PBS at room temperature for 5 mins and, then, permeabilized with 0.1% Triton X-100 for further 5 mins at room temperature.
F-actin cytoskeleton was stained with 50 mg/L of tetramethyl rhodamine B isothiocyanate-conjugated phalloidin (Sigma-Aldrich, St. Louis, MO, USA) for 40 mins at room temperature, followed by incubation with 5 mg/L of trihydrochloride Hoechst 33342 (Thermo Fisher Scientific, Eugene, OR, USA) for 10 mins in the dark to stain cells nuclei. Samples were then washed with PBS, mounted and examined using LSM 880 upright confocal laser scanning microscope with Airyscan (Zeiss, Oberkochen, Germany) with a 63× magnification objective. The following settings were employed to excite the dyes and acquire the images: λex 540–545 nm and λex 340 nm; λem 570–573 nm and λem 488 nm for tetramethyl rhodamine B isothiocyanate-conjugated phalloidin and Hoechst, respectively. The obtained images were processed using ZEN imaging software (Zeiss).29
Statistical analysis
One-way ANOVA was performed to assess the statistical significance of results between groups at a level p<0.05. All data were expressed as mean±SD from at least three independent values.
Results
TEM and particles size
Particles appeared cubical and spherical-like shaped (Figure 1A–C) with relatively narrow distributed diameters (Table 1). LbL did not noticeably impact the size of the particles; however, after 10 quadruple layers, some particles agglomeration could be observed.
 |
Table 1 Average diameter size of TiO2 nanoparticles bare, after functionalization and LbL deposition determined from TEM images |
Thermogravimetric analysis (TGA)
As visible in Figure 1D, the thermograms of all coated nanoparticles showed a plateau from around 650°C that is due to the inorganic fraction (in this work made of TiO2) of the nanoparticles remaining after the organic components have been burnt.30,31 However, the mass loss at this point does not correspond entirely to the amount of deposited polyelectrolyte and drug as the weight loss occurring around 100°C is due to the vaporization of entrapped water not a result of degradation of organic compounds (alginate/POLY/DEX-P); therefore, this mass loss needs offsetting in the calculations.32
The organic content (Table 2) of uncoated TiO2 (bare) nanoparticles was the lowest (1.4±0.2%); while after amino functionalization, the particles presented 4.2±0.9% of organic fraction. Moreover, in LbL-coated particles, the weight loss at 800°C depended on the number of quadruple layers deposited, from the highest for Q10 (17.5±1.4%) and the lowest for Q1 (6.6±0.4). As the organic content is a depended on the film thickness, Q10 was the highest film coating, followed by Q7, Q5, Q3 and Q1.
 |
Table 2 Percentage of organic material in multi-layered DEX-LbL-loaded Ti-O-NH2 surface after addition of various quadruple layers (Q1, Q3, Q5, Q7 and Q10) |
Drug loading
The amount of DEX loaded on the particles increased with increasing number of quadruples layers deposited (Table 3), starting from 0.021 mg DEX per mg of particles (2.1% w/w) after one quadruple layers to 0.044 mg DEX per mg of particles (4.5% w/w) when 10 quadruples layers were deposited.
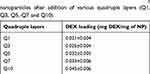 |
Table 3 DEX loading on multi-layered LbL-coated Ti-O-NH2 nanoparticles after addition of various quadruple layers (Q1, Q3, Q5, Q7 and Q10) |
DEX release quantification
The amount of DEX released from the LbL-coated Ti-O-NH2 surfaces monotonically increased with time and overall the entire amount of loaded drug was released at pH=5 regardless of the number of quadruple layers deposited while at pH=7.3 not all the loaded drug was released; the amount of DEX remained entrapped in the coating decreased with increasing number of quadruple layers deposited. DEX was detectable in the release media for longer periods of time with increasing numbers of quadruple layers; moreover, drug release was detectable for up to 20–30 days for both pH conditions (Figure 2).
At pH=7.3, DEX was detected in the release buffer for up to 25 days compared to 30 days for drug release at pH=5; the same pattern (longer release at pH=5 than pH=7.3) was observed for all the particles investigated regardless of number of layers. Furthermore, the total amount of DEX released was higher at pH=5 than the one released at pH=7.3 for comparable numbers of quadruples layers.
In-vitro inflammation model: LPS-activated human monocytes and macrophages
Mitochondrial activity
Cellular mitochondrial activity of macrophages after exposure for 6 and 24 hrs to particle release buffer containing 0.3 mg/L of DEX or media containing and equivalent amount of steroidal drug as DEX-P was assessed employing MTT assay and it did not reveal any detrimental effect (p>0.05) (Figure 3).
Inflammation markers (IL-6 and TNFα)
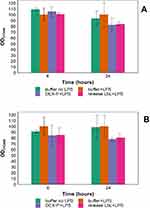
Activated human monocytes (macrophages) did not produce either noticeable amount of IL-6 or TNFα when not exposed to LPS. When inflammation was induced, IL-6 reached ~1.2 ng/mL and ~1.4 ng/mL after 6 and 24 hrs of exposure (Figure 4A–B); similarly TNFα concentration was about ~30 and ~33 ng/mL after the same exposure (Figure 4C–D). The addition of DEX-P suppressed IL-6 production to about a third after both 6 and 24 hrs; TNFα concentration was reduced to half after 6 hrs and about a third after 24 hrs. The use of an equivalent dose of DEX release from LbL coating had similar reduction patterns for both inflammation markers but the efficacy of the release drug was about 20–30% inferior to pure DEX (p<0.05).
The concentration of both inflammation markers (Figure 4) released by human monocytes (non-activate) was lower compared to macrophages, but the same pattern was observed with release media containing DEX being capable of reducing the levels of inflammation as pure DEX-P.
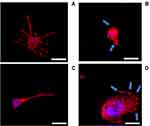
Cell morphology
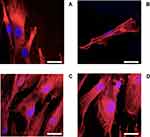
Additionally, cell morphology and cytoskeletal properties were evaluated by staining actin rings using confocal microscopy (Figure 5). The results showed that, after 24 hrs growth in media without LPS and DEX (Figure 5A), the human macrophages were spread over the surface demonstrating a regular morphology. LPS exposure (1 µg/mL) induced a reorganization in actin distribution of the macrophages resulting in evident difference in cell morphology: cells showed an oval-shaped configuration (Figure 5B), after addition of DEX the cell size increased remaining oval shape (Figure 5C-D).
Safety assessment for orthopedic application using osteoblasts (Saos-2) and fibroblasts
Mitochondrial activity
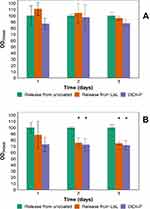
The mitochondrial activity of human osteoblasts and fibroblasts exposed to DEX-P or the equivalent dose in release buffers at pH=7.3 increased with exposing time (Figure 6). No difference was observed when comparing control cells to either DEX-P or DEX in release buffers (p>0.05) for osteoblasts; while for fibroblasts no difference was detected between DEX-P and DEX released from LbL coatings (p>0.05); although both these were lower than the control.
Cell morphology
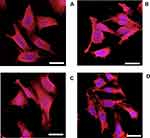
Osteoblast cells appeared spread with well organized actin filaments regardless of the presence of LPS, DEX-P or DEX from release buffers (Figure 7).
Similarly, the organization of the actin filaments in fibroblast cells was unaffected by the presence of LPS, DEX-P or DEX from release buffers (Figure 8).
Discussion
We successfully developed novel LbL coatings for the release of anti-inflammatory drugs from uncemented joint replacement devices as a preventive approach to aseptic loosening. The properties of these materials were determined to establish, not only the successful preparation of the coatings and the desired drug release profile, but also the active reduction of the inflammatory process and the absence of major cytotoxic effects on the various types of cells the materials would be in contact with.
The size of bare particles determined was in agreement with the manufacturer stated dimensions; amino functionalization did not impact on the nanoparticles size, while the small size increase calculated from TEM images observed after coating with ten quadruple layers is consistent with polyelectrolyte layers thickness under a few nanometers, similar to that reported in other studies.27,33 As observed in other studies, polyelectrolyte deposition on nanoparticles surfaces originates agglomeration as the polyelectrolytes can deposit simultaneously over more than one particle during LbL assembly.34 These aggregates during subsequent layer deposition behave as one individual particle leading to further agglomeration. However, agglomeration appears to be minimal as LbL protocol was optimized to minimize such occurrence through polyelectrolytes excess.
The amount of organic content on the surface of multi-layered DEX-LbL loaded Ti-O-NH2 particles, after the deposition of each quadruple layer (alginate, POLY, DEX and alginate) was evaluated by TGA (Figure 1D). Thermogravimetric assay is typically conducted to confirm and quantify the attachment of organic molecules on surfaces either by conjugation35 or LbL.27 The organic fraction for the amino functionalized nanoparticles (Table 2) was the results of the amino silanol conjugating to the titanium surfaces; moreover, the organic fraction increased with growing number of quadruple layers: Q1, Q3, Q5, Q7, Q10 (Table 2). The organic content of the nanoparticles after each quadruple layer observed in this work was similar to LbL-coated silica nanoparticles containing gentamicin.27 The amount was almost linear with the number of quadruple layers up to Q7 but the rate of organic fraction deposition decreased after this, because, as already seen in TEM images, LbL is progressively hindered by agglomeration and the available surface diminish accordingly.
In-vitro release studies were performed in buffers at two different pH values to simulate different joint conditions: healthy joints (pH=7.3)36 and inflamed joints, which are associated with local acidosis (pH=5).37 Only DEX-P and not DEX was detected in both the release media (pH=5 and pH=7.3). Drug release from LbL constructs is the sum of two simultaneous and independent processes; the progressive detachment of the coating layers (delamination) and the diffusion of the drug through the deposited layers.21 The kinetic of the two phenomena depends on the nature of the polyelectrolytes employed and pH;27 these two parameters directly influence the hydrolysis kinetic of the polyelectrolytes, that governs the delamination process, and the electrostatic attractions between layers that control the drug diffusion coefficient.21,27 Moreover, if diffusion is the predominant mechanism of release the profile exhibits an exponential behavior with the highest drug release at the beginning that gradually drops to zero. On the contrary, pure delamination provides a zero-order kinetic of release (constant rate until the LbL is fully degraded).21,27 The release profiles observed in this work (Figure 2) are attributable to release mechanism dominated by diffusion, similar profiles were observed for other LbL coatings prepared with the positive charged antibiotic gentamicin18,27 or an osteoinductive protein.38 However, DEX-P exhibited higher release at pH=5 than pH=7.3 while gentamicin exhibited the opposite behavior.27 Higher DEX release kinetic in acidic conditions compared to neutral were also observed39 but determined by the hydrolysis of the ester bond employed to conjugate the steroid. Since only DEX-P was observed in the release buffers, the opposite impact of pH on the release of DEX-P and gentamicin can be assumed to depend on the charge of the two drugs at the pHs tested. POLY is almost completely deprotonated at pH=7.3, with a charge close to zero, while in mild acidic conditions (pH=5) is fully protonated and positively charged; DEX-P acid groups are deprotonated in both conditions and the drug is negatively charged, analogously alginate is negatively charged in both conditions; both molecules are more negatively charged at pH=7.3 than pH=5. POLY hydrolysis is slow at pH=5,27 more a month to reach a 80% reduction of the polyelectrolyte molecular weight, thus higher delamination does not contribute to the release of DEX-P at pH=5 as it is also revealed by the release profile that is not zero order. Because of the different protonation levels of the LbL components, it can be hypothesized that diffusion of DEX-P deposited in the inner layers of the coatings would be electrostatically hindered at pH=7.3 more than at pH=5. POLY can counteract the negative charges of alginate only in acidic conditions as it is almost neutral at pH=7.3 (Figure 2) hence DEX-P molecules face more electrostatic repulsion from alginate at pH=7.3. This also explains the small impact of the number of deposited quadruple layers on the overall drug release observed at pH=7.3 (Figure 2).
Moreover, drug release had been observed for about 4 weeks and this length of time is compatible with the requirements to prevent the acute host inflammatory reaction that occurs immediately after device implantation. This reaction is due to both the injury tissues undergo during surgery to place the device and the immunological response to the material itself.40 Acute host inflammatory reaction can result in difficult device integration with compromised functionality and longevity.41
Inflammation is accompanied by the release of numerous makers in the surrounding tissues (ILs such as IL-6 and TNFα), thus their quantification is employed for diagnostic purposes, including aseptic loosening.42–44 These inflammation markers induce both osteoclast differentiation and inhibit osteoblast differentiation resulting in an overall bone loss; furthermore, they are also responsible for attracting further macrophages, osteoclasts and lymphocytes to the site aggravating the inflammation.45 Both monocytes and macrophages are known to release ILs and TNFα during inflammation46 and such human monocytes and activated human monocytes (THP-1)-derived macrophages, as model human macrophages, were used in this study to test the anti-inflammatory properties of the coatings. LPS concentration of 1 µg/mL is routinely employed to simulate inflammation in human macrophages47,48 while IL-6 and TNFα were chosen to quantify the inflammation process as they are common markers employed in aseptic loosening research.49,50 Cells were exposed to LPS and to either DEX-P or the same drug dose using the elutes collected from the DEX release studies at pH=7.3 to confirm that the anti-inflammatory activity of DEX, on inflammation markers such as TNFα and IL-6, was retained when released from the LbL construct. The elutes from the drug release studies performed at pH=5 were not considered due to the low cellular viability they caused as determined in preliminary studies (date not shown). As we have shown (Figure 2) that DEX concentration in the release media varied according to the number of layers deposited onto the Ti-O-NH2 surfaces, only elutes from DEX released from 10QL assembly were used as maximum concentration of released DEX was obtained.
LbL coatings release not only DEX but also polyelectrolytes or the products of POLY hydrolysis, therefore we determined whether both DEX activity was retained once released, and LbL decomposition products potential toxicity and inflammatory activity. DEX activity once released was marginally reduced compared to the equivalent amount of DEX-P likely as the drug was deposited and not conjugated to the surface, thus avoiding reactions for DEX active groups.
Beside IL-6 and TNFα production (Figure 4), LPS stimulation (1 µg/mL) was shown to induce significant changes on the cytoskeletal properties and morphology of the cells (Figure 5) compared to the untreated cells (control group). Multiple pseudopods with abundant actin filaments were clearly visible only on cells exposed to LPS; these membrane features did not disappear when DEX was added. Similar images were presented by Liu et al51 and Qin et al.49 As monocytes/macrophages phagocytosis is key in their immunological activity, structural changes may have negative impacts on monocytes ability to perform the assigned tasks.52
The development and evaluation of any new biomaterial must comprise not only the assessment of functionally to the desired level, but also the demonstration of no adverse effects caused to tissues and cells that the material will be in contact with. Osteoblasts are the fundamental cells of bones and they are routinely employed in in-vitro testing of novel orthopedic materials,53,54 while fibroblasts are present in connective tissues.55 Moreover, the impact of the coating on human monocytes (THP-1) and derived macrophages was also determined to exclude drug toxicity toward these cell types. It was not possible to grow cells directly onto the LbL-coated surfaces as we employed titanium nanoparticles as a model for titanium devices; hence, we tested the response of already established osteoblast cultures to media containing either DEX-P or released DEX to the response to sterile PBS. Moreover, the tests were conducted only from release buffer pH=7 as the acidic buffer (pH=5) exhibited toxic activity.
DEX released from the LbL coating did not negatively impact osteoblasts (Figure 6), fibroblast (Figure 7) and, as such, these materials are not inferior to standard titanium in regards to osteoblasts growth. Furthermore, mitochondrial activity of monocytes and macrophages was not affected by the coatings. Because titanium exhibits sufficient cytocompatibility toward osteoblasts, it was not necessary for the LbL coating to improve such properties and non-inferiority was deemed sufficient. POLY belongs to a class of known biocompatible polyelectrolytes hence the lack of toxic was expected.
In this work, we employed nanoparticles as model for implant surfaces, the next development stage will be the assessment of whether coatings on larger surfaces (i.e. coupons) would still provide an effective drug dose in light of the lower surface area/unit volume or the amount of quadruple layers necessary to achieve the required drug concentration.
Conclusion
Longevity of joint replacement devices is severely impacted by inflammatory processes that can lead to bone loss and aseptic loosening of the prosthesis. We have successfully controlled the release of DEX (a widely used anti-inflammatory steroidal drug) for about a month. This material did not impact on osteoblasts or fibroblasts proliferation and the anti-inflammatory activity of the realized drug was retained. These materials, therefore, appear a potential tool for reducing the number of revision surgeries necessary when aseptic loosening develops. Uncemented hip replacement devices have a porous or textured surface to allow mechanical interlocking with the bone; hence, during insertion only the outer part of the surface contacts the bone. Because of this, our proposed coating deposited within the porous surface will not be subjected to friction and will remain unaffected by the handling and mechanical actions associated with the surgery.
Acknowledgments
This work was supported by funding from the Princess Nourah Bint Abdulrahman University, Saudi Arabia, and Arthritis Research UK (ARUK: 18461).
Disclosure
The authors have no conflicts of interest to declare.
References
1. UK national joint registry 15th annual report [press release]; 2018.
2. Aprato A, Risitano S, Sabatini L, Giachino M, Agati G, Massè A. Cementless total knee arthroplasty. Ann Transl Med Epidemiol. 2016;4(7):129. doi:10.21037/atm.2016.01.34
3. Donaldson AJ, Thomson HE, Harper NJ, Kenny NW. Bone cement implantation syndrome. Br J Anaesth. 2009;102(1):12–22. doi:10.1093/bja/aen328
4. Maggs J, Wilson M. The relative merits of cemented and uncemented prostheses in total hip arthroplasty. Indian J Orthop. 2017;51(4):377–385. doi:10.4103/ortho.IJOrtho_405_16
5. Landgraeber S, Jäger M, Jacobs JJ, Hallab NJ. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediators Inflamm. 2014;2014:9. doi:10.1155/2014/185150
6. Ulrich SD, Seyler TM, Bennett D, et al. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 2008;32(5):597–604. doi:10.1007/s00264-007-0364-3
7. Rozkydal Z, Janik P, Janicek P, Kunovsky R. [Revision knee arthroplasty due to aseptic loosening]. Acta Chir Orthop Traumatol Cech. 2007;74(1):5–13.
8. Goriainov V, Cook RB, Latham J, Dunlop D, Oreffo R. Bone and metal - an orthopaedic perspective on osseointegration of metals. Acta Biomater. 2014;10(10):4043–4057. doi: 10.1016/j.actbio.2014.06.004
9. Wu K, Chen YC, Hsu YM, Chang CH. Enhancing drug release from antibiotic-loaded bone cement using porogens. J Am Acad Orthop Surg. 2016;24(3):188–195. doi:10.5435/JAAOS-D-15-00469
10. Li D, Guo G, Fan R, et al. PLA/F68/dexamethasone implants prepared by hot-melt extrusion for controlled release of anti-inflammatory drug to implantable medical devices: I. Preparation, characterization and hydrolytic degradation study. Int J Pharm. 2013;441(1–2):365–372. doi:10.1016/j.ijpharm.2012.11.019
11. National Institute Clinical Excellence. Total hip replacement and resurfacing arthroplasty for end-stage arthritis of the hip. Technology appraisal guidance [TA304]; 2014.
12. Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. BioTechniques. 2011;51(4):239–passim. doi:10.2144/000113754
13. Dumont CM, Park J, Shea LD. Controlled release strategies for modulating immune responses to promote tissue regeneration. J Control Release. 2015;219:155–166. doi:10.1016/j.jconrel.2015.08.014
14. Dang TT, Bratlie KM, Bogatyrev SR, Chen XY, Langer R, Anderson DG. Spatiotemporal effects of a controlled-release anti-inflammatory drug on the cellular dynamics of host response. Biomaterials. 2011;32(19):4464–4470. doi:10.1016/j.biomaterials.2011.02.048
15. Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23(7):1649–1656. doi:10.1016/s0142-9612(01)00291-5
16. Zhou G, Ma L, Jing J, Jiang H. A meta-analysis of dexamethasone for pain management in patients with total knee arthroplasty. Medicine. 2018;97(35):e11753. doi:10.1097/MD.0000000000011753
17. Tsurufuji S, Kurihara A, Ojima F. Mechanisms of anti-inflammatory action of dexamethasone: blockade by hydrocortisone mesylate and actinomycin D of the inhibitory effect of dexamethasone on leukocyte infiltration in inflammatory sites. J Pharmacol Exp Ther. 1984;229(1):237–243.
18. Chuang HF, Smith RC, Hammond PT. Polyelectrolyte multilayers for tunable release of antibiotics. Biomacromolecules. 2008;9(6):1660–1668. doi:10.1021/bm800185h
19. Hammond PT. Building biomedical materials layer-by-layer. Mater Today. 2012;15(5):196–206. doi:10.1016/S1369-7021(12)70090-1
20. Macdonald ML, Samuel RE, Shah NJ, Padera RF, Beben YM, Hammond PT. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials. 2011;32(5):1446–1453. doi:10.1016/j.biomaterials.2010.10.052
21. Smith RC, Riollano M, Leung A, Hammond PT. Layer-by-layer platform technology for small-molecule delivery. Angew Chem Int Ed Engl. 2009;48(47):8974–8977. doi:10.1002/anie.200902782
22. Wong SY, Moskowitz JS, Veselinovic J, et al. Dual functional polyelectrolyte multilayer coatings for implants: permanent microbicidal base with controlled release of therapeutic agents. J Am Chem Soc. 2010;132(50):17840–17848. doi:10.1021/ja106288c
23. Al Thaher Y, Perni S, Prokopovich P. Nano-carrier based drug delivery systems for sustained antimicrobial agent release from orthopaedic cementous material. Adv Colloid Interface Sci. 2017;249:234–247. doi:10.1016/j.cis.2017.04.017
24. Al Thaher Y, Yang L, Jones SA, Perni S, Prokopovich P, Xu B. LbL-assembled gentamicin delivery system for PMMA bone cements to prolong antimicrobial activity. PLoS One. 2018;13(12):e0207753. doi:10.1371/journal.pone.0207753
25. Rivera MC, Perni S, Sloan A, Prokopovich P. Anti-inflammatory drug-eluting implant model system to prevent wear particle-induced periprosthetic osteolysis. Int J Nanomedicine. 2019;14:1069–1084. doi:10.2147/IJN.S188193
26. Lynn DM, Langer R. Degradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122(44):10761–10768. doi:10.1021/ja0015388
27. Al Thaher Y, Latanza S, Perni S, Prokopovich P. Role of poly-beta-amino-esters hydrolysis and electrostatic attraction in gentamicin release from layer-by-layer coatings. J Colloid Interface Sci. 2018;526:35–42. doi:10.1016/j.jcis.2018.04.042
28. Wall IB, Moseley R, Baird DM, et al. Fibroblast dysfunction is a key factor in the non-healing of chronic venous leg ulcers. J Invest Dermatol. 2008;128(10):2526–2540. doi:10.1038/jid.2008.114
29. Perni S, Yang L, Preedy EC, Prokopovich P. Cobalt and titanium nanoparticles influence on human osteoblast mitochondrial activity and biophysical properties of their cytoskeleton. J Colloid Interface Sci. 2018;531:410–420. doi:10.1016/j.jcis.2018.07.028
30. Mai TB, Tran TN, Rafiqul Islam M, Park JM, Lim KT. Covalent functionalization of silica nanoparticles with poly(N-isopropylacrylamide) employing thiol-ene chemistry and activator regenerated by electron transfer ATRP protocol. J Mater Sci. 2014;49(4):1519–1526. doi:10.1007/s10853-013-7833-4
31. Zhong G, Guo W, Liu Y, et al. Preparation of tetrasulfide-functionalized silica particles by hydrothermal assisted grafting method for removal of lead (II) via dynamic solid phase extraction. Colloids Surf A Physicochem Eng Asp. 2015;485(Complete):63–72. doi:10.1016/j.colsurfa.2015.09.006
32. Du P, Zhao X, Zeng J, Guo J, Liu P. Layer-by-layer engineering fluorescent polyelectrolyte coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled release. Appl Surf Sci. 2015;345:90–98. doi:10.1016/j.apsusc.2015.03.151
33. Feng W, Nie W, He C, et al. Effect of pH-responsive alginate/chitosan multilayers coating on delivery efficiency, cellular uptake and biodistribution of mesoporous silica nanoparticles based nanocarriers. ACS Appl Mater Interfaces. 2014;6(11):8447–8460. doi:10.1021/am501337s
34. Gosens I, Post JA, de la Fonteyne LJ, et al. Impact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammation. Part Fibre Toxicol. 2010;7(1):37. doi:10.1186/1743-8977-7-37
35. Gil-Tomás J, Tubby S, Parkin IP, et al. Lethal photosensitisation of staphylococcus aureus using a toluidine blue O–tiopronin–gold nanoparticle conjugate. J Mater Chem. 2007;17(35):3739–3746. doi:10.1039/b706615e
36. Goldie I, Nachemson A. Synovial pH in rheumatoid knee joints: II. The effect of local corticosteroid treatment. Acta Orthop Scand. 1970;41(3):354–362. doi:10.3109/17453677008991521
37. de Nadai TR, de Nadai MN, Albuquerque AA, de Carvalho MT, Celotto AC, Evora PR. Metabolic acidosis treatment as part of a strategy to curb inflammation. Int J Inflam. 2013;2013:601424. doi:10.1155/2013/601424
38. Shah NJ, Hyder MN, Moskowitz JS, et al. Surface-mediated bone tissue morphogenesis from tunable nanolayered implant coatings. Sci Transl Med. 2013;5(191):191ra183–191ra183. doi:10.1126/scitranslmed.3005576
39. Wang D, Miller SC, Liu X-M, Anderson B, Wang XS, Goldring SR. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res Ther. 2007;9(1):R2. doi:10.1186/ar2106
40. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi:10.1016/j.smim.2007.11.004
41. Bridges AW, García AJ. Anti-inflammatory polymeric coatings for implantable biomaterials and devices. J Diabetes Sci Technol. 2008;2(6):984–994. doi:10.1177/193229680800200628
42. Vasconcelos DM, Ribeiro-da-Silva M, Mateus A, et al. Immune response and innervation signatures in aseptic hip implant loosening. J Transl Med. 2016;14(1):205. doi:10.1186/s12967-016-0950-5
43. Jiranek WA, Machado M, Jasty M, et al. Production of cytokines around loosened cemented acetabular components. analysis with immunohistochemical techniques and in situ hybridization. J Bone Joint Surg Am. 1993;75(6):863–879. doi:10.2106/00004623-199306000-00007
44. Goldring SR, Schiller AL, Roelke M, Rourke CM, O’Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am. 1983;65(5):575–584.
45. Gu Q, Shi Q, Yang H. The role of TLR and chemokine in wear particle-induced aseptic loosening. J Biomed Biotechnol. 2012;2012:596870. doi:10.1155/2012/596870
46. Wolf Y, Shemer A, Polonsky M, et al. Autonomous TNF is critical for in vivo monocyte survival in steady state and inflammation. J Exp Med. 2017;214(4):905–917. doi:10.1084/jem.20160499
47. Glue C, Hansen JB, Schjerling P, Jinquan T, Poulsen LK. LPS-induced cytokine production in the monocytic cell line THP-1 determined by multiple quantitative competitive PCR (QC-PCR). Scand J Clin Lab Invest. 2002;62(6):405–412.
48. Moreira-Tabaka H, Peluso J, Vonesch J-L, et al. Unlike for human monocytes after LPS activation, release of TNF-α by THP-1 cells is produced by a TACE catalytically different from constitutive TACE. PLoS One. 2012;7(3):e34184. doi:10.1371/journal.pone.0034184
49. Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis. 2012;221(1):2–11. doi:10.1016/j.atherosclerosis.2011.09.003
50. Cochran FR, Finch-Arietta MB. Interleukin-6 can prime THP-1 macrophages for enhanced production of tumor necrosis factor-alpha in response to LPS. Immunopharmacology. 1992;23(2):97–103.
51. Liu D-Q, Li L-M, Guo Y-L, et al. Signal regulatory protein α negatively regulates β2 integrin-mediated monocyte adhesion, transendothelial migration and phagocytosis. PLoS One. 2008;3(9):e3291. doi:10.1371/journal.pone.0003291
52. Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112(4):935–945. doi:10.1182/blood-2007-12-077917
53. Mikulewicz M, Chojnacka K. Cytocompatibility of medical biomaterials containing nickel by osteoblasts: a systematic literature review. Biol Trace Elem Res. 2011;142(3):865–889. doi:10.1007/s12011-010-8798-7
54. Baranowski A, Klein A, Ritz U, et al. Surface functionalization of orthopedic titanium implants with bone sialoprotein. PLoS One. 2016;11(4):e0153978. doi:10.1371/journal.pone.0153978
55. Chaffey N, Alberts B, Johnson A, et al. Molecular biology of the cell. 4th edn. Ann Bot. 2003;91(3):401. doi:10.1093/aob/mcg023
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.