Back to Journals » International Journal of Nanomedicine » Volume 18
Nanoparticle-Based Combination Therapy for Ovarian Cancer
Authors Wu Y , Yang Y , Lv X , Gao M , Gong X , Yao Q, Liu Y
Received 23 October 2022
Accepted for publication 19 March 2023
Published 12 April 2023 Volume 2023:18 Pages 1965—1987
DOI https://doi.org/10.2147/IJN.S394383
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Lei Yang
Yingli Wu,1– 3,* Yu Yang,1– 3,* Xiaolin Lv,1– 3 Menghan Gao,1 Xujin Gong,1– 3 Qingqiang Yao,1– 4 Yanna Liu1– 3
1School of Pharmacy and Pharmaceutical Sciences & Institute of Materia Medica, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, Shandong, 250117, People’s Republic of China; 2NHC Key Laboratory of Biotechnology Drugs (Shandong Academy of Medical Sciences), Jinan, Shandong, 250117, People’s Republic of China; 3Key Laboratory for Rare & Uncommon Diseases of Shandong Province, Jinan, Shandong, 250117, People’s Republic of China; 4Jining Medical University, Jining, Shandong, 272067, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qingqiang Yao, Jining Medical University, No. 133 HeHua Road, Jinan, Shandong, 272067, People’s Republic of China, Email [email protected] Yanna Liu, Shandong First Medical University, No. 6699 Qingdao Road, HuaiYin District, Jinan, Shandong, 250117, People’s Republic of China, Email [email protected]
Abstract: Ovarian cancer is one of the most common malignant tumors in gynecology with a high incidence. Combination therapy, eg, administration of paclitaxel followed by a platinum anticancer drug is recommended to treat ovarian cancer due to its advantages in, eg, reducing side effects and reversing (multi)drug-resistance compared to single treatment. However, the benefits of combination therapy are often compromised. In chemo and chemo/gene combinations, co-deposition of the combined therapeutics in the tumor cells is required, which is difficult to achieve due to dramatic pharmacokinetic differences between combinational agents in free forms. Moreover, some undesired properties such as the low-water solubility of chemodrugs and the difficulty of cellular internalization of gene therapeutics also hinder the therapeutic potential. Delivery of dual or multiple agents by nanoparticles provides opportunities to tackle these limits. Nanoparticles encapsulate hydrophobic drug(s) to yield aqueous dispersions facilitating its administration and/or to accommodate hydrophilic genes facilitating its access to cells. Moreover, nanoparticle-based therapeutics can not only improve drug properties (eg, in vivo stability) and ensure the same drug disposition behavior with controlled drug ratios but also can minimize drug exposure of the normal tissues and increase drug co-accumulation at targeted tissues via passive and/or active targeting strategies. Herein, this work summarizes nanoparticle-based combination therapies, mainly including anticancer drug-based combinations and chemo/gene combinations, and emphasizes the advantageous outcomes of nanocarriers in the combination treatment of ovarian cancer. In addition, we also review mechanisms of synergetic effects resulting from different combinations.
Keywords: ovarian cancer, nanoparticles, combination therapy, chemotherapy, gene therapy
Introduction
Ovarian cancer is one of the most common gynecologic malignancies with high morbidity.1 Due to its relatively asymptomatic nature, about 70% of patients are diagnosed at an advanced stage and have poor prognosis, leading to high mortality rates.2,3 The clinical treatment of ovarian cancer mainly relies on chemotherapy after tumor debulking. Chemotherapy exerts the growth inhibition or control of cancer cells by utilizing a single chemotherapeutic drug. However, the therapeutic efficacy of monotherapy is compromised mainly due to the emergence of multidrug resistance (MDR) and undesired side effects.4
Since it can overcome the drawbacks of single drug treatment to some extent, combination therapy has been recommended for the treatment of ovarian cancer. Combination therapy is developed by combining two or more therapeutic agents in free forms and in a manner wherein anticancer mechanisms of action synergistically complement each other.5 Different combination strategies such as combinations of chemical drugs, combinations of chemo/gene therapy and combinations of chemo/immunotherapy have been explored for different purposes and applied in clinical practice (Table 1).6–8 Combinations of chemodrugs with various action mechanisms could exert antitumor effects cooperatively, which can reduce some cases of side effects.5 For instance, doxorubicin (DOX) in combination with free radical scavenger curcumin has been shown to reduce the cardiotoxicity side effect of DOX.5 In addition, the synergistic interactions of the combined drugs prevent cancer cells to form compensatory resistance mechanisms and thus improve the therapeutic efficacy.5 For instance, doxorubicin combined with Olaparib has been indicated against the drug-resistant ovarian tumor.9 However, the synergistic anticancer effects of combination therapy based on chemodrugs are highly dependent on the accurate molar ratio of the drugs accumulated in the targeted site. This is often limited by intrinsic differences between combinational therapeutic agents in free forms, thus resulting in dramatic pharmacokinetic differences, ie, the uncoordinated cellular uptake and distribution of various drugs at the targeted site, as presented in Figure 1. In addition, some undesired properties resulting from free forms of chemotherapeutic agents in systemic administration such as low-water solubility, instability in biological fluids, renal clearance and limited tumor specificity have to be optimized to fully exploit the therapeutic potential for the treatment of ovarian cancer.10–12 For instance, paclitaxel (PTX) has poor water solubility requiring the use of toxic excipients, Cremophor EL, in clinical formulations, which can cause severe hypersensitivity reactions in patients.13
 |
Table 1 Examples of Drug Combinations for the Treatment of Ovarian Cancer in Clinical Trials |
Because ovarian cancer is a disease with genetic and acquired genetic defects, gene therapy and chemotherapy can be combined to manipulate the gene content of tumor cells and kill cancer cells at the same time, thus improving the therapeutic outcomes. In addition, MDR in cancers can be caused by adaptive gene expression. For instance, P-glycoprotein (P-gp) as a typical drug efflux pump is usually overexpressed in drug-resistant cells and has become the main contributor to the reduced intracellular drug accumulation, especially for hydrophobic drugs like PTX. The co-administration of chemodrug and drug resistance related small interfering RNAs (siRNAs) targeting P-gp has been reported to restore the sensitivity of tumor cells to the used chemodrug and thus effectively reverse cancer resistance.14 To achieve the maximal synergetic effects, temporal colocalization of the combined drug and genes in the tumor cells is required. However, compared to chemodurgs, intrinsic differences between small molecular drugs and macromolecular gene therapeutics are even more prominent, making co-deposition of them more challenging. In addition, gene therapeutics are typically characterized by hydrophilicity, large molecular weight and negatively-charged, making them difficult to enter cells.15
Chemotherapy in combination with immunotherapy may improve the curative effect as chemotherapy can mobilize anticancer immunity and generate antigenic molecules while immunotherapy can evade immunological destruction and counterbalance the acute immunosuppression induced by chemotherapy.16 However, similar to chemotherapy, immunotherapeutic agents also face problems such as instability, immune-related adverse effects and inefficient delivery.16
Codelivery of dual or multiple agents by nanoparticulate drug delivery systems provides opportunities to tackle the limits above mentioned. A delivery system co-encapsulating different therapeutic agents can control drug ratios and ensure colocalization of the combined therapeutics at the tumor site.17 Nanoparticulate drug delivery systems can encapsulate hydrophobic therapeutic agent(s), such as PTX to yield aqueous dispersions facilitating its administration and/or to accommodate hydrophilic agent(s) such as RNA facilitating its access to cells.15,17–19 Nanoparticle-based therapeutics typically consist of nanosized particles with a diameter in the range of 10 to 200 nm, favorable for increasing accumulation of delivered cargoes in the tumor via passive targeting, ie, enhanced permeability and retention (EPR) effect (Figure 1).20,21 Nanoparticles can also be modified with targeting molecules such as growth factors, antibodies, antibody fragments, or peptides, which can specifically bind to receptors overexpressed by tumor cells, leading to enhanced internalization of drug loaded nanoparticles by targeted cells and thus improved selectivity and therapeutic response by so-called active targeting (Figure 1).22 In addition, by tailoring the type and characteristics, eg, molecular weight, compositions and architectures, of the nanoparticle-forming materials, nanoparticles can also be adjusted to improve their properties and thus their payload properties such as in vivo stability, drug retention, circulation time and renal clearance.17 Many studies on encapsulation of different therapeutic agents into various types of nanoparticles, eg, polymeric micelles, lipid-related nanoparticles, dendrimers, and liposomes (Figure 2) have been carried out and shown to be effective in the treatment of different cancers including ovarian cancer.23–25 Herein, this work summarizes nanoparticle-based combination therapies, mainly including anticancer drug-based combinations and chemo/gene combinations, and emphasizes the advantageous outcomes of nanocarriers in the combination treatment of ovarian cancer. In addition, we also review mechanisms of synergetic effects resulting from different combinations. It is noted that combinations of chemo/immunotherapy are beyond our discussion because reports simultaneously involving combined chemo/immunotherapy, delivery systems, and ovarian cancer treatment are not found, which leaves room for investigation.
Anticancer Drug-Based Combinations
As combination chemotherapy is currently the first-line therapy for ovarian cancer, in this section combinational anticancer drug(s) and small molecule therapeutic agents with different action mechanisms by nanoparticulate delivery systems for the treatment of ovarian cancer are summarized (Table 2). In addition, mechanisms of synergetic effects resulting from different combinations are introduced.
 |
Table 2 Summary of Anticancer Drug-Based Combinations Achieved by Various Drug Delivery Systems for the Treatment of Ovarian Cancer |
Taxane-Based Combinations
Taxanes represented by PTX and docetaxel (DTX) are one of the most effective therapeutic agents in gynecological cancers including ovarian cancer.26 Taxanes prevent depolymerization of the protofilament substructure within the microtubule by binding to and stabilizing the β-tubulin subunits, thus causing G2/M arrest and apoptosis through cell-signaling cascades.27 Although taxanes are widely used in cancer treatment, the clinical application is limited by, eg, low water solubility, non-selective distribution and drug resistance.28 Therefore, taxane-based combinations mediated by nanomedicines formed in an aqueous solution provide opportunities to overcome these obstacles and improve the therapeutic efficacy of taxanes (Table 2). For instance, to enhance specificity and therapeutic efficacy in ovarian cancer, Jain et al codelivered PTX and topotecan by liposomes consisting of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene glycol) (DSPE-PEG) dipalmitoylphosphatidylcholine (DPPC), dimyristoyl phosphatidylglycerol (DMPG).29 The liposomes were decorated by folate acid facilitating folate receptor targeting, which is overexpressed more than 90% in ovarian cancer.29,30 In this combination regimen, PTX blocks cell division at the G2 and M phases by stabilization of microtubules whereas topotecan induces cell cycle arrest in the S phase by converting DNA topoisomerase I into a cellular toxin.29 Indeed, the synergistic combination of PTX and topotecan mediated by liposomes exhibited superior therapeutic activities in vitro and in vivo compared to the combination of free PTX and topotecan and PTX and topotecan coloaded liposomes without folate modification.
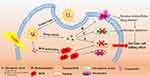
One of the main contributors of MDR is related to P-gp, which is located in the cellular membrane and uses adenosine triphosphate (ATP) to actively pump cytotoxic drugs out from the cell.31 Therefore, PTX combined with P-gp inhibitor has been reported to overcome drug-resistance. For example, Zou et al reported the combination of PTX and Borneol, a natural compound with P-gp inhibition effect, using polyamindoamine (PAMAM) dendrimers as a nanocarrier for drug resistance reversal in ovarian cancer.32,33 As a result, when compared with PTX alone, nanoparticles containing PTX and Borneol exhibited higher cytotoxicity and apoptosis induction activity on drug-resistance A2780/PTX ovarian cancer cells in vitro and improved growth inhibition efficacy for drug-resistance A2780/PTX tumor in vivo. The synergistic effect of PTX and Borneol combination on drug-resistance reversal is attributed to decrease P-gp mediated drug efflux by P-gp inhibition activity of Borneol, thus leading to enhanced intracellular concentration of PTX in PTX-resistance A2780/PTX cells, as indicated in Figure 3.
Since it has been reported that curcumin as a natural polyphenol compound can suppress the expression of P-gp, Zhao et al designed hyaluronic acid decorated polyethyleneimine (PEI) nanoparticles for codelivery of curcumin and PTX to reverse the drug-resistance of ovarian cancer.34,35 In this system, hyaluronic acid as an active targeting ligand can target cancer cells with over-expressed CD44 on cancer cell membranes while the amphiphilic graft copolymer PEI-SA containing a hydrophobic segment of SA and a hydrophilic block of PEI self-assemble to nanoparticles.36 Curcumin and PTX coloaded by the PEI-based nanoparticles exerted synergistic anticancer activities on chemosensitive SKOV3 and multidrug resistant SKOV3-TR30 ovarian cancer cells in vitro and had a good therapeutic effect on drug-resistant SKOV3-TR30 tumors in vivo. A clear synergistic effect against multidrug-resistant SKOV3-TR ovarian cancer cells in vitro and in vivo was also observed when curcumin and PTX were coloaded in micelles consisting of a mixture of DSPE-PEG and vitamin E.37 In addition, codelivery of PTX and a synthetic analog of curcumin, namely di-fluorinated curcumin, by albumin-based nanoparticles also demonstrated a synergistic anticancer effect on SKOV3 ovarian cancer cells.38 In this system, the surface of the nanoparticles was modified by folic acid for actively targeting folate receptors, leading to improve tumor specificity. The mechanism of the synergistic effect is related to the fact that curcumin inhibits the drug efflux of P-gp and changes the paclitaxel signaling pathway, which increases the intracellular PTX concentration resulting in reversing the resistance and increasing anti-tumor activity of PTX.34
In addition, although DTX as the second-generation anti-tumor taxane drug has some advantages over the first-generation taxane, ie, PTX, the bioavailability of DTX is still suboptimal due to the effect of cytochrome P450 (CYP3A) and P-gp. Considering that the activity of CYP3A enzyme and the expression of P-gp can be inhibited by curcumin, DTX was codelivered with curcumin by poly(ethylene glycol)-poly(L-lactic acid) (PEG-PLA) based polymeric micelles to improve therapeutic efficacy on ovarian cancer.28 The DTX and curcumin coloaded micelles demonstrated stronger anti-proliferative and pro-apoptotic effects on A2780 ovarian cancer cells and significantly enhanced antitumor effects in terms of inhibiting tumor growth, suppressing tumor angiogenesis and promoting tumor cell apoptosis when compared to DTX or curcumin mono-loaded micelles.
Cisplatin-Based Combinations
Platinum-based anticancer drugs, such as cisplatin, are standard chemotherapeutic agents in ovarian cancer therapy.39,40 This class of drugs can penetrate the nucleus of cancer cells and form adducts with genomic or mitochondrial DNA to block the production of DNA, mRNA, and proteins, arrest DNA replication, and activate several transduction pathways, which finally lead to necrosis or apoptosis.41 Nanoparticle-mediated combination therapy based on cisplatin has been shown a promising strategy in improving water solubility, enhancing therapeutic potential, reducing side effects and reversing drug resistance (Table 2).6 For instance, Shen et al codelivered cisplatin and PTX using an injectable prodrug hydrogel based on poly(lactic-co-glycolic acid)-b-poly(ethylene glycol) (PLGA-PEG) polymer−platinum (IV) conjugate for ovarian cancer treatment.42 The dual-drug system exhibited a synergistic anticancer effect against SKOV3 ovarian cancer cells and excellent in vivo anticancer efficacy with significantly reduced side effects in SKOV3 ovarian cancer xenograft mouse model. Likewise, the synergistic anticancer effect was also found when PTX and alkylated cisplatin prodrug were coloaded in polymeric micelles based on poly(2-methyl-2-oxazoline-b-2-butyl-2-oxazoline-b-2-methyl-2-oxazoline) (P(MeOx-BuOx-MeOx).43 The PTX and cisplatin coloaded polymeric micelles exhibited superior antitumor activity in cisplatin-resistant A2780 ovarian xenograft tumor model in comparison with single drug loaded micelles.
In addition to combination with the classical anticancer drug PTX, cisplatin as a DNA-damaging agent in combination with drugs that interfere with DNA synthesis and/or DNA damage repair has been reported to exhibit synergistic effects on ovarian cancer treatment.34 For example, Mensah et al used the electrostatic layer-by-layer liposomal nanoparticles based on 1.2-distearoylsn-glycero-3-phosphocholine (DSPC), 1-palmitoyl-2-oleoyl-sn-glycero3-phospho-(10-rac-glycerol) sodium salt (POPG) and cholesterol to codeliver cisplatin and poly(ADP-ribose) polymerase (PARP) inhibitors (Olaparib or Talazoparib) for the treatment of advanced ovarian cancers.41 The liposomal nanoparticles contain a terminal hyaluronic acid layer for selective targeting of high-grade serous ovarian cancer cells with overexpressed CD44 receptors while both cisplatin and the PARPi drug were loaded within the liposomal core and bilayer. Compared to monotherapy, the combination therapy mediated by liposomal nanoparticles led to significantly reduced tumor metastasis, extended survival, and moderated systemic toxicity in orthotopic OVCAR8 high-grade serous ovarian cancer xenograft tumor-bearing mice. In this combination regimen, PARPs as a family of nuclear DNA damage repair enzymes are responsible for the repair of DNA single-strand breaks (SSBs). The inhibition of SSB repair by PARP inhibitors causes double-strand breaks (DSB) at replication forks, which induces synthetic lethality and confers sensitivity in cells with defective BRCA1- and BRCA2-mediated homologous recombination (HR)-directed DSB repair.44 Therefore, cisplatin combined with PARP inhibitors exhibited synergistic activity by interfering with DNA repair and thus boosting the activity of the DNA-damaging agent. In addition, PLGA-PEG-based nanoparticles were used to encapsulate both cisplatin and wortmannin for the treatment of platinum-resistant ovarian cancer.45 In this combination system, wortmannin, a phosphoinositide 3-kinase inhibitor, can potently block downstream DNA repair pathways that are involved in cisplatin resistance.46,47 As a result, wortmannin in dual-drug loaded PLGA-PEG nanoparticles synergistically enhanced the cytotoxicity of cisplatin on ovarian cancer in vitro and improved its therapeutic efficacy of cisplatin in both platinum-sensitive and platinum-resistant A2780/cisplatin ovarian cancer xenograft models as compared to combinations of free drugs or single-drug loaded nanoparticles.45 Except for the inhibition of DNA repair resulting from wortmannin, the resistance reversal of cisplatin is partially attributed to improved cellular uptake of cisplatin resulting from PLGA-PEG nanoparticles. Besides, Hung et al also used PLGA-PEG-based nanoparticles to codeliver cisplatin and gemcitabine to overcome gemcitabine-chemoresistance in ovarian cancer subtypes.48 The synergistic mechanism of both drugs is related to the fact that gemcitabine inhibition of ribonucleotide reductase leads to increased formation of platinum-DNA adduct, improved gemcitabine triphosphate incorporation into DNA, which hinders DNA replication and repair.49–53 The results suggest that compared to its free drug combination, nanoparticle mediated delivery of gemcitabine further enhanced synergistic interactions with cisplatin in different ovarian cancer subtypes including high drug-resistant cancer subtypes such as PA-1 teratocarcinoma and TOV-21G clear cell carcinoma. This improved synergistic effect is also partially attributed to the improved cellular accumulation of gemcitabine resulting from nanoparticle delivery that can bypass the inherent nucleoside transporters, required for intracellular delivery of gemcitabine in its free form but defective in high drug-resistant cancer subtypes such as teratocarcinoma.48
Doxorubicin-Based Combinations
Doxorubicin (DOX) is an anthracycline anticancer antibiotic that is widely used for the treatment of various cancers including ovarian cancer.54 The mechanisms for anticancer effects of DOX are involved in insertion into DNA to inhibit the production of topoisomerase II and production of reactive oxygen species (ROS), resulting in damage of the membrane of cell and mitochondria and apoptosis.55–57 Due to the undesired clinical effect of DOX alone, many researchers have explored the combined effect of DOX and other drugs by codelivering them with various nanoparticles for the treatment of ovarian cancer (Table 2).58–63 For example, Zheng et al encapsulated DOX and verapamil, a P-gp inhibitor, in PEG-PLA-based nanoparticles.64 In vitro cytotoxicity shows that single DOX had limited intracellular concentration in DOX-resistant A2780/DOX and SKOV3/DOX ovarian cancer cells while the addition of verapamil significantly elevated the DOX accumulation in both cells due to inhibiting the DOX efflux induced by P-gp overexpression. More importantly, DOX and verapamil coloaded nanoparticles showed efficient drug resistance reversal in DOX-resistant A2780/DOX and SKOV3/DOX ovarian cancer both in vitro and in vivo, along with enhanced curative effects and reduced systemic toxicity when compared with single DOX and free verapamil and DOX combination of free verapamil and DOX. In addition, PEG-PLA nanoparticles simultaneously encapsulating DOX and chloroquine also led to significantly improved anticancer effects of DOX in vivo along with reduced side effects when compared with DOX alone regardless of its forms.61 The synergistic effect is contributed to the fact that chloroquine as a lysosome inhibitor efficiently reversed the DOX degradation and sequestration effects induced by lysosomes, leading to the enhanced tumor suppression of chemotherapeutic agents. Except for the physical loading of drugs in PEG-PLA nanoparticles, synergistic cytotoxic effects were observed by chemical conjugation of DOX and DTX to PEG-PLA polymeric micelles modified by folate acid that have targeting ability of folate receptors, as indicated by enhanced therapeutic efficacy in vitro at low doses of each drug.65 Similarly, DSPE-PEG conjugated with DOX and d-tocopheryl polyethylene glycol succinate (TPGS) conjugated a lipophilic anthraquinone Chinese herbal medicine, rhein, were mixed to form polymeric micelles for drug delivery.66 Compared to the free DOX alone, free rhein and free DOX/rhein group, DOX and rhein loaded polymeric micelles elevated effects on cell cycle arrest in G0/G1 phase and apoptosis induction in DOX-resistant SKOV3/DOX ovarian cancer cells, which led to enhanced cytotoxicity in DOX-resistant SKOV3/DOX ovarian cancer cells. In vivo study shows that DOX and rhein loaded polymeric micelles had better cancer targeting and improved tumor efficacy without obvious toxicity on SKOV3/DOX tumor-bearing mice compared to free DOX/rhein combination group.
Cardiotoxicity is a major adverse effect of DOX, limiting its clinical use of DOX. It has been reported that the cardiotoxicity of DOX is associated with increased oxidative stress resulting from increased levels of ROS and lipid peroxidation.67 Natural polyphenols such as quercetin, resveratrol, and curcumin are known free radical scavengers and have demonstrated cardioprotective effects through, eg, mechanisms of antioxidant and free radical scavenging.68 In addition, these natural products have been reported to have chemosensitizing effects when used in combination with conventional chemotherapeutics.60 Considering their low aqueous solubility and low oral bioavailability, and short half-lives, these free radical scavengers are usually delivered by nanocarriers when in combination with DOX to fully assess their capabilities of cardioprotection and chemosensitization in cancer treatment.60 For instance, it has been demonstrated that curcumin and resveratrol coloaded Pluronic®F127 micelles in combination with DOX enhanced the antiproliferative effect of DOX on SKOV3 and DOX-resistant SKOV3/DOX ovarian cancer cells in vitro while mitigating doxorubicin-induced cardiotoxicity both in vitro and in vivo.58,59 In separate studies, they also show that combinatorial resveratrol and quercetin by Pluronic®F127 micelles when co-administered with DOX had cardioprotective effects in vitro and in vivo. Meanwhile, quercetin/resveratrol coloaded Pluronic®F127 micelles combined with DOX increased the DOX efficacy against DOX-sensitive (SKOV3 and ES2) and DOX-resistant (A2780/DOX) ovarian cancer cells in vitro and significantly lowered tumor growth in both ES2 and A2780/DOX mouse xenograft models when compared to DOX alone or quercetin/resveratrol loaded micelles alone.59,69
Other Combinations
Except for combinations based on first-line chemodrugs, combinations between other drugs have also been studied for the treatment of ovarian cancer. For example, Zhou et al co-encapsulated a H2S-releasing nonsteroidal anti-inflammatory prodrug, SH-aspirin, and curcumin in PEG-PLGA-based nanoparticles to solve their drawbacks such as the relatively low anticancer activity of SH-aspirin (eg, IC50 of SH-aspirin were 56.1 µg/mL on SKOV3 cells) and poor water solubility of both drugs.70 Codelivery of SH-aspirin and curcumin by nanoparticles led to better anticancer effects than SH-single aspirin or curcumin loaded nanoparticles on both SKOV3 and ES-2 human ovarian carcinoma cells in vitro due to the synergistic effects of both drugs. The synergistic anticancer effects are associated with the induction of apoptosis through activating caspase-3 and caspase-9 to trigger the mitochondria-dependent apoptotic signaling pathway.
Triptolide is a predominant active component of Chinese herbal medicine and has antitumor effects on ovarian cancer.71 However, the therapeutic potential is hindered by its high toxicity to the liver, kidney and reproductive system.72 It has been reported that curcumin can protect liver and kidney by resisting oxidative stress caused by chemodrugs.73 Therefore, to enhance therapeutic effects and reduce the side effects of triptolide, nanoparticles composed of 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene glycol) and calcium phosphate (DPPE-PEG/calcium) were developed to codeliver triptolide and curcumin.74 This study indicates that triptolide and curcumin coloaded nanoparticles exhibited synergistic in vitro proliferation inhibition effects on the SKOV3 cell line by arresting the cell cycle in the S and G2/M phases and inducing cellular apoptosis through inhibiting the expression of related heat shock protein in cells. In vivo study shows that nanoparticles loaded with both drugs could greatly reduce the toxicity of free triptolide to liver and kidney through anti-oxidative stress effects resulting from curcumin and exhibited favorable curative effects on the SKOV3 tumor-bearing mice (Table 2).
Combination of Chemotherapy and Gene Therapy
Gene therapy introduces exogenous genetic material(s), ie, nucleic-acid-based bioactives to regulate gene expression in specific cells and overcome the cellular pathways to treat diseases including cancers caused by aberrant gene expression.75 Among nucleic-acid-based bioactive compounds, small interfere RNAs (siRNAs) are extensively studied as therapeutic agents, which can induce specific cleavage through its complementary pairing with mRNA, thus leading to degradation of mRNA and failure of the corresponding protein expression.4 Since naked-siRNA is difficult to enter cells due to its hydrophilicity, large molecular weight and negatively-charged feature resulting in low transfection efficiency, nanoparticle-based carriers are required for delivery of genes. Encouragingly, many siRNA-based therapeutic agents delivered by different nanocarriers have been developed and used for clinical trials.15 It has been demonstrated that a combinational therapy consisting of siRNA and chemotherapy is effective in the treatment of various cancers including ovarian cancer.4,19
Codelivery of siRNA and Anticancer Drug for Overcoming Resistance
As the primary reason for the poor efficacy of ovarian cancer treatment, resistance to chemotherapy is related to abnormal gene expression. Expression of several genes such as MDR1 and Bcl-2 has been reported to be associated with the reduced sensitivity of cancer cells to anti-cancer drugs.76 Therefore, chemotherapy in combination with siRNA-based gene therapy is shown to be effective in reversing MDR of ovarian cancer.4,19 In this section, we summarize nanoparticulate delivery systems for codelivery of different gene silencing siRNA and anticancer drug to treat ovarian cancer, especially MDR ovarian cancer (Table 3).
 |
Table 3 Summary of Combination of Chemotherapy and Gene Therapy |
Codelivery of siRNA and Anticancer Drug for Overcoming Pump-Related Resistance
One of the main development mechanisms of MDR is associated with overexpression of ATP-binding cassette (ABC) transporter family of transmembrane proteins that can form membrane-bound ATP-dependent active drug efflux pumps (Figure 3).76 ABC transporters utilize the energy of ATP to actively pump chemotherapeutic agents out of the cell, which can significantly decrease the intracellular accumulation of the drug and thereby increase the therapeutic efficacy. Suppression of the expression of MDR associated proteins can be achieved via selective silencing of the corresponding genes by siRNA, which is a promising approach to reverse the resistance of anticancer drugs (Figure 3).
Among MDR associated proteins, P-gp encoded by the ABCB1 or MDR1 gene is the first identified ABC transporter and is known to be the main protein that induces MDR in solid tumors.77–79 Therefore, many different nano-delivery systems have been developed to codelivery MDR1 gene silencing siRNA (MDR1 siRNA) and chemotherapeutics for overcoming MDR resistance of chemodrug(s) (Table 3).80 For example, nanoparticles composed of poly(ethylene oxide)-b-poly(beta-aminoester) (PEO-PbAE) and poly(ethylene oxide)-b-poly(ɛ-caprolactone) (PEO-PCL) were developed to deliver MDR1 siRNA and PTX, respectively.81 Compared to PTX nanoparticles only, administration of MDR1 siRNA and PTX coloaded nanoparticles resulted in a significant increase in intracellular PTX levels, apoptotic rate and the cytotoxic chemotherapeutic effect in drug-resistant SKOV3-TR ovarian cancer cells. Wang et al designed a biomimetic lipid/dextran hybrid nanocarrier to coload MDR1 siRNA and PTX, which also showed better therapeutic effects on highly PTX-resistant OVCAR-3 ovarian cancer cells both in vitro and in vivo as compared to PTX-only system did.82 The synergistic effects were also found when MDR1 siRNA delivered by hyaluronic-acid-based PEI/PEG nanoparticles in combination with PTX.14 It is shown that compared to treatment of the PTX alone, administration of nanoparticles loaded with MDR1 siRNA followed by PTX effectively downregulated P-gp expression, potentially increased apoptosis and significantly inhibited tumor growth in MDR SKOV3-TR ovarian cancer mice model. In addition, He et al reported a self-assembled core−shell nanoscale coordination polymer-based nanoparticles for the codelivery of MDR1 siRNA with cisplatin or cisplatin plus gemcitabine, in which siRNAs were located in the lipid layer and chemotherapeutic agent(s) was loaded in the core. Strong synergic effects among chemotherapeutic agent(s) and MDR1 siRNA in the nanoparticles were indicated by, eg, efficiently induced cell apoptosis and drastically enhanced anticancer efficacy in subcutaneous and intraperitoneal xenograft mouse models of cisplatin resistant ovarian cancer, as compared to that provided by mono-chemotherapeutic nanoparticles or free drug(s).83 Joshi et al delivered DOX and MDR1 siRNA using a novel hypoxia-sensitive nanoparticle system composed of PEG, azobenzene (Azo), polyethyleneimine (PEI) and 1,2-dioleyl-sn-glycero-3-phosphoethanolamine (DOPE) units (PEG-Azo-PEI-DOPE), where Azo imparts hypoxia sensitivity.84 The treatment of PEG-Azo-PEI-DOPE nanoparticles containing MDR1 siRNA and DOX significantly enhanced the cytotoxicity effect of DOX on the DOX-resistant A2780/DOX ovarian cancer cells. For instance, cell viability was approx. 90% at DOX monotherapy of 3 µM whereas only 25% cell viability was observed when MDR1 siRNA (500 nM) was combined with DOX (3 µM) under hypoxia. Dendrimer micelles composed of polyamidoamine-g-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(methoxy (polyethylene glycol)) (PMMA-PEG-DOPE) and PEG-DOPE were developed to codeliver MDR1 siRNA and DOX to the tumor, in which siRNA was loaded in PAMAM moieties and hydrophobic DOX were encapsulated into the lipid core.85 Dendrimer micelles loaded with both DOX and MDR1 siRNA led to significantly higher cytotoxicity on DOX-resistant A2780/DOX cell lines compared to micelles containing DOX only and free DOX due to MDR1 siRNA induced downregulation of the P-gp efflux pump and thus increased amount of DOX inside cells.
In addition, CD44 as a cell-surface glycoprotein involves in cell interactions, cell adhesion, and migration.86 It has been reported that CD44 expression is related to the expression of P-gp, resistance to chemotherapy, and progression of metastases.87–89 Therefore, Shah et al established a polypropylenimine (PPI) dendrimer-based drug delivery system consisting of the mixture of PTX conjugated PPI (PTX-PPI) and CD44 targeted siRNA-PPI complexes (CD44 siRNA-PPI), which simultaneously deliver PTX and CD44 siRNA to ovarian cancer cells to induce cell death and prevent metastases.90 In this system, PPI dendrimers were decorated by luteinizing hormone–releasing hormone (LHRH) peptide for active targeting LHRH receptor that is overexpressed in ovarian cancer cells.91,92 The results show that the delivered siRNA targeting to CD44 effectively suppressed CD44 mRNA and MDR1 mRNA, primarily responsible for multidrug resistance in ovarian cancer cells. Such inhibition significantly improved the potent of PTX to induce cell death by apoptosis and, as a result, the PTX-PPI and CD44 siRNA-PPI exhibited significantly enhanced antitumor efficiency in vitro and in vivo while preventing adverse side effects on healthy organs. For instance, within the 28-day study period, when treated by monotherapy, ie, PTX-PPI or CD44 siRNA-PPI alone, the tumor derived from ovarian cancer cells isolated from malignant ascites from patients with advanced ovarian carcinoma continued growth whereas the combination of PTX and CD4 siRNA delivered by the nanocarrier led to the almost complete tumor shrinkage.
In addition to MDR1 gene encoding P-gp, other genes encoding drug efflux pump-related proteins such as focal adhesion kinase (FAK) can also regulate the drug efflux pump, thus serving as an important defense mechanism for ovarian cancer resistance (Figure 3).93–95 It is reported that down-regulation of FAK can restore the sensitivity of tumor cells to chemotherapy.96 Therefore, Byeon et al designed hyaluronic acid modified PLGA nanoparticles to encapsulate PTX and siRNA inhibiting FAK expression (FAK siRNA), in which hyaluronic acid can actively target CD44 overexpressed ovarian cancer cells.97 When compared with those without FAK siRNA loading, the nanoparticles loaded with both PTX and FAK siRNA induced a significant increase in cell death and apoptosis in multidrug-resistant HeyA8-MDR and PTX-resistant SKOV3-TR cells. In vivo study shows that the combined delivery of PTX and FAK siRNA resulted in significant inhibition of tumor growth not only in drug-resistant HeyA8-MDR and SKOV3-TR xenograft mouse models but also in a drug-resistant, patient-derived xenograft model.
Codelivery of siRNA and Anticancer Drug for Overcoming Nonpump-Related Resistance
Except for the above-mentioned efflux pump-based resistance, non-efflux pump resistance also play an important role in drug resistance.98 This resistance is involved in various proteins like MCL-1, Bcl-2, Toll-like receptor 4, surviving and vascular endothelial growth factor (VEGF).99 Instead of affecting the accumulation of drugs in tumor cells, nonpump resistance protects tumor cells from anticancer drugs by altering the checkpoints in the cell cycle, activation of detoxifying systems, the escape from drug-induced apoptosis and impaired DNA repair.99 Among the aforementioned mechanisms, the proteins associated with the antiapoptotic defense are extensively studied to overcome cancer resistance. For instance, Bcl-2 protein encoded by the Bcl-2 gene is a well-known anti-apoptotic protein that is an activation of cellular anti-apoptotic defense to prevent cell death.99 Overexpression of Bcl-2 protein in MDR cancer cells can cause the formation of a large number of Bcl-2/Bcl-2 homodimer on the surface of mitochondria, which inhibits the formation of apoptosomes in the external membrane of mitochondria and thus results in bypassing the regular apoptotic pathway in tumor cells.100,101 Therefore, Chen et al designed mesoporous silica nanoparticles to coload DOX and siRNA targeting the Bcl-2 gene (Bcl-2 siRNA) for overcoming drug resistance in ovarian cancer.99 The result showed that due to the significant suppression of Bcl-2 mRNA by Bcl-2 siRNA, simultaneously delivered Bcl-2 siRNA and DOX by the silica nanoparticles resulted in a 64- and 132-fold increase in cytotoxicity of DOX on multidrug-resistance A2780/DOX human ovarian cancer cells as compared to that of mono-DOX loaded nanoparticles and free DOX alone (IC50: 0.017 μM vs 1.07 μM and 2.25 μM), respectively. In addition, Zou et al developed a folate-targeted nanocarrier based on PEG–PEI–PCL triblock copolymer for codelivering DOX and Bcl-2 siRNA into folate receptor overexpressed SKOV3 ovarian cancer cells.102 Upon the codelivery of Bcl-2 siRNA and DOX using the folate-targeted nanocarrier, DOX-induced apoptosis in the SKOV3 cells significantly enhanced through downregulating the antiapoptotic protein Bcl-2, while simultaneously upregulating the proapoptotic protein Bax. As a result, siRNA and DOX codelivered by the folate-targeted PEG–PEI–PCL nanocarrier exhibited higher cytotoxicity on the SKOV3 cells than a single therapeutic agent loaded targeting nanoparticles or dual-agent loaded nontargeting nanoparticles.
Another example of acting on the anti-apoptotic pathway is DJ-1 protein that can bind to p53 tumor suppressor protein, thereby impeding the cell cycle arrest function of p53 and facilitating the enhanced expression of various anti-apoptotic proteins.103,104 Given the role of the DJ-1 protein, Taratula group constructed the PPI dendrimer-based nanoplatform to deliver DJ-1 targeted siRNA (DJ-1 siRNA), which is combined with a low dose of cisplatin for the treatment of platinum-resistant ovarian cancer.105 It is shown that the combinatorial approach is superior to both siRNA-mediated DJ-1 knockdown and cisplatin treatment alone in therapeutic response, especially in the platinum-resistant A2780/cisplatin cancer cells characterized by the highest basal level of DJ-1 protein. In addition to being an anti-apoptotic agent in cancer cells, it has been reported that DJ-1 is associated with invasion and metastasis by modulating different oncogenic pathways.103,104 Therefore, in the follow-up study, Schumann et al demonstrated the synergistic effects of combinatorial DJ-1-siRNA delivered by PPI nanoparticles and cisplatin at low dose on the treatment of metastatic ovarian cancer.105 For example, compared to incomplete remission of cancer in mice treated by DJ-1 siRNA or cisplatin monotherapy, complete eradication of ovarian metastatic tumors with no cancer recurrence during the 35-week trial was observed in mice with metastatic ovarian cancer treated by sequentially intraperitoneal injection of DJ-1 siRNA loaded nanoparticles and low-dose cisplatin.
In addition to the anti-apoptotic defense mechanism, the development of nonpump-related MDR can also be due to ineffective induction of cell death by, eg, affecting the cell cycle.76 For instance, kinesin spindle protein (KSP), a member of kinesin superfamily, facilitates bipolar spindle assembly during an early stage of mitosis and eventually leads to centrosome separation.106 KSP inhibitors as therapeutic agents can cause cell cycle arrest during mitosis by inhibiting KSP function, ultimately leading to cell death. However, a different endogenous kinesin Kif15 is capable of driving bipolar spindle assembly/maintenance in the absence of KSP activity, which leads to resistance against KSP inhibitors.106–108 It has been reported that Kif15 behavior is associated with dynamic microtubules, ie, Kif15 motility is suppressed by the reduction of microtubule growth with a microtubule-directed antimitotic agent like PTX.109–111 Therefore, Lee et al paired KSP silencing siRNA (KSP siRNA) as KSP inhibitor with PTX as Kif15 inhibitor by PEGylated cationic liposomes composed of cholesterol, DOPE, and DSPE-PEG to overcome KSP resistance and enhance the therapeutic effects of PTX in drug-resistant ovarian cancer.112 The results show that combined KSP siRNA and PTX in liposomes completely blocked bipolar spindle assembly in mitosis through simultaneous downregulation of KSP and Kif15. This dual action led to the synergistic therapeutic efficacy of combined therapy in vitro and in vivo. This is supported by the fact that siKSP/PTX coloaded liposomes inhibited cell proliferation more potently, induced cell death more prominently on drug-resistant HeyA8-MDR cells and suppressed tumor growth more efficiently in the drug-resistant in vivo models, including drug-resistant cell line and patient-derived xenografts when compared to mono PTX or KSP siRNA loaded liposomes and free PTX alone.
Reduced expression of the β5 subunit of the proteasome and/or enhanced expression of the autophagy regulatory protein P62/SQSTM1 (P62) protein have been demonstrated to contribute to cancer drug resistance in ovarian cancer cells.113–115 To reverse cisplatin resistance, Babu et al developed multifunctional nanoparticles based on chitosan coated poly (D, L -lactide) (PLA) to codeliver cisplatin, siRNA for P62/SQSTM1 gene silencing (P62 siRNA) and a plasmid DNA for restoring β5 expression (pβ5).116 In the nanoparticles, cisplatin was loaded in the inner core formed by PLA while the negatively charged P62 siRNA and pβ5 were located at the outer layer of the cationic chitosan polymer through electrostatic interactions, as indicated by Figure 2C (the third nanoparticles). Codelivered P62 siRNA and pβ5 by the nanoparticles caused P62 knockdown and restored expression of β5 in cisplatin-resistant 2008/C13 ovarian cancer cells, resulting in restoration of sensitivity to cisplatin and enhanced cell killing as evidenced by a marked decrease of IC50 value of cisplatin on 2008/C13 cells compared to single P62 siRNA or pβ5 and cisplatin coloaded nanoparticles, single cisplatin loaded nanoparticles and free cisplatin.
Codelivery of Multiple siRNAs and Anticancer Drug for Overcoming Both Pump and Nonpump-Related Resistance
Targeting a single factor responsible for drug resistance is often insufficient in reversal MDR due to the simultaneous activation of both pump and non-pump resistances in heterogeneous tumors. Therefore, He et al developed a nanoscale coordination polymer system consisting of cisplatin prodrug, 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), cholesterol and DSPE-PEG for codelivery of cisplatin and pooled siRNAs targeting three MDR genes including anti-apoptotic proteins (Bcl-2 and survivin) and P-gp to overcome drug resistance of ovarian cancer.117 The resulting siRNAs and cisplatin loaded particles promoted cellular uptake of cisplatin in cisplatin-resistant ovarian cancer cells, which is attributed to the decreased nanoparticle/drug efflux pump by downregulating the P-gp expression. Codelivery of cisplatin and pooled siRNAs significantly enhanced the chemotherapeutic efficacy of cisplatin as indicated by the drastically decreased IC50 values of cisplatin in different types of cisplatin-resistant ovarian cancer cell lines (ES2, OVCAR-3, SKOV3, and A2780/cisplatin cells) compared to either free cisplatin or cisplatin coordinated particles. For instance, in ES2 and SKOV3, the IC50 of cisplatin induced by siRNAs and cisplatin loaded nanoparticles showed a 102- and 140-fold decrease compared to single cisplatin coordinated particles. In addition, the codelivery of cisplatin and pooled siRNAs drastically enhanced the in vivo chemotherapeutic effects in cisplatin-resistant SKOV3 ovarian cancer mouse model while no side effects such as immunogenic response and nephrotoxicity were observed. The drastically enhanced anticancer efficacy of the combination treatment benefits from the synergy between downregulation of MDR genes by pooled siRNAs and chemotherapeutic effects of cisplatin.
Codelivery of Viral Gene and Anticancer Drug
Viral-mediated gene therapy (virotherapy) is an approach to cancer treatment, in which the replicating virus itself is the anticancer agent and exploits the lytic property of virus replication to kill tumor cells. A variety of viral species have been adopted as virotherapy agents, such as Adenoviruses, retroviruses, lentiviruses, and herpes simplex viruses (Figure 4).118–121 It has been reported that virotherapy combined with chemotherapy is a potential therapeutic modality for the improvement of transfection efficiency and therapeutic efficacy for ovarian cancer.122,123 Zhang et al demonstrated a synergistic effect in the combination of a novel surviving promoter-based conditionally replicating adenovirus and cisplatin in the enhancement of the antitumor efficacy in ovarian cancer while Franke et al reported that tobacco mosaic virus delivered cisplatin restored efficacy in platinum-resistant ovarian cancer cells.122,124 In addition, It is reported that some microtubule-disrupting drugs, such as PTX, can improve gene transfection efficiency at a non-cytotoxic dosage.125–129 Therefore, Long et al reported that the therapeutic gene targeting vesicular stomatitis virus matrix protein (VSVMP), ie, VSVMP gene, was codelivered with low-dosage of PTX by nanoparticles composed of DOTAP and PEG-PLA for combinational treatment of ovarian cancer.116 Vesicular stomatitis virus (VSV) is an oncolytic virus that preferentially replicates in various tumor cells and has antitumor effects.118,130–132 VSVMP as a key structural component of the virion is the main contributor to VSV-induced antitumor activities, which has exhibited antitumor effects in multiple tumor-bearing models.133–136 As expected, VSVMP gene and low-dosage PTX coloaded in nanoparticles exhibited synergistic anticancer effects in SKOV3 ovarian cancer treatment.137 The results show that in the codelivery system, the PTX significantly improved the gene delivery efficiency of VSVMP gene, leading to enhanced VSVMP expression and VSVMP-induced anti-proliferation and apoptosis in SKOV3 cells. Intraperitoneal injection of nanoparticles coloaded with VSVMP and PTX more effectively inhibited the intraperitoneal metastasis of SKOV3 ovarian cancer, as compared to that delivered either VSVMP or PTX only.
 |
Figure 4 Representative images of the viral vectors with their features including genome sizes and advantages for virotherapy. |
Combination of Other Therapies
Photodynamic therapy (PDT) is an emerging non-invasive treatment modality that is based on a photochemical reaction between a light-activable molecule, namely photosensitizer, and light to generate ROS to kill cancer cells (Figure 5).138–141 Current research reveals that the combination of PDT and chemotherapy is a feasible and effective strategy for the improvement of the therapeutic outcomes of ovarian cancer therapy due to the synergistic effect as presented in Figure 5.142 For instance, a reduction-sensitive drug codelivery system based on polymeric prodrug poly(ethylene glycol)-b-poly(5-methyl-5-propargyl-1,3 dioxan-2-one)-g-paclitaxel (PEG-b-PMPMC-g-PTX) was constructed to coload a near-infrared (NIR) fluorophore, 4.8-bis((2-ethylhexyl)oxy)benzo[1,2-b-4,5-b’]dithiophene-1,1,5,5-tetraoxide (BDTO) derivative (namely, DEB) and tariquidar to treat drug-resistant ovarian cancer.143 In this codelivery system, DEB functions as a photosensitizer for image-guided PDT, while PTX and tariquidar act as a cytotoxic agent and a drug-resistant (P-gp) inhibitor, respectively. In vitro study shows that DEB and tariquidar loaded in PEG-b-PMPMC-g-PTX prodrug formed micelles substantially increased the accumulation of PTX in drug-resistant SKOV3/MDR ovarian cancer cells due to the suppression of P-gp by tariquidar and displayed a prominent synergistic enhancement effect of PDT and chemotherapy in terms of anti-proliferation of SKOV3 and SKOV3/MDR cells. In the SKOV3/MDR tumor-bearing mouse model, the multidrug resistance of tumors was successfully reversed and the tumor growth was significantly inhibited by combining PDT and chemotherapy. Moreover, Pan et al reported nanoparticles based on poly-ε-caprolactone using bovine albumin as a stabilizer to coload IR780, a hydrophobic NIR photosensitizer, and PTX for combinational PDT and chemotherapy.144 IR780 and PTX coloaded nanoparticles demonstrated an excellent synergistic therapeutic effect against PTX-resistant SKOV3-TR30 cells in vitro, which is evident by a substantial decrease in the IC50 value (19.8 nM) compared to that of the corresponding monotherapy (IC50 of chemotherapy only and PDT only was 1285 and 158 nM, respectively). In drug-resistant SKOV3-TR30 tumor-bearing mice treated simultaneously with NIR light-induced PDT and chemotherapy (ie, IR780-PTX loaded nanoparticles + light group), tumors were completely eradicated without recurrence in the entire experimental period and the survival of mice was greatly prolonged to over 65 days. In contrast, after treatment with one of the corresponding two therapies, the tumors were destroyed at the beginning but followed by regrowth and tumor-bearing mice had a medium survival rate of 35–37 days.
In addition to the advantages for MDR ovarian cancer, cooperative therapy of chemotherapy and PDT also provides a great opportunity for metastasis, which is one of the major obstacles to the successful treatment of ovarian cancer.145 Many small molecule inhibitors, such as KBU2046, BENC-511, MPT0G211, DSHN, BDP5290 and BMS-777607, have been reported for effectively inhibiting the metastasis of primary tumors.146,147 However, no cytotoxic effect of these inhibitors, responsible for continuous proliferation of tumor cells in situ, is a defect in the treatment of tumors. Cooperative therapy through the introduction of PDT provides a possibility to compensate for their congenital defects. Dai et al used DSPE-PEG-based nanoparticles to codeliver small molecule inhibitor (KBU2046) and a photosensitizer, BDTO derivative (namely TB), in which the former drug has the effect of anti-metastasis of the primary tumors and the latter photosensitizer can suppress the growth of the primary tumors under irradiation.148 In vitro studies show that KBU2046 and TB coloaded nanoparticles exhibited the anti-migration and anti-proliferation for SKOV3 cells under irradiation. In line with this, in vivo study shows that cooperation therapy achieved anti-tumor growth and anti-metastasis in the subcutaneous SKOV3 tumor model and effectively suppressed the recurrence and metastasis of tumor after surgery in an orthotopic ovarian tumor model.
In addition to the combination of PDT and chemotherapy, chemotherapy associated with ultrasound or tumor treating fields has also been demonstrated to enhance therapeutic outcomes of ovarian cancer.149,150 For example, Baghbaniet al demonstrate that DOX and curcumin coloaded alginate/perfluorohexane nanodroplets in combination with ultrasound irradiation led to significantly increased drug internalization in a very short time and cytotoxicity on DOX-resistant A2780/DOX ovarian cancer cells compared to the non-sonicated group. In vivo study shows that DOX and curcumin coloaded nanodroplets resulted in complete eradication of DOX-resistant A2780/DOX ovarian tumors while tumor growth was only inhibited to some extent in the non-ultrasound irradiation group.149
Except for the combination of chemotherapy and PDT, PDT is also reported to have synergistic effects when in combination with gene therapy (Figure 5). For instance, Clements et al exploited Generation 4 PPI dendrimer-based nanoplatforms for the sequential delivery of a NIR photosensitizer, phthalocyanine, and siRNA targeting DJ-1 gene (as a DJ-1 gene suppressor), a key player in protecting cancer cells from ROS mediated apoptosis.104,151,152 It is validated that compared to that provided by PDT alone, the anticancer activity and intracellular ROS levels induced by phthalocyanine mediated PDT in combination with siRNA delivery dramatically increased in DOX-resistant A2780/DOX human ovarian carcinoma cells characterized by higher basal levels of DJ-1 due to siRNA mediated silencing of DJ-1 expression. In A2780/DOX ovarian cancer mice model, after the treatment with PDT alone, tumors were almost eliminated but followed by relapse while tumors were completely eradicated and, importantly, no evidence of cancer recurrence was exhibited when treated with a single dose of a combinatorial therapy (PPI-siRNA followed by PPI- phthalocyanine).
Conclusion and Perspective
Combined different therapy modality by nanomedicine has advantages in terms of improving drug water-solubility, controlling drug ratios, ensuring the same drug disposition behavior at the tumor site, decreasing non-specific toxicities and increasing drug co-accumulation at tumor sites, which provides an unprecedented opportunity for the treatment of ovarian cancer. Various nanocarriers have been developed for different combination purposes and nanoparticle-based combinations indeed have been demonstrated the outperformed therapeutic outcomes in ovarian cancer treatment compared to drug alone or free drug combination. Codelivery of chemodrugs with other small molecule drugs by nanoparticles solves the problems of conventional combinations such as low-water solubility, non-specific distribution, and different pharmacokinetics of combined drugs. As a result, the synergistic interactions of the different combinations are dramatically enhanced, leading to improved antitumor effects, increased tumor targeting and reversed MDR. Combination delivery of chemodrug and siRNA using nanoparticles minimizes the accumulation of the drug/siRNA in the normal tissues, achieves temporal co-deposition of the combined agents in tumors and efficient delivery of siRNA into cells. Combined with the fact that gene therapy can tune the expression of proteins (eg, P-gp, Bcl-2) by manipulating the gene content of tumor cells, the drug/gene combination leads to the excellent performance of anticancer drug(s) in overcoming MDR and enhancing therapeutic response. In addition, other combinations like PDT in combination with chemotherapy or gene therapy also exhibit superior therapeutic outcomes compared to single therapy alone.
Although nanoparticles as codelivery systems are extensively used for the combination treatment of ovarian cancer, they are still challenged by their instability in circulation, which often leads to premature drug release, diminished ability to selectively reach target sites, and suboptimal therapeutic efficacy. In addition, concerns regarding drug loading capacity, flexibility in changing the drug ratio, release timing of different therapeutic agents (mainly for chemo/gene combination) and biosafety of the delivery materials also remain in the field of nanoparticle-based combination therapies. Therefore, future work should concentrate on optimizing carrier systems or implementing innovative delivery strategies such as carrier-free drug delivery systems to address these challenges and thus generate a maximum effect of the combination therapy in the preclinical trials.
Despite the superior advantages of nanomedicine in the combination treatment for ovarian cancer compared to traditional therapy, the number of nanomedicines used clinically is rare, let alone as first-line treatment options. The clinical translation failure of many nanomedicines is partially due to a lack of understanding of the different physiology and pathology between animal models and humans.153 Except for the differences across species, heterogeneity amongst patients is another contributor to hindering the clinical translation of nanomedicines. Therefore, future research can explore how the gap between animals and humans and how heterogeneity resulting from, eg, the growth, structure and physiology of pathological tissue amongst patients influence the behavior, distribution, functionality and efficacy of nanomedicines in the human body. This research will provide an important reference for the rational design of nanomedicines. On the other hand, the current nanoparticle design mainly focuses on developing delivery systems with a one-size-fits-all solution. Considering the heterogeneous barriers, future research also should focus on advanced intelligent nanoparticle design, which can utilize tailored designs for precision applications while improving therapeutic effects in conventional delivery applications, ultimately enhancing the overall therapeutic outcomes in patients.
Acknowledgments
This work is supported by Academic Promotion Program of Shandong First Medical University (No 2019LJ003) and the Natural Science Foundation of Shandong Province (ZR202103020117).
Disclosure
The authors declare no conflict of interest.
References
1. Zhang Y, Luo G, Li M, et al. Global patterns and trends in ovarian cancer incidence: age, period and birth cohort analysis. BMC Cancer. 2019;19(1):984. doi:10.1186/s12885-019-6139-6
2. Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81(1):17–38. doi:10.1007/s00280-017-3501-8
3. Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi:10.3322/caac.21456
4. Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release. 2014;194:238–256. doi:10.1016/j.jconrel.2014.09.001
5. Zhu L, Guo Y, Qian Q, et al. Carrier-free delivery of precise drug-chemogene conjugates for synergistic treatment of drug-resistant cancer. Angew Chem Int Ed Engl. 2020;59(41):17944–17950. doi:10.1002/anie.202006895
6. Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New England J Med. 2006;354(1):34–43. doi:10.1056/NEJMoa052985
7. Levasseur LM, Greco WR, Rustum YM, Slocum HK. Combined action of paclitaxel and cisplatin against wild type and resistant human ovarian carcinoma cells. Cancer Chemother Pharmacol. 1997;40(6):495–505. doi:10.1007/s002800050693
8. Gilmore KA, Lampley MW, Boyer C, Harth E. Matrices for combined delivery of proteins and synthetic molecules. Adv Drug Deliv Rev. 2016;98:77–85. doi:10.1016/j.addr.2015.11.018
9. Perez-Fidalgo JA, Cortés A, Guerra E, et al. Olaparib in combination with pegylated liposomal doxorubicin for platinum-resistant ovarian cancer regardless of BRCA status: a GEICO Phase II trial (ROLANDO study). ESMO Open. 2021;6(4):1-9. doi:10.1016/j.esmoop.2021.100212
10. Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017;35(3):222–229. doi:10.1038/nbt.3802
11. Ivanov AI, Christodoulou J, Parkinson JA, et al. Cisplatin binding sites on human albumin. J Biol Chem. 1998;273(24):14721–14730. doi:10.1074/jbc.273.24.14721
12. Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235(1–2):179–192. doi:10.1016/S0378-5173(01)00986-3
13. Galic VL, Wright JD, Lewin SN, Herzog TJ. Paclitaxel poliglumex for ovarian cancer. Expert Opin Investig Drugs. 2011;20(6):813–821. doi:10.1517/13543784.2011.576666
14. Yang X, Iyer AK, Singh A, et al. MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci Rep. 2015;5:8509. doi:10.1038/srep08509
15. Aghamiri S, Mehrjardi KF, Shabani S, et al. Nanoparticle-siRNA: a potential strategy for ovarian cancer therapy? Nanomedicine. 2019;14(15):2083–2100. doi:10.2217/nnm-2018-0379
16. Yang C, Xia BR, Zhang ZC, et al. Immunotherapy for ovarian cancer: adjuvant, combination, and neoadjuvant. Front Immunol. 2020;11:577869. doi:10.3389/fimmu.2020.577869
17. Bozzer S, Dal Bo M, Grimaldi MC, Toffoli G, Macor P. Nanocarriers as a delivery platform for anticancer treatment: biological limits and perspectives in B-cell malignancies. Pharmaceutics. 2022;14 (9) :1965. doi:10.3390/pharmaceutics14091965
18. Liu Y, Fens M, Lou B, et al. π-π-stacked poly(ɛ-caprolactone)-b-poly(ethylene glycol) micelles loaded with a photosensitizer for photodynamic therapy. Pharmaceutics. 2020;12(4):338. doi:10.3390/pharmaceutics12040338
19. Tsouris V, Joo MK, Kim SH, Kwon IC, Won YY. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnol Adv. 2014;32(5):1037–1050. doi:10.1016/j.biotechadv.2014.05.006
20. Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10(17):7921–7924. doi:10.7150/thno.49577
21. Maeda H. Polymer therapeutics and the EPR effect. J Drug Target. 2017;25(9–10):781–785. doi:10.1080/1061186X.2017.1365878
22. Rai S, Singh N, Bhattacharya S. Concepts on smart nano-based drug delivery system. Recent Pat Nanotechnol. 2022;16(1):67–89. doi:10.2174/1872210515666210120113738
23. Li Y, Gao Y, Zhang X, Guo H, Gao H. Nanoparticles in precision medicine for ovarian cancer: from chemotherapy to immunotherapy. Int J Pharm. 2020;591:119986. doi:10.1016/j.ijpharm.2020.119986
24. Wang X, Xiong T, Cui M, et al. A novel targeted co-delivery nanosystem for enhanced ovarian cancer treatment via multidrug resistance reversion and mTOR-mediated signaling pathway. J Nanobiotechnol. 2021;19(1):444. doi:10.1186/s12951-021-01139-1
25. Barani M, Bilal M, Sabir F, Rahdar A, Kyzas GZ. Nanotechnology in ovarian cancer: diagnosis and treatment. Life Sci. 2021;266:118914. doi:10.1016/j.lfs.2020.118914
26. Das T, Anand U, Uttpal SK et al. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist Updat. 2021;55:100754. doi:10.1016/j.drup.2021.100754
27. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665–667. doi:10.1038/277665a0
28. Hu Y, Ran M, Wang B, et al. Co-delivery of docetaxel and curcumin via nanomicelles for enhancing anti-ovarian cancer treatment. Int J Nanomed. 2020;15:9703–9715. doi:10.2147/IJN.S274083
29. Jain A, Jain SK. Multipronged, strategic delivery of paclitaxel-topotecan using engineered liposomes to ovarian cancer. Drug Dev Ind Pharm. 2016;42(1):136–149. doi:10.3109/03639045.2015.1036066
30. Chelvi TP, Ralhan R. Hyperthermia potentiates antitumor effect of thermosensitive-liposome-encapsulated melphalan and radiation in murine melanoma. Tumour Biol. 1997;18(4):250–260. doi:10.1159/000218038
31. Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi:10.1146/annurev.med.53.082901.103929
32. Zou L, Wang D, Hu Y, et al. Drug resistance reversal in ovarian cancer cells of paclitaxel and borneol combination therapy mediated by PEG-PAMAM nanoparticles. Oncotarget. 2017;8(36):60453–60468. doi:10.18632/oncotarget.19728
33. He H, Shen Q, Li J. Effects of borneol on the intestinal transport and absorption of two P-glycoprotein substrates in rats. Arch Pharm Res. 2011;34(7):1161–1170. doi:10.1007/s12272-011-0714-y
34. Zhao MD, Li JQ, Chen FY, et al. Co-delivery of curcumin and paclitaxel by “core-shell” targeting amphiphilic copolymer to reverse resistance in the treatment of ovarian cancer. Int J Nanomed. 2019;14:9453–9467. doi:10.2147/IJN.S224579
35. Valentine SP, Le Nedelec MJ, Menzies AR, et al. Curcumin modulates drug metabolizing enzymes in the female Swiss webster mouse. Life Sci. 2006;78(20):2391–2398. doi:10.1016/j.lfs.2005.09.017
36. Wang J, He H, Xu X, et al. Far-red light-mediated programmable anti-cancer gene delivery in cooperation with photodynamic therapy. Biomaterials. 2018;171:72–82. doi:10.1016/j.biomaterials.2018.04.020
37. Abouzeid AH, Patel NR, Torchilin VP. Polyethylene glycol-phosphatidylethanolamine (PEG-PE)/vitamin E micelles for co-delivery of paclitaxel and curcumin to overcome multi-drug resistance in ovarian cancer. Int J Pharm. 2014;464(1–2):178–184. doi:10.1016/j.ijpharm.2014.01.009
38. Gawde KA, Sau S, Tatiparti K, et al. Paclitaxel and di-fluorinated curcumin loaded in albumin nanoparticles for targeted synergistic combination therapy of ovarian and cervical cancers. Colloid Surface B. 2018;167:8–19. doi:10.1016/j.colsurfb.2018.03.046
39. Romanini A, Tanganelli L, Carnino F, et al. First-line chemotherapy with epidoxorubicin, paclitaxel, and carboplatin for the treatment of advanced epithelial ovarian cancer patients. Gynecol Oncol. 2003;89(3):354–359. doi:10.1016/S0090-8258(03)00128-8
40. Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33(2 Suppl 6):S3–S11. doi:10.1053/j.seminoncol.2006.03.011
41. Mensah LB, Morton SW, Li J, et al. Layer-by-layer nanoparticles for novel delivery of cisplatin and PARP inhibitors for platinum-based drug resistance therapy in ovarian cancer. Bioeng Transl Med. 2019;4(2):e10131. doi:10.1002/btm2.10131
42. Shen W, Chen X, Luan J, et al. Sustained codelivery of cisplatin and paclitaxel via an injectable prodrug hydrogel for ovarian cancer treatment. ACS Appl Mater Interfaces. 2017;9(46):40031–40046. doi:10.1021/acsami.7b11998
43. Wan X, Beaudoin JJ, Vinod N, et al. Co-delivery of paclitaxel and cisplatin in poly(2-oxazoline) polymeric micelles: implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials. 2019;192:1–14. doi:10.1016/j.biomaterials.2018.10.032
44. Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Bio. 2006;7(7):517–528. doi:10.1038/nrm1963
45. Zhang M, Hagan CTT, Min Y, et al. Nanoparticle co-delivery of wortmannin and cisplatin synergistically enhances chemoradiotherapy and reverses platinum resistance in ovarian cancer models. Biomaterials. 2018;169:1–10. doi:10.1016/j.biomaterials.2018.03.055
46. Hashimoto M, Rao S, Tokuno O, et al. DNA-PK: the major target for wortmannin-mediated radiosensitization by the inhibition of DSB repair via NHEJ pathway. J Radiat Res. 2003;44(2):151–159. doi:10.1269/jrr.44.151
47. Okayasu R, Suetomi K, Ullrich RL. Wortmannin inhibits repair of DNA double-strand breaks in irradiated normal human cells. Radiat Res. 1998;149(5):440–445. doi:10.2307/3579783
48. Hung SW, Marrache S, Cummins S, et al. Defective hCNT1 transport contributes to gemcitabine chemoresistance in ovarian cancer subtypes: overcoming transport defects using a nanoparticle approach. Cancer Lett. 2015;359(2):233–240. doi:10.1016/j.canlet.2015.01.017
49. Kawaguchi H, Terai Y, Tanabe A, et al. Gemcitabine as a molecular targeting agent that blocks the Akt cascade in platinum-resistant ovarian cancer. J Ovarian Res. 2014;7:38. doi:10.1186/1757-2215-7-38
50. Van Moorsel CJ, Smid K, Voorn DA, et al. Effect of gemcitabine and cis-platinum combinations on ribonucleotide and deoxyribonucleotide pools in ovarian cancer cell lines. Int J Oncol. 2003;22(1):201–207. doi:10.3892/ijo.22.1.201
51. Moufarij MA, Phillips DR, Cullinane C. Gemcitabine potentiates cisplatin cytotoxicity and inhibits repair of cisplatin-DNA damage in ovarian cancer cell lines. Mol Pharmacol. 2003;63(4):862–869. doi:10.1124/mol.63.4.862
52. van Moorsel CJ, Pinedo HM, Veerman G, et al. Mechanisms of synergism between cisplatin and gemcitabine in ovarian and non-small-cell lung cancer cell lines. Br J Cancer. 1999;80(7):981–990. doi:10.1038/sj.bjc.6690452
53. Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ. Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res. 1996;2(3):521–530.
54. Yahuafai J, Asai T, Oku N, Siripong P. Anticancer efficacy of the combination of berberine and PEGylated liposomal doxorubicin in meth a sarcoma-bearing mice. Biol Pharm Bull. 2018;41(7):1103–1106. doi:10.1248/bpb.b17-00989
55. Meredith AM, Dass CR. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J Pharm Pharmacol. 2016;68(6):729–741. doi:10.1111/jphp.12539
56. Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. doi:10.1124/pr.56.2.6
57. Chen C, Lu L, Yan S, et al. Autophagy and doxorubicin resistance in cancer. Anti-Cancer Drug. 2018;29(1):1–9. doi:10.1097/CAD.0000000000000572
58. Carlson LJ, Cote B, Alani AW, Rao DA. Polymeric micellar co-delivery of resveratrol and curcumin to mitigate in vitro doxorubicin-induced cardiotoxicity. J Pharm Sci. 2014;103(8):2315–2322. doi:10.1002/jps.24042
59. Fatease AA, Shah V, Nguyen DX, et al. Chemosensitization and mitigation of adriamycin-induced cardiotoxicity using combinational polymeric micelles for co-delivery of quercetin/resveratrol and resveratrol/curcumin in ovarian cancer. Nanomed-nanotechnol. 2019;19:39–48. doi:10.1016/j.nano.2019.03.011
60. Vinod BS, Maliekal TT, Anto RJ. Phytochemicals as chemosensitizers: from molecular mechanism to clinical significance. Antioxid Redox Sign. 2013;18(11):1307–1348. doi:10.1089/ars.2012.4573
61. Shao M, Zhu W, Lv X, et al. Encapsulation of chloroquine and doxorubicin by MPEG-PLA to enhance anticancer effects by lysosomes inhibition in ovarian cancer. Int J Nanomed. 2018;13:8231–8245. doi:10.2147/IJN.S174300
62. Takahashi S, Takekuma M, Tamura K, et al. A Phase I study of combined trabectedin and pegylated liposomal doxorubicin therapy for advanced relapsed ovarian cancer. Int J Clin Oncol. 2021;26(10):1977–1985. doi:10.1007/s10147-021-01973-1
63. Zhang G, Zhu Y, Wang Y, et al. pH/redox sensitive nanoparticles with platinum(iv) prodrugs and doxorubicin enhance chemotherapy in ovarian cancer. RSC Adv. 2019;9(36):20513–20517. doi:10.1039/c9ra04034j
64. Zheng W, Li M, Lin Y, Zhan X. Encapsulation of verapamil and doxorubicin by MPEG-PLA to reverse drug resistance in ovarian cancer. Biomed Pharmacother. 2018;108:565–573. doi:10.1016/j.biopha.2018.09.039
65. Hami Z, Rezayat SM, Gilani K, Amini M, Ghazi-Khansari M. In-vitro cytotoxicity and combination effects of the docetaxel-conjugated and doxorubicin-conjugated poly(lactic acid)-poly(ethylene glycol)-folate-based polymeric micelles in human ovarian cancer cells. J Pharm Pharmacol. 2017;69(2):151–160. doi:10.1111/jphp.12675
66. Han NN, Li X, Tao L, Zhou Q. Doxorubicin and rhein loaded nanomicelles attenuates multidrug resistance in human ovarian cancer. Biochem Biophys Res Commun. 2018;498(1):178–185. doi:10.1016/j.bbrc.2018.01.042
67. Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115(2):155–162. doi:10.1159/000265166
68. Vittorio O, Curcio M, Cojoc M, et al. Polyphenols delivery by polymeric materials: challenges in cancer treatment. Drug Deliv. 2017;24(1):162–180. doi:10.1080/10717544.2016.1236846
69. Cote B, Carlson LJ, Rao DA, Alani AWG. Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J Control Release. 2015;213:128–133. doi:10.1016/j.jconrel.2015.06.040
70. Zhou L, Duan X, Zeng S, et al. Codelivery of SH-aspirin and curcumin by mPEG-PLGA nanoparticles enhanced antitumor activity by inducing mitochondrial apoptosis. Int J Nanomed. 2015;10:5205–5218. doi:10.2147/IJN.S84326
71. Westfall SD, Nilsson EE, Skinner MK. Role of triptolide as an adjunct chemotherapy for ovarian cancer. Chemotherapy. 2008;54(1):67–76. doi:10.1159/000112419
72. Phillips PA, Dudeja V, McCarroll JA, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67(19):9407–9416. doi:10.1158/0008-5472.CAN-07-1077
73. Peng JR, Qian ZY. Drug delivery systems for overcoming the bioavailability of curcumin: not only the nanoparticle matters. Nanomedicine. 2014;9(6):747–750. doi:10.2217/nnm.14.21
74. Liu L, Xiong X, Shen M, et al. Co-delivery of triptolide and curcumin for ovarian cancer targeting therapy via mPEG-DPPE/CaP nanoparticle. J Biomed Nanotechnol. 2018;14(10):1761–1772. doi:10.1166/jbn.2018.2633
75. Sun W, Shi Q, Zhang H, et al. Advances in the techniques and methodologies of cancer gene therapy. Discov Med. 2019;27(146):45–55.
76. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. doi:10.1038/nrc3599
77. Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. doi:10.1038/nrd1984
78. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi:10.1038/nrc706
79. Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets. 2003;3(1):1–19. doi:10.2174/1568009033333754
80. Haque A, Sait KHW, Alam Q, et al. MDR1 gene polymorphisms and its association with expression as a clinical relevance in terms of response to chemotherapy and prognosis in ovarian cancer. Front Genet. 2020;11:516. doi:10.3389/fgene.2020.00516
81. Yadav S, van Vlerken LE, Little SR, Amiji MM. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother Pharmacol. 2009;63(4):711–722. doi:10.1007/s00280-008-0790-y
82. Wang C, Guan W, Peng J, et al. Gene/paclitaxel co-delivering nanocarriers prepared by framework-induced self-assembly for the inhibition of highly drug-resistant tumors. Acta Biomater. 2020;103:247–258. doi:10.1016/j.actbio.2019.12.015
83. He C, Poon C, Chan C, Yamada SD, Lin W. Nanoscale coordination polymers codeliver chemotherapeutics and siRNAs to eradicate tumors of cisplatin-resistant ovarian cancer. J Am Chem Soc. 2016;138(18):6010–6019. doi:10.1021/jacs.6b02486
84. Joshi U, Filipczak N, Khan MM, Attia SA, Torchilin V. Hypoxia-sensitive micellar nanoparticles for co-delivery of siRNA and chemotherapeutics to overcome multi-drug resistance in tumor cells. Int J Pharm. 2020;590:119915. doi:10.1016/j.ijpharm.2020.119915
85. Pan J, Mendes LP, Yao M, et al. Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance. Eur J Pharm Biopharm. 2019;136:18–28. doi:10.1016/j.ejpb.2019.01.006
86. Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25(11):1679–1691. doi:10.1038/sj.onc.1209377
87. Patil ML, Zhang M, Taratula O, et al. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery: effect of the degree of quaternization and cancer targeting. Biomacromolecules. 2009;10(2):258–266. doi:10.1021/bm8009973
88. Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22(53):8581–8589. doi:10.1038/sj.onc.1207113
89. Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4(1):1–6.
90. Shah V, Taratula O, Garbuzenko OB, et al. Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: an optimal delivery of siRNA and anticancer drug. Clin Cancer Res. 2013;19(22):6193–6204. doi:10.1158/1078-0432.CCR-13-1536
91. Dharap SS, Qiu B, Williams GC, et al. Molecular targeting of drug delivery systems to ovarian cancer by BH3 and LHRH peptides. J Control Release. 2003;91(1–2):61–73. doi:10.1016/S0168-3659(03)00209-8
92. Li X, Taratula O, Taratula O, Schumann C, Minko T. LHRH-targeted drug delivery systems for cancer therapy. Mini Rev Med Chem. 2017;17(3):258–267. doi:10.2174/1389557516666161013111155
93. Kang Y, Hu W, Ivan C, et al. Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J Natl Cancer Inst. 2013;105(19):1485–1495. doi:10.1093/jnci/djt210
94. Haemmerle M, Bottsford-Miller J, Pradeep S, et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J Clin Invest. 2016;126(5):1885–1896. doi:10.1172/JCI85086
95. Freimund AE, Beach JA, Christie EL, Bowtell DDL. Mechanisms of drug resistance in high-grade serous ovarian cancer. Hematol Oncol Clin North Am. 2018;32(6):983–996. doi:10.1016/j.hoc.2018.07.007
96. Wang B, Li S, Meng X, Shang H, Guan Y. Inhibition of mdr1 by G-quadruplex oligonucleotides and reversal of paclitaxel resistance in human ovarian cancer cells. Tumour Biology. 2015;36(8):6433–6443. doi:10.1007/s13277-015-3333-2
97. Byeon Y, Lee JW, Choi WS, et al. CD44-targeting PLGA nanoparticles incorporating paclitaxel and FAK siRNA overcome chemoresistance in epithelial ovarian cancer. Cancer Res. 2018;78(21):6247–6256. doi:10.1158/0008-5472.CAN-17-3871
98. Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21(9):3233. doi:10.3390/ijms21093233
99. Chen AM, Zhang M, Wei D, et al. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5(23):2673–2677. doi:10.1002/smll.200900621
100. Oakes SA, Scorrano L, Opferman JT, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102(1):105–110. doi:10.1073/pnas.0408352102
101. Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19(5):488–496. doi:10.1016/j.coi.2007.05.004
102. Zou S, Cao N, Cheng D, et al. Enhanced apoptosis of ovarian cancer cells via nanocarrier-mediated codelivery of siRNA and doxorubicin. Int J Nanomed. 2012;7:3823–3835. doi:10.2147/IJN.S29328
103. Fan J, Ren H, Jia N, et al. DJ-1 decreases Bax expression through repressing p53 transcriptional activity. J Biol Chem. 2008;283(7):4022–4030. doi:10.1074/jbc.M707176200
104. Cao J, Lou S, Ying M, Yang B. DJ-1 as a human oncogene and potential therapeutic target. Biochem Pharmacol. 2015;93(3):241–250. doi:10.1016/j.bcp.2014.11.012
105. Schumann C, Chan S, Khalimonchuk O, et al. Mechanistic nanotherapeutic approach based on siRNA-mediated DJ-1 protein suppression for platinum-resistant ovarian cancer. Mol Pharm. 2016;13(6):2070–2083. doi:10.1021/acs.molpharmaceut.6b00205
106. Sarli V, Giannis A. Targeting the kinesin spindle protein: basic principles and clinical implications. Clin Cancer Res. 2008;14(23):7583–7587. doi:10.1158/1078-0432.CCR-08-0120
107. Tanenbaum ME, Macurek L, Janssen A, et al. Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr Biol. 2009;19(20):1703–1711. doi:10.1016/j.cub.2009.08.027
108. Olzmann JA, Brown K, Wilkinson KD, et al. Familial Parkinson’s disease-associated L166P mutation disrupts DJ-1 protein folding and function. J Biol Chem. 2004;279(9):8506–8515. doi:10.1074/jbc.M311017200
109. Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10(4):947–959. doi:10.1091/mbc.10.4.947
110. Rath O, Kozielski F. Kinesins and cancer. Nat Rev Cancer. 2012;12(8):527–539. doi:10.1038/nrc3310
111. Myers SM, Collins I. Recent findings and future directions for interpolar mitotic kinesin inhibitors in cancer therapy. Future Med Chem. 2016;8(4):463–489. doi:10.4155/fmc.16.5
112. Lee J, Cho YJ, Lee JW, Ahn HJ. KSP siRNA/paclitaxel-loaded PEGylated cationic liposomes for overcoming resistance to KSP inhibitors: synergistic antitumor effects in drug-resistant ovarian cancer. J Control Release. 2020;321:184–197. doi:10.1016/j.jconrel.2020.02.013
113. Puissant A, Fenouille N, Auberger P. When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res. 2012;2(4):397–413.
114. Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7(3):279–296. doi:10.4161/auto.7.3.14487
115. Yu H, Su J, Xu Y, et al. p62/SQSTM1 involved in cisplatin resistance in human ovarian cancer cells by clearing ubiquitinated proteins. Eur J Cancer. 2011;47(10):1585–1594. doi:10.1016/j.ejca.2011.01.019
116. Babu A, Wang Q, Muralidharan R, et al. Chitosan coated polylactic acid nanoparticle-mediated combinatorial delivery of cisplatin and siRNA/Plasmid DNA chemosensitizes cisplatin-resistant human ovarian cancer cells. Mol Pharm. 2014;11(8):2720–2733. doi:10.1021/mp500259e
117. He C, Liu D, Lin W. Self-assembled nanoscale coordination polymers carrying siRNAs and cisplatin for effective treatment of resistant ovarian cancer. Biomaterials. 2015;36:124–133. doi:10.1016/j.biomaterials.2014.09.017
118. Mathis JM, Stoff-Khalili MA, Curiel DT. Oncolytic adenoviruses - selective retargeting to tumor cells. Oncogene. 2005;24(52):7775–7791. doi:10.1038/sj.onc.1209044
119. Sayed N, Allawadhi P, Khurana A, et al. Gene therapy: comprehensive overview and therapeutic applications. Life Sci. 2022;294:120375. doi:10.1016/j.lfs.2022.120375
120. Uchitel J, Kantor B, Smith EC, Mikati MA. Viral-mediated gene replacement therapy in the developing central nervous system: current status and future directions. Pediatr Neurol. 2020;110:5–19. doi:10.1016/j.pediatrneurol.2020.04.010
121. Peng J, Zou WW, Wang XL, et al. Viral-mediated gene therapy in pediatric neurological disorders. World J Pediatr. 2023. doi:10.1007/s12519-022-00669-4
122. Zhang B, Liu Y, Zhang P, et al. A novel CRAd in combination with cisplatin enhanced the antitumor efficacy in ovarian cancer. Int J Gynecol Cancer. 2011;21(9):1540–1546. doi:10.1097/IGC.0b013e31823105ed
123. Tuppurainen L, Sallinen H, Karvonen A, et al. Combined gene therapy using AdsVEGFR2 and AdsTie2 with chemotherapy reduces the growth of human ovarian cancer and formation of ascites in mice. Int J Gynecol Cancer. 2017;27(5):879–886. doi:10.1097/IGC.0000000000000973
124. Franke CE, Czapar AE, Patel RB, Steinmetz NF. Tobacco mosaic virus-delivered cisplatin restores efficacy in platinum-resistant ovarian cancer cells. Mol Pharm. 2018;15(8):2922–2931. doi:10.1021/acs.molpharmaceut.7b00466
125. Wang Y, Gao S, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5(10):791–796. doi:10.1038/nmat1737
126. Zhan C, Wei X, Qian J, et al. Co-delivery of TRAIL gene enhances the anti-glioblastoma effect of paclitaxel in vitro and in vivo. J Control Release. 2012;160(3):630–636. doi:10.1016/j.jconrel.2012.02.022
127. Wang J, Lu Z, Wang J, et al. Paclitaxel tumor priming promotes delivery and transfection of intravenous lipid-siRNA in pancreatic tumors. J Control Release. 2015;216:103–110. doi:10.1016/j.jconrel.2015.08.012
128. Qiu N, Liu X, Sui M, Tang J, Shen Y. Paclitaxel improved gene transfection efficiency through cell synchronization in SW480 cells. J Control Release. 2015;213:e83. doi:10.1016/j.jconrel.2015.05.138
129. Wong HL, Shen Z, Lu Z, Wientjes MG, Au JL. Paclitaxel tumor-priming enhances siRNA delivery and transfection in 3-dimensional tumor cultures. Mol Pharm. 2011;8(3):833–840. doi:10.1021/mp1004383
130. Chiocca EA, Blair D, Mufson RA. Oncolytic viruses targeting tumor stem cells. Cancer Res. 2014;74(13):3396–3398. doi:10.1158/0008-5472.CAN-14-0290
131. Eiselein JE, Biggs MW, Walton JR. Treatment of transplanted murine tumors with an oncolytic virus and cyclophosphamide. Cancer Res. 1978;38(11 Pt 1):3817–3822.
132. Sze DY, Reid TR, Rose SC. Oncolytic virotherapy. J Vasc Interv Radiol. 2013;24(8):1115–1122. doi:10.1016/j.jvir.2013.05.040
133. Luo S, Chen P, Luo ZC, et al. Combination of vesicular stomatitis virus matrix protein gene therapy with low-dose cisplatin improves therapeutic efficacy against murine melonoma. Cancer Sci. 2010;101(5):1219–1225. doi:10.1111/j.1349-7006.2010.01507.x
134. Shi W, Tang Q, Chen X, et al. Antitumor and antimetastatic activities of vesicular stomatitis virus matrix protein in a murine model of breast cancer. J Mol Med. 2009;87(5):493–506. doi:10.1007/s00109-009-0444-5
135. Lin X, Chen X, Wei Y, et al. Efficient inhibition of intraperitoneal human ovarian cancer growth and prolonged survival by gene transfer of vesicular stomatitis virus matrix protein in nude mice. Gynecol Oncol. 2007;104(3):540–546. doi:10.1016/j.ygyno.2006.09.022
136. Zhong Q, Wen YJ, Yang HS, et al. Efficient inhibition of cisplatin-resistant human ovarian cancer growth and prolonged survival by gene transferred vesicular stomatitis virus matrix protein in nude mice. Ann Oncol. 2008;19(9):1584–1591. doi:10.1093/annonc/mdn167
137. Long J, Yang Y, Kang T, et al. Ovarian cancer therapy by VSVMP gene mediated by a paclitaxel-enhanced nanoparticle. ACS Appl Mater Interfaces. 2017;9(45):39152–39164. doi:10.1021/acsami.7b10796
138. Wu W, Mao D, Hu F, et al. A highly efficient and photostable photosensitizer with near-infrared aggregation-induced emission for image-guided photodynamic anticancer therapy. Adv Mater. 2017;29(33):1700548. doi:10.1002/adma.201700548
139. Dong Z, Feng L, Hao Y, et al. Synthesis of hollow biomineralized CaCO3-polydopamine nanoparticles for multimodal imaging-guided cancer photodynamic therapy with reduced skin photosensitivity. J Am Chem Soc. 2018;140(6):2165–2178. doi:10.1021/jacs.7b11036
140. Yang G, Xu L, Xu J, et al. Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano lett. 2018;18(4):2475–2484. doi:10.1021/acs.nanolett.8b00040
141. Wang H, Chao Y, Liu J, et al. Photosensitizer-crosslinked in-situ polymerization on catalase for tumor hypoxia modulation & enhanced photodynamic therapy. Biomaterials. 2018;181:310–317. doi:10.1016/j.biomaterials.2018.08.011
142. Zheng M, Yue C, Ma Y, et al. Single-step assembly of DOX/ICG loaded lipid--polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013;7(3):2056–2067. doi:10.1021/nn400334y
143. Zhen S, Yi X, Zhao Z, et al. Drug delivery micelles with efficient near-infrared photosensitizer for combined image-guided photodynamic therapy and chemotherapy of drug-resistant cancer. Biomaterials. 2019;218:119330. doi:10.1016/j.biomaterials.2019.119330
144. Pan Q, Tian J, Zhu H, et al. Tumor-targeting polycaprolactone nanoparticles with codelivery of paclitaxel and IR780 for combinational therapy of drug-resistant ovarian cancer. ACS Biomater Sci Eng. 2020;6(4):2175–2185. doi:10.1021/acsbiomaterials.0c00163
145. Liu R, Gao Y, Liu N, Suo Y. Nanoparticles loading porphyrin sensitizers in improvement of photodynamic therapy for ovarian cancer. Photodiagnosis Photodyn. 2021;33:102156. doi:10.1016/j.pdpdt.2020.102156
146. Zhang Q, Zhou L, Guan Y, Cheng Y, Han X. BENC-511, a novel PI3K inhibitor, suppresses metastasis of non-small cell lung cancer cells by modulating beta-catenin/ZEB1 regulatory loop. Chem Biol Interact. 2018;294:18–27. doi:10.1016/j.cbi.2018.08.010
147. Yang S, Wu J, Zuo Y, et al. ZD6474, a small molecule tyrosine kinase inhibitor, potentiates the anti-tumor and anti-metastasis effects of radiation for human nasopharyngeal carcinoma. Curr Cancer Drug Tar. 2010;10(6):2475–2484. doi:10.2174/156800910791859506
148. Dai J, Xu M, Wang Q, et al. Cooperation therapy between anti-growth by photodynamic-AIEgens and anti-metastasis by small molecule inhibitors in ovarian cancer. Theranostics. 2020;10(5):2385–2398. doi:10.7150/thno.41708
149. Baghbani F, Moztarzadeh F. Bypassing multidrug resistant ovarian cancer using ultrasound responsive doxorubicin/curcumin co-deliver alginate nanodroplets. Colloid Surface B. 2017;153:132–140. doi:10.1016/j.colsurfb.2017.01.051
150. Voloshin T, Munster M, Blatt R, et al. Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo. Int J Cancer. 2016;139(12):2850–2858. doi:10.1002/ijc.30406
151. Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103(41):15091–15096. doi:10.1073/pnas.0607260103
152. Schumann C, Taratula O, Khalimonchuk O, et al. ROS-induced nanotherapeutic approach for ovarian cancer treatment based on the combinatorial effect of photodynamic therapy and DJ-1 gene suppression. Nanomed-nanotechnol. 2015;11(8):1961–1970. doi:10.1016/j.nano.2015.07.005
153. Mitchell MJ, Billingsley MM, Haley RM, et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–124. doi:10.1038/s41573-020-0090-8
154. Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10(4):211–224. doi:10.1038/nrclinonc.2013.5
155. Del Campo JM, Munoz-Couselo E, Diaz de Corcuera I, Oaknin A. Trabectedin combined with liposomal doxorubicin in women with relapsed ovarian cancer. Expert Rev Anticancer Ther. 2010;10(6):795–805. doi:10.1586/era.10.59
156. Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, Phase 3 study. Lancet Oncol. 2021;22(7):1034–1046. doi:10.1016/S1470-2045(21)00216-3
157. Lee MW, Ryu H, Song IC, et al. Efficacy of cisplatin combined with topotecan in patients with advanced or recurrent ovarian cancer as second- or higher-line palliative chemotherapy. Medicine. 2020;99(17):e19931. doi:10.1097/MD.0000000000019931
158. Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2003;21(17):3194–3200. doi:10.1200/JCO.2003.02.153
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.