Back to Journals » Drug Design, Development and Therapy » Volume 9
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) as second-line chemotherapy in HER2-negative, taxane-pretreated metastatic breast cancer patients: prospective evaluation of activity, safety, and quality of life
Authors Palumbo R, Sottotetti F, Trifirò G, Piazza E, Ferzi A, Gambaro A, Spinapolice EG, Pozzi E, Tagliaferri B, Teragni C, Bernardo A
Received 19 December 2014
Accepted for publication 30 January 2015
Published 15 April 2015 Volume 2015:9 Pages 2189—2199
DOI https://doi.org/10.2147/DDDT.S79563
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Raffaella Palumbo,1 Federico Sottotetti,1 Giuseppe Trifirò,2 Elena Piazza,3 Antonella Ferzi,4 Anna Gambaro,3 Elena Giulia Spinapolice,2 Emma Pozzi,1 Barbara Tagliaferri,1 Cristina Teragni,1 Antonio Bernardo1
1Departmental Unit of Oncology, IRCCS Fondazione Salvatore Maugeri, Pavia, Italy; 2Unit of Nuclear Medicine, IRCCS Fondazione Salvatore Maugeri, Pavia, Italy; 3Medical Oncology Luigi Sacco Hospital, Milano, Italy; 4Medical Oncology, Legnano Hospital, Legnano, Italy
Background: A prospective, multicenter trial was undertaken to assess the activity, safety, and quality of life of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) as second-line chemotherapy in HER2-negative, taxane-pretreated metastatic breast cancer (MBC).
Patients and methods: Fifty-two women with HER2-negative MBC who were candidates for second-line chemotherapy for the metastatic disease were enrolled and treated at three centers in Northern Italy. All patients had previously received taxane-based chemotherapy in the adjuvant or first-line metastatic setting. Single-agent nab-paclitaxel was given at the dose of 260 mg/m2 as a 30-minute intravenous infusion on day 1 each treatment cycle, which lasted 3 weeks, in the outpatient setting. No steroid or antihistamine premedication was provided. Treatment was stopped for documented disease progression, unacceptable toxicity, or patient refusal.
Results: All of the enrolled patients were evaluable for the study endpoints. The objective response rate was 48% (95% CI, 31.5%–61.3%) and included complete responses from 13.5%. Disease stabilization was obtained in 19 patients and lasted >6 months in 15 of them; the overall clinical benefit rate was 77%. The median time to response was 70 days (range 52–86 days). The median progression-free survival time was 8.9 months (95% CI, 8.0–11.6 months, range 5–21+ months). The median overall survival point has not yet been reached. Toxicities were expected and manageable with good patient compliance and preserved quality of life in patients given long-term treatment.
Conclusion: Our results showed that single-agent nab-paclitaxel 260 mg/m2 every 3 weeks is an effective and well tolerated regimen as second-line chemotherapy in HER2-negative, taxane-pretreated MBC patients, and that it produced interesting values of objective response rate and progression-free survival without the concern of significant toxicity. Specifically, the present study shows that such a regimen is a valid therapeutic option for that ‘difficult to treat’ patient population represented by women who at the time of disease relapse have already received the most active agents in the adjuvant and/or metastatic setting (ie, conventional taxanes).
Keywords: nab-paclitaxel, metastatic breast cancer, taxanes, quality of life
Introduction
Metastatic breast cancer (MBC) has always been a challenging disease to treat because of its poor prognosis and 5-year survival rate of only 23%–26%.1,2 Data from population-based studies and analysis of clinical trials show that the outcome for women with MBC is slowly but steadily improving, as the risk of death is decreasing by 1%–2% each year3,4 and the median overall survival (OS) has increased from 18 to 28 months in recent years.5–8 It is likely the greatest improvement is related to the development and widespread availability of modern systemic therapies, including combinations with targeted biological agents in different breast cancer subtypes, that have been proved effective in increasing response rates, progression-free survival (PFS), and OS.7,9–11 However, therapeutic goals in the metastatic setting remain palliative in nature and are aimed at controlling symptoms, improving and maintaining quality of life (QoL), and prolonging survival, all while carefully balancing treatment efficacy and toxicity.12–14
Currently, taxanes are considered the most effective cytotoxic drugs for the treatment of MBC, both in monotherapy and combined schedules, and have a proven survival benefit greater than those of other types of chemotherapy.15,16 According to the most recent international guidelines, paclitaxel and docetaxel, the two most commonly used taxanes against breast cancer, are the agents of choice in patients progressing after anthracycline-containing chemotherapy.17,18 Despite their clinical activity, the use of taxanes could be limited by significant toxicities observed in treated patients; most notably, effects such as hypersensitivity reactions and peripheral neuropathy remain major challenges. Premedication with corticosteroids and antihistamines before taxane administration is mandatory but causes additional side effects.19–21
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a solvent-free colloidal suspension of paclitaxel and human serum albumin; this medication exploits the physiological transport of albumin from the bloodstream via the endothelium of the blood vessels. This system may also allow better delivery of the drug to the tumor microenvironment, and thus it is associated with more linear pharmacokinetics.22 Nab-paclitaxel was developed to take advantages of the antitumor activity of conventional paclitaxel. After the completion of Phase I and pharmacokinetic studies to determine the maximum tolerated dose and optimal dosing,23,24 a 300 mg/m2 regimen every three weeks (q3w) of nab-paclitaxel was tested in a Phase II trial on 63 MBC women, 59% of whom had prior exposure to anthracyclines. An objective response rate (ORR) of 48% was achieved (41% in the pretreated patients, 64% in those chemotherapy-naïve for the metastatic disease); median time to progression and OS were 26.6 weeks and 62.6 weeks, respectively.25
The efficacy and safety of nab-paclitaxel in the first- and second-line treatment of MBC was demonstrated in a large randomized Phase III trial comparing q3w nab-paclitaxel 260 mg/m2 and q3w paclitaxel 175 mg/m2. The study showed the statistically significant superiority of nab-paclitaxel in terms of ORR (33% for nab-paclitaxel versus 19% for paclitaxel, P=0.001). In particular, the ORR was 42% for nab-paclitaxel and 27% paclitaxel in the first-line setting (P=0.029); in second-line-or-greater setting, the ORR was 27% for nab-paclitaxel and 13% for paclitaxel (P=0.006). In patients with visceral dominant lesions, the tumor response rate was significantly higher (P=0.002) with nab-paclitaxel (34%) than with paclitaxel (19%). In patients <65 years of age, the tumor response rate was significantly higher (P<0.001) with nab-paclitaxel (34%) than with paclitaxel (19%). In the experimental arm, PFS was significantly longer with nab-paclitaxel than with paclitaxel (23 weeks versus 16.9 weeks, P=0.006). A trend in favor of nab-paclitaxel for OS was also observed (65.0 versus 55.7 months, P=0.046). Patients randomized in the experimental arm had a lower incidence of grade 4 neutropenia (9% versus 22%, P=0.046) despite a 49% higher taxane dose. Grade 3 sensory neuropathy was more common in the nab-paclitaxel arm (10% versus 2%, P<0.01), with a median time of improvement to a lower grade of 22 days for the nab-paclitaxel group and 79 days for the paclitaxel group, respectively.26 The results from the pivotal Phase III study led to the regulatory approval of nab-paclitaxel for the treatment of MBC by the US Food and Drug Administration in 2005 as monotherapy with a recommended dose of 260 mg/m2 as a q3w regimen.27 In Europe it is licensed for use in adult patients with disease progression despite the type of first-line treatment for metastatic disease and in whom standard, anthracycline-containing therapy is not indicated.28
The next logical step in the clinical development of nab-paclitaxel was the investigation of a weekly schedule. In a direct comparison between weekly (100 mg/m2 or 150 mg/m2) nab-paclitaxel, q3w nab-paclitaxel (300 mg/m2), and docetaxel (100 mg/m2), each type of dose and schedule of nab-paclitaxel was superior to docetaxel in terms of ORR and PFS as a first-line treatment for MBC, and nab-paclitaxel had a favorable toxicity profile.29,30 Additional studies have further demonstrated that the administration of weekly nab-paclitaxel is both safe and effective, even in heavily pretreated, taxane-refractory patients31 or in combined regimens with other cytotoxic or targeted agents.32–35 To date, little information is available regarding the approved q3w schedule in the real life clinical context, because most of the data have been provided by post hoc or retrospective analyses.36–38 Finally, the impact of such a treatment option on patient QoL has not been specifically evaluated in this setting.
Presented here are the results of a single-arm, multicenter, prospective study undertaken to assess the activity, safety, and impact on QoL of q3w nab-paclitaxel as second-line chemotherapy in HER2-negative MBC patients previously treated with taxanes in the adjuvant or metastatic setting.
Patients and methods
Study design and endpoints
This prospective, multicenter trial was designed to evaluate the antitumor activity, safety, and QoL of q3w nab-paclitaxel in patients with MBC who were previously treated with taxanes. The study was conducted in compliance with the Helsinki Declaration.39 The primary efficacy endpoint was the overall ORR, defined as the percentage of patients having either a complete response (CR) or partial response (PR). The exact binomial for a 95% confidence interval (CI) for the ORR was used. A sample size of 52 MBC patients was targeted to ensure that the lower limit of the 95% CI exceeded 50% of the overall response rate. Secondary objectives included safety, QoL and treatment compliance evaluation, PFS, and OS.
Patient selection
Each eligible patient had to fulfill all the following criteria: 1) be histologically or cytologically confirmed to have locally advanced or metastatic breast cancer; 2) have HER2-negative disease, defined as an immunohistochemistry score of 0–1+ or have an immunohistochemistry score of 2+ and no gene amplification by fluorescence in situ hybridization; 3) have no more than one prior chemotherapy for metastatic disease; 4) have an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2; 5) be at least 18 years of age; 6) have adequate bone marrow (absolute neutrophil count ≥1,500 cells/μL, hemoglobin ≥9.5 g/dL, and platelet count >100,000 cells/μL), hepatic functions (serum bilirubin ≤2.0 mg/dL; alanine transaminase, aspartate aminotransferase, and alkaline phosphatase ≤ double upper normal limit), and renal functions (serum creatinine ≤1.1 mg/dL); 7) have no active concomitant malignancies; 8) have a life expectancy >3 months; and 9) have at least one bidimensionally measurable target lesion documented by computed tomography scan or magnetic resonance imaging according to the Response Evaluation Criteria in Solid Tumors.40 Patients may have had previous hormonal therapy as adjuvant treatment and/or treatment for metastatic disease if they had progressive disease and they discontinued hormone therapy at study entry. Neoadjuvant and/or adjuvant chemotherapy was allowed. Patients had to be treated with taxane-containing chemotherapy as adjuvant or first-line treatment for the metastatic disease. Previous radiation therapy was allowed if the measurable lesions were completely outside the radiation field and 4 weeks had elapsed prior to study entry. Bisphosphonate therapy for bone metastases was allowed; however, treatment must have been initiated prior to the first dose of the study medication. Patients were excluded if they met any one of the following conditions: 1) had clinical signs of a central nervous system disorder and brain metastases or leptomeningeal infiltration; 2) had history of other cancers except for radically resected carcinoma in the uterine cervix or nonmelanoma skin cancer; 3) had poorly controlled medical disorders (diabetes, hypertension, infection); 4) had pre-existing peripheral neuropathy of grade >1 based on National Cancer Institute Common Toxicity Criteria (NCICTC) Version 2.0;41 5) were pregnant or lactating. The had baseline staging consisted of a complete clinical examination; chest and abdomen computed tomography scans with contrast enhancement, positron emission tomography, or computed tomography and/or X-ray and abdominal ultrasound; bone isotope scan; electrocardiogram and echocardiography with left ventricular ejection fraction evaluation; complete blood count; and routine biochemistry. Written informed consent was obtained from each patient before enrollment in the study.
Treatment and procedures
All of the enrolled patients were treated in the outpatient setting. Single agent nab-paclitaxel was given at the dose of 260 mg/m2 as a 30-minute intravenous infusion on day 1 of each 3-week cycle. A standard antiemetic regimen with 5-HT3 receptor antagonists was given; no premedication to prevent hypersensitivity reactions was provided. Treatment could be delayed for a maximum of 2 weeks in case of hematological toxicity, febrile neutropenia, sepsis, or any other grade 3–4 nonhematological toxicity. Dose adjustments for nab-paclitaxel (with a dose reduction first by 25% and then by 50%) were planned to correspond with type and grade of observed toxicity when appropriate. If an adverse event required dose interruption, the nab-paclitaxel dose was reinitiated at the start of a treatment cycle if the patient’s absolute neutrophil count was ≥1,500 cells/μL, the patient's platelet count was ≥100,000 cells/μL, and any other toxicity resolved to grade 1. Patients experiencing grade 3–4 neutropenia, with or without fever, or grade 2 symptomatic anemia could receive hematological support with granulocyte colony-stimulating factor or erythropoietin. Treatment was administered until documented disease progression, unacceptable toxicity, or patient refusal.
Treatment activity was assessed in accordance with the Response Evaluation Criteria in Solid Tumors.40 Responses were evaluated during every second chemotherapy cycle with repeated clinical and appropriate radiological assessments based on the extent of the disease defined at baseline. A patient was considered assessable for response if she received a minimum of two cycles of treatment. Overall response was defined as the best confirmed response detected in each patient from the date of enrollment until the end of the study. Response duration was computed from the initiation of treatment to the first evidence of disease progression for all responsive patients. Objective response rates (ORR) and clinical benefit rates (defined as the sum of the number of patients who achieved a CR, the number of patients who achieved a PR, and the number of patients whose disease remained stable for a minimum of 6 months) were tabulated together with 95% CI by following the exact method. Subset analysis according to baseline characteristics was performed for ORR; PFS, defined as the time from the date of enrollment to the first documented progression, and OS, defined as the time between study enrollment and date of death, were estimated using the Kaplan–Meier method.42 All treated patients were included in the intent-to treat (ITT) analysis and were analyzed for safety.
Toxicity was monitored by clinical evaluation, complete blood cell count, and full serum chemistry before each cycle. Cardiac assessment was performed by clinical evaluation and by electrocardiography and echocardiography with left ventricular ejection fraction measurements at baseline and, thereafter, when clinically indicated. Toxicity was graded according to NCICTC, version 2.0.41 Patients who received at least one cycle of therapy were considered evaluable for safety analysis. QoL was measured at baseline and then at the start of every cycle by using the self-administered European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Breast 23 (EORTC QLQ-BR23, Italian translation).43 For a more accurate evaluation of treatment compliance, patients were also asked to concomitantly complete an institutional validated questionnaire in which patients’ subjective perceptions of tolerability of the most recent therapy prior to the start of the study and of the therapy provided during the study was graded as ‘very good’, ‘good’, ‘satisfactory’, or ‘insufficient’.
Results
Patient population
From February 4, 2011 to May 11, 2013, 52 consecutive MBC patients were enrolled and treated in three centers in Northern Italy. The main patient characteristics at baseline are reported in Table 1. The median age was 53 years (range 33–71 years), and the ECOG performance status was 0–1 in 92% of cases. The median time from initial diagnosis was 28 months (range 19–57 months); about 35% of patients had a disease-free interval <2 years. Visceral involvement was present in 67% of cases, and more than 70% of patients had metastases to >2 sites. All patients received prior adjuvant therapy, anthracycline-based in 27% and taxane-based in 65% of cases. Moreover, all of the patients had received one prior regimen as first-line treatment for the metastatic disease; this treatment consisted of taxane-based chemotherapy in 46% of cases (weekly paclitaxel/bevacizumab in 14% of patients and docetaxel/capecitabine in 10% of patients), and the remaining patients were treated with all-oral vinorelbine/capecitabine combination or capecitabine/vinorelbine monotherapy (16 and 12 patients, respectively).
Treatment activity
All patients were evaluable for the primary study endpoint (Table 2). The ORR was 48% (95% CI, 31.5%–61.3%) and included CRs in 13.5%. Disease stabilization was reached in 19 patients and lasted more than 6 months in 15 of them; the overall clinical benefit rate was 77%. The median time to response was 70 days (range 52–86 days).
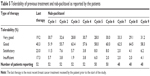
In Table 3, the rate of responding patients in the whole series is scattered by the main baseline characteristics of patients, tumor and pretreatment that could potentially affect the chance of response. The CIs suggest that none of the considered variables significantly affected the probability of response. However, with limitations due to the small sample size, it appears that elderly patients (those older than 65 years of age), patients whose ECOG performance status was poor (1–2), and patients whose breast cancer onset was ≥2 years had low response rates. By contrast, young patients (those ≤65 years of age) with triple negative disease, patients whose disease-free interval from breast cancer diagnosis was <2 years, and patients whose predominant metastases were hepatic were highly responsive to treatment.
The curve based on Kaplan–Meier estimates of PFS in the ITT population is reported in Figure 1. The median PFS was 8.9 months (95% CI, 8.0–11.6 months, range 5–21+ months). As of the data cut-off, 11 women (21%) had died. Therefore, the median OS was not reached because the data were not mature (ie, >75% of the patients were censored for the endpoint). Overall, 36 women received further chemotherapy at the time of disease relapse, three had third-line hormone therapy, and two other patients were treated with palliative radiotherapy for symptomatic metastatic bone disease.
Toxicity and compliance
All of the enrolled patients were assessable for safety analysis (Table 4). A total of 378 chemotherapy cycles were given to the 52 patients. The median number of courses was six per patient (range 4–26 cycles). Treatment was well tolerated; 92% of patients received nab-paclitaxel at the protocol-specified dose throughout the study, and 40% of them had ≥9 cycles. The median relative dose intensity was 98%. Neutropenia was the most commonly seen effect of hematological toxicity; grade 3–4 toxicity was observed in 11 patients (21%) and corresponds to 5% of administered cycles. Granulocyte colony-stimulating factor support was given for five patients (9.6%) during eight cycles of treatment. Neutropenia was usually brief and not cumulative, and no episodes of febrile neutropenia occurred; chemotherapy administration was delayed by 1 week in nine out of 52 women (17%) because of hematological toxicity or patient convenience (six and three patients, respectively).
  | Table 4 Treatment-related toxicity |
As expected, peripheral neuropathy was the most significant effect of nonhematological toxicity: 22 women (42%) experienced grade 1–2 neuropathy during treatment. Three patients (5.8%; one at cycle 3, one at cycle 5, and one at cycle 8) required dose reduction by 25% because of grade 3 sensory neuropathy. The onset of such a toxicity occurred after a median of six treatment cycles (range 3–14 cycles); the median time to improvement to a lower grade was 19 days (range 16–26 days).
Nausea/vomiting was mild on standard antiemetic regimens. Self-limited mucositis was detected in ten patients, eight of whom had grade 1 severity and two of whom had grade 2 severity. Transient and reversible increases in serum transaminases were observed in four patients. Grade 2 fatigue occurred in three women. All patients experienced treatment-related alopecia, which was of grade 1 in 51% of cases and grade 2 in 42% of cases; in four patients, grade 3 hair loss occurred over 32 treatment courses. No hypersensitivity reactions were documented. All of the observed treatment-related adverse events were fully resolved at the time of the first follow-up visit, and no long-term toxicity was detected.
Overall, the toxicity profile in women aged ≥65 years did not significantly differ from that of younger patients.
Short-term infusion and not needing premedication allowed good patient compliance throughout the whole population. Information on treatment tolerability was available on all of the treated patients (Table 5). ‘Very good’ tolerability was reported in 28%–33% of the patients who received nab-paclitaxel, a proportion that was higher than the 19% that was reported for the patients’ last therapy. The percentage of patients reporting ‘insufficient’ tolerability did not exceed 6%. Overall, 60% of all of the patients reported an improvement in tolerability after switching to nab-paclitaxel from their last therapies, mostly from ‘satisfactory’ to ‘good’ or from ‘good’ to ‘very good’.
QoL assessment
The QLQ-BR 23 complementary questionnaire was found to be feasible and easily completed by the majority of patients: 50/52 (96%) women returned the completed modules at the start of each chemotherapy cycle. Figure 2 provides an overall profile of the investigated parameters of QoL of all enrolled patients during the first nine cycles of treatment. No significant deterioration of QoL was observed for most of the evaluated aspects, such as systemic therapy side effects, breast and arm symptoms, and distress over hair loss; a non statistically significant decrease in median score regarding body image was observed during cycles 5–6, while scores of future perspectives improved during treatment. Interestingly, such an improvement was maintained in women receiving prolonged treatments (eight courses and over).
Discussion
The treatment of MBC is evolving as researchers continue aiming to improve the QoL, the duration of remission, and, in the last couple of decades, the OS. Today, there is no standard of care for a disease as heterogeneous and complex as HER2-negative MBC, and many criteria will need consideration when selecting not only the best drug but also the best regimen.12–14 Weekly 80 mg/m2 paclitaxel and q3w 75–100 mg/m2 docetaxel are considered the gold standard in MBC on the basis of results of randomized clinical trials.44–48 Moreover, how these agents stand relative to each other in terms of efficacy remains difficult to judge. The issue of the sequential versus the combined chemotherapy approach in the metastatic setting remains unresolved.49–52 Therefore, the choice of the optimal therapeutic strategy is made on an individual basis and with the consideration of both objective clinical/biological parameters (age, HER2 status, disease-free interval, previous neo- and adjuvant treatments, metastatic sites, predominant symptoms) and the patient’s attitudes and preferences. Indeed, the increasing use of anthracycline- and taxane-based chemotherapy in the early stage of breast cancer makes the management of relapsing disease more difficult, and new active therapeutic options need to be identified in such a ‘difficult to treat’ patient population. Despite the current lack of a standard of care for the metastatic disease, a considerable proportion of women receive multiple lines of treatment, including taxane rechallenge, that are prescribed on the basis of previous efficacy and tolerance; the results of these treatments justify this practice.53–56 However, there are very few data available that detail the outcomes of this pragmatic approach for MBC.30,57–59 Specifically, in the second-line treatment, the challenge is how to deliver full doses of the chosen agents without causing unacceptable levels of toxicity.
The primary objective of this prospective, multicenter study was to assess the activity and tolerability of the approved single agent q3w nab-paclitaxel regimen as a second-line treatment in women previously treated with taxane-based chemotherapy in the adjuvant or metastatic setting. To the best of our knowledge, this is the first prospective study specifically focused on this issue, since currently available data are derived from trials testing the nab-paclitaxel weekly schedule or from post hoc retrospective analyses.
A clinical demonstration that nab-paclitaxel does not demonstrate absolute cross-resistance with first-generation taxanes was firstly provided by Blum et al in 2007.31 In this Phase II trial treatment with weekly nab-paclitaxel, 100 mg/m2 and 125 mg/m2 were both associated with an ORR of 14%–16%, a median PFS of 3.0–3.5 months, and a median OS of 9.1–9.2 months in 181 women with MBC who were heavily pretreated with taxanes. Among 75 women given 125 mg/m2 nab-paclitaxel, the disease control rate was 45% in those treated with conventional paclitaxel and 46% in those with prior exposure to docetaxel; in the whole population, median survival was similar for responding patients and those with disease stabilization ≥16 weeks.31
The activity of q3w nab-paclitaxel observed in our study was higher than that previously reported in taxane-pretreated MBC patients,31,36,37 but a cross-comparison of results is difficult because of the different characteristics of enrolled patients. We reported an ORR of 48%, including 13% complete responses, in 52 evaluable patients; 13 out of 24 women (54%) previously given paclitaxel/bevacizumab or docetaxel/capecitabine as a first-line treatment for the metastatic disease demonstrated an objective response. Overall, 77% of patients had a clinical benefit from their second-line treatment with nab-paclitaxel, since 15 stable diseases lasting more than 6 months were observed. A short time to response was also noted; 98% of responder patients achieved maximum response by cycle 3. These findings appear of importance in the practical management of MBC patients, since tumor response to chemotherapy can lead to restoration of organ function, symptom relief, and improvement in patient QoL. On the other hand, in this setting, obtaining prolonged stabilization of disease can provide the same clinical advantage as exhibiting an objective response. Findings from the randomized Phase II and III studies and subsequent exploratory analyses suggest that patients treated with nab-paclitaxel achieved a CR or a PR, and they appeared to live longer than those who did not receive nab-paclitaxel. This trend was observed across various patient subgroups. However, whether tumor response could be indicative of a survival benefit with nab-paclitaxel is unknown, and the role of surrogate endpoints to predict OS benefit to chemotherapy remains unclear as well.60–62
The results of our statistical analysis, which was performed in order to identify factors potentially predictive of treatment response and clinical outcome, suggest a higher chance of response for women who are usually in the ‘poor prognosis’ subset: women who are <65 years of age; women who are affected with triple negative subtype; women whose disease free interval (DFI) from the time of diagnosis is short; and women whose predominant metastatic disease is in the liver. Similar data were previously reported in a post hoc analysis of two randomized trials of nab-paclitaxel that aimed to examine whether patients with DFI ≤2 years and visceral dominant metastases demonstrate outcomes similar to the ITT population in these studies. The results of the analysis showed that the treatment benefits observed with nab-paclitaxel, but not with paclitaxel or docetaxel, in these trials also apply to women with poor prognostic factors.38 Recently reported data further support the effectiveness of the drug in MBC patients with features typically associated with more aggressive disease (including the triple-negative phenotype, a higher number of metastatic sites, the presence of visceral metastases and a short DFI), both in the first-line setting and in the context of progressive or resistant disease.63–65
The secondary efficacy endpoint of our study was treatment safety and tolerability, including a prospective assessment of QoL. Overall, treatment-related toxicity was manageable in the outpatient setting, and in no case did treatment have to be stopped because of unacceptable side effects or patient refusal. Specifically, the incidence of severe peripheral neuropathy was less frequent than expected and less frequent than previously reported in taxane-pretreated populations:26,31 only three patients, all previously given docetaxel-based chemotherapy for the metastatic disease, experienced grade 3 sensory neuropathy, and these episodes occurred during the third, fifth, or eighth cycle of treatment. All of these episodes were easily managed with dose reduction and treatment delay until improvement to grade 2. No significant differences in the whole safety profile in elderly patients were detected; this finding confirms results previously reported for weekly nab-paclitaxel in patients ≥65 years old.26,29,66
As described previously, in our study, nab-paclitaxel given at 260 mg/m2 every 3 weeks resulted in a good patient compliance even for patients given long-term treatment. Treatment tolerability, as reported by the patients, was ‘very good’ or ‘good’ in more than 80% of the whole cohort. Interestingly, 31 patients (60%) reported better tolerability of therapy with nab-paclitaxel than with their last therapy, which consisted of docetaxel or paclitaxel-based chemotherapy in 46% of them.
No significant deterioration of QoL for most of the evaluated aspects over treatment was detected. The non statistically significant decrease we observed in median scores regarding the body image during cycles 5–6 of therapy is probably related to the onset of sensorial neuropathy, which impacts daily activities. Interestingly, we observed that scores for the item of future perspectives improved over treatment. Because the time of responding to the QoL questionnaires coincided with the instrumental re-evaluation of the disease, this finding could reflect the better functioning of patients continuing therapy, who were informed by the physician that the treatment had a positive effect.
Despite more than 40 years of clinical research, treatment choices beyond the first line in MBC are still difficult to determine. Drug selection and combination are complicated because the majority of patients were exposed to docetaxel and/or paclitaxel at the time of disease relapse. The introduction of nab-paclitaxel opened a novel scenario in the treatment of MBC. For choosing the best drug for each patient for a particular set of benefits, more options are now available that allow for the possibility of tailoring taxane-based therapy in the decision making process. The challenge to pick the adequate dose for the individual patient will depend on the therapeutic index of the different possible regimens. The issue with the use of nab-paclitaxel in clinical practice is linked to the probability of sensory neuropathy. As elegantly highlighted in a recent editorial,67 further investigation is required to better manage this ‘difficult-to-quantify’ toxicity, since data in MBC are equivocal at the present time. For clinical practice, the time to reversibility of neuropathy appears to be an important variable to be considered when choosing the dose and schedule of nab-paclitaxel for treating MBC patients. The data reported in this study confirm that sensorial neuropathy occurs late in the course of treatment with the q3w schedule also in taxane-pretreated patients, and adequate management by dose reductions or treatment delays allows the maintenance of an adequate dose-intensity of the drug.
Conclusion
In conclusion, our study demonstrated that q3w nab-paclitaxel produces good antitumor activity with manageable toxicity and no significant deterioration of QoL as second-line chemotherapy in MBC patients, confirming the previously reported efficacy data. Specifically, our study shows that such a regimen is a valid therapeutic option for that ‘difficult to treat’ patient population represented by women who at time of disease relapse have already received the most active agents in the adjuvant and/or metastatic setting, such as taxanes.
To further optimize the role of nab-paclitaxel in the management of taxane-pretreated patients, future clinical research in this setting should include investigating specific patient and tumor characteristics that can be used as biomarkers to potentially predict the response to this therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
American Cancer Society. Cancer Facts and Figures 2014. Atlanta: American Cancer Society; 2014. Available from: http://wiki.dovepress.com/tiki-index.php?page=Reference+types+and+formatting&structure=Style+Guide. Accessed January 1, 2015. | ||
Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Andre F, Slimane K, Bachelot T, et al. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol. 2004;22(16):3302–3308. | ||
Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Cancer. 2004;100:44–52. | ||
Mauri D, Polyzos NP, Salanti G, Pavlidis N, Ioannidis, JP. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008;100(24):1780–1791. | ||
Dawood S, Broglio K, Gonzalez-Angulo AM, Buzdar AU, Hortobagyi GN, Giordano SH. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26(30):4891–4898. | ||
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010; 28(1):92–98. | ||
Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol. 2011;29(16):2144–2149. | ||
O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(Suppl 3):20–29. | ||
Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005;104(8):1742–1750. | ||
Chia S, Speers H, D’yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110(5):973–979. | ||
Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. Oncologist. 2003;8(6):514–520. | ||
Smith I. Goals of treatment for patients with metastatic breast cancer. Semin Oncol. 2006;33(1 Suppl 2):S2–S5. | ||
Jones SE. Metastatic breast cancer: the treatment challenge. Clin Breast Cancer. 2008;8(3):224–233. | ||
Gradishar WJ. Taxanes for the treatment of metastatic breast cancer. Breast Cancer. 2012;6:159–171. | ||
Ghersi D, Wilcken N, Simes J, Donoghue E. Taxane containing regimens for metastatic breast cancer. Cochrane Database Syst Reviews. 2003;3:CD003366. | ||
Cardoso F, Harbeck N, Fallowfield S, et al. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow up. Ann Oncol. 2012;23(Suppl 7):vii11–vii19. | ||
The National Comprehensive Cancer Network, ed. National comprehensive network clinical practice guidelines in oncology, breast cancer, version 1.2015; Fort Washington: The National Comprehensive Cancer Network; 2015. Available from: http://www.nccn.org/. Accessed January 10, 2015. | ||
Weiss RB, Donehower PH, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8(7):1263–1268. | ||
Sparreboom A, van Zuylen L, Brouwer E, et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: clinical pharmacokinetic implications. Cancer Res. 1999;59(7):1454–1457. | ||
Ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42(7):665–685. | ||
Henderson IC, Bathia V. Nab-paclitaxel for breast cancer: a new formulation with an improved safety profile and greater efficacy. Expert Rev Anticancer Res. 2007;7(7):919–943. | ||
Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–1044. | ||
Nyman DW, Campbell KJ, Hersh E, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23(31):7785–7793. | ||
Ibrahim NK, Samuels B, Page R, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23(25):6019–6026. | ||
Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(1):7794–7803. | ||
Paclitaxel albumin-stabilized nanoparticle formulation [webpage on Internet]. Bethesda: National Institutes of Health; 2006 [updated November 18, 2013]. Available from: http://www.cancer.gov/cancertopics/druginfo/nanoparticlepaclitaxel. Accessed January 10, 2015. | ||
EMA. Abraxane Summary of Product Characteristics, 2008. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/000778/WC500020435.pdf. Accessed January 10, 2015. | ||
Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first line therapy for metastatic breast cancer. J Clin Oncol. 2009; 27(22):3611–3619. | ||
Gradishar WJ, Krasnojon D, Cheporov S, et al. Phase II trial of nab-paclitaxel compared with docetaxel as first-line chemotherapy in patients with metastatic breast cancer: final analysis of overall survival. Clin Breast Cancer. 2012;12(5):313–321. | ||
Blum JL, Savin MA, Edelman G, et al. Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer. 2007;7(11):850–856. | ||
Roy V, LaPlant BR, Gross GG, Bane CL, Palmieri FM; North Central Cancer Treatment Group. Phase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane®) in combination with gemcitabine in patients with metastatic breast cancer (N0531). Ann Oncol. 2009;20(3):449–453. | ||
Conlin AK, Seidman AD, Bach A, et al. Randomized phase II trial of nanoparticle albumin-bound paclitaxel with carboplatin and trastuzumab as first line therapy for women with HER2-overexpressing metastatic breast cancer. Clin Breast Cancer. 2010;10(4):281–287. | ||
Mirtsching B, Cosgriff T, Harker G, Keaton M, Chidiac T, Min M. A phase II study of weekly nanoparticle albumin-bound paclitaxel with or without trastuzumab in metastatic breast cancer. Clin Breast Cancer. 2011;11(2):121–128. | ||
Yardley DA, Hart L, Bosserman L, et al. Phase II study evaluating lapatinib in combination with nab-paclitaxel in HER2-overexpressing metastatic breast cancer patients who have received no more than one prior chemotherapeutic regimen. Breast Cancer Res Treat. 2013;137(2):457–464. | ||
Dent S, Fraser J, Graham N, Campbell M, Hopkins S, Dranitsaris G. Clinical outcomes of women with metastatic breast cancer treated with nab-paclitaxel: experience from a single academic cancer centre. Curr Oncol. 2013;20(1):24–29. | ||
Lohmann AE, Speers CH, Chia SK. Evaluation of the clinical benefit of nanoparticle albumin-bound paclitaxel in women with metastatic breast cancer in British Columbia. Curr Oncol. 2013;20(2):97–103. | ||
O’Shaughnessy J, Gradishar WJ, Bhar P, Iglesias J. Nab-paclitaxel for first-line treatment of patients with metastatic breast cancer and poor prognostic factors: a retrospective analysis. Breast Cancer Res Treat. 2013;138(3):829–837. | ||
WMA Declaration of Helsinki - Ethical principles for medical research involving human subjects [webpage on the Internet]. Ferney-Voltaire: WMA World Medical Association, Inc. Available from: www.wma.net/en/30publications/10policies/b3. Accessed January 2, 2015. | ||
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. | ||
Common Terminology Criteria for Adverse Events Version 2.0 [webpage on Internet]. Bethesda: National Cancer Institute; 2010. Available from: http://ctep.cancer.gov/reporting/ctc.html. Accessed January 10, 2015. | ||
Kaplan EL, Meier P. Non parametric estimation from incomplete observation. Journal of American Statistical Association. 1958;53(282): 457–481. | ||
Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768. | ||
Burstein HJ, Manola J, Younger J, et al. Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol. 2000;18(6): 1212–1219. | ||
Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23(24):5542–5551. | ||
Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every 3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER2-overexpressors and random assignment to trastuzumab or not in HER2 nonoverexpressors: final results of cancer and leukemia group B protocol 9840. J Clin Oncol. 2008;26(10):1642–1649. | ||
Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(20):3239–3247. | ||
Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26): 2666–2676. | ||
Wilcken N, Dear R. Chemotherapy in metastatic breast cancer: a summary of all randomized trials reported 2000–2007. Eur J Cancer. 2008; 44(15):2218–2225. | ||
Cardoso F, Bedard PL, Winer EP, et al. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101(17):1174–1181. | ||
Guarneri V, Conte P. Metastatic breast cancer: therapeutic options according to molecular subtypes and prior adjuvant therapy. Oncologist. 2009;14(7):645–656. | ||
Fossati R, Confalonieri C, Torri V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women. J Clin Oncol. 1998;16(10): 3439–3460. | ||
Roché H, Vahdat LT. Treatment of metastatic breast cancer: second line and beyond. Ann Oncol. 2011;22:1000–1010. | ||
Planchat E, Abrial C, Thivat E, et al. Late lines of treatment benefit survival in metastatic breast cancer in current practice? Breast. 2011;20(6):574–578. | ||
Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21(3):242–252. | ||
Palumbo R, Sottotetti F, Riccardi A, et al. Which patients with metastatic breast cancer benefit from subsequent lines of treatment? An update for clinicians. Ther Adv Med Oncol. 2013;5(6):334–350. | ||
Toulmonde M, Madranges N, Brouste V, et al. Docetaxel rechallenge after a first response in non-resistant metastatic breast cancer: significant activity with manageable toxicity. Breast Cancer Res Treat. 2012; 134(1):325–332. | ||
Andreupoulou E, Sparano JA. Chemotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer: an overview. Curr Breast Cancer Rep. 2013;5(1):42–50. | ||
Palmieri C, Krell J, James CR, et al. Rechallenging with anthracyclines and taxanes in metastatic breast cancer. Nat Rev Clin Oncol. 2010; 7(10):561–574. | ||
Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56(1):67–78. | ||
Pierga JY, Robain M, Jouve M, et al. Response to chemotherapy is a major parameter influencing long-term survival of metastatic breast cancer patients. Ann Oncol. 2001;12(2):231–237. | ||
Bruzzi P, Del Mastro L, Sormani MP, et al. Objective response to chemotherapy as a potential surrogate end point of survival in metastatic breast cancer patients. J Clin Oncol. 2005;23(22):5117–5125. | ||
Glück S. Nab-paclitaxel for the treatment of aggressive metastatic breast cancer. Clin Breast Cancer. 2014;14(4):221–227. | ||
Ciruelos E, Jackisch C. Evaluating the role of nab-paclitaxel (Abraxane) in women with aggressive metastatic breast cancer. Expert Rev Anticancer Ther. 2014;14(5):511–521. | ||
von Minckwitz G, Martin M, Wilson G, et al. Optimizing taxane use in MBC in the emerging era of targeted chemotherapy. Crit Rev Oncol Hematol. 2013;85(3):315–331. | ||
Aapro M, Tjulandin S, Bhar P, Gradishar W. Weekly nab-paclitaxel is safe and effective in ≥65 years old patients with metastatic breast cancer. A post-hoc analysis. Breast. 2011;20(5):468–474. | ||
Kudlowitz D, Muggia F. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel): extending its indications. Expert Opin Drug Saf. 2014;13(6):681–685. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.