Back to Journals » International Journal of Nanomedicine » Volume 15
Nano-Silicate-Reinforced and SDF-1α-Loaded Gelatin-Methacryloyl Hydrogel for Bone Tissue Engineering
Authors Shi Z , Xu Y , Mulatibieke R, Zhong Q, Pan X, Chen Y, Lian Q, Luo X, Shi Z, Zhu Q
Received 9 July 2020
Accepted for publication 2 November 2020
Published 24 November 2020 Volume 2020:15 Pages 9337—9353
DOI https://doi.org/10.2147/IJN.S270681
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Video abstract of "GelMA-SN-SDF-1α hydrogel for bone tissue engineering" [ID 270681].
Views: 797
Zhe Shi,1,* Yichuan Xu,1,* Ruzha Mulatibieke,2 Qiang Zhong,1 Xin Pan,1 Yuhang Chen,1 Qiang Lian,1 Xin Luo,3 Zhanjun Shi,1 Qingan Zhu1
1Department of Orthopedics, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China; 2Department of Plastic Surgery, The Third Affiliated Hospital of Sun Yet-Sen University, Guangzhou, People’s Republic of China; 3Rehabilitation Medical School, Guangzhou International Economics College, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhanjun Shi; Qingan Zhu Tel +86 2062787924
; +86 20622787195
Email [email protected]; [email protected]
Purpose: Autologous bone grafts are the gold standard for treating bone defects. However, limited bone supply and morbidity at the donor site restrict its extensive use. Therefore, developing bone graft materials as an alternative to autologous grafts has gained considerable attention. Injectable hydrogels endowed with osteogenic potential have the ability to fill irregular bone defects using minimally invasive procedures and have thus been attracting researchers’ attention. However, from a clinical perspective, most fabrication methods employed for the current injectable osteogenic hydrogels are difficult and inconvenient. In the current study, we fabricated an injectable osteogenic hydrogel using a simple and convenient strategy.
Materials and Methods: Gelatin-methacryloyl (GelMA) pre-polymer was synthetized. Nano silicate (SN) and stromal cell-derived factor-1 alpha (SDF-1α) were introduced into the pre-polymer to achieve injectability, controlled release property, excellent osteogenic ability, and efficient stem cell homing.
Results: The GelMA-SN-SDF-1α demonstrated excellent injectability via a 17-G needle at room temperature. The loaded SDF-1α exhibited a long-term controlled release pattern and efficiently stimulated MSC migration and homing. The GelMA-SN-SDF-1α hydrogel amplified cell spreading, migration, osteogenic-related biomarker expression, and matrix mineralization. The GelMA-SN-SDF-1α hydrogel filled critical-sized calvaria defects in rats and demonstrated excellent bone regeneration ability, as assessed using micro-CT scanning and histomorphometric staining.
Conclusion: The GelMA-SN-SDF-1α hydrogel provides a simple and convenient strategy for the fabrication of injectable osteogenic graft materials.
Keywords: bone defects, gelatin methacryloyl hydrogels, injectable, nano-silicate, SDF-1α, osteogenic
Introduction
Surgical reconstruction of bone defects resulting from trauma, tumors, or infection (osteomyelitis) has been a significant challenge for orthopedic surgeons.1 The annual medical expenditure for bone defect treatment in the US is estimated to be $5 billion, of which a significant part is attributed to bone grafts with defective fracture healing.2 Conventionally, bone autografts have been considered the gold standard for treating bone defects in terms of osteoconduction and osteoinduction.1,3,4 However, the availability of autografts is limited, and the accompanying morbidity at the donor site continues to be a concern.3 Bone ceramic materials and metallic bone substitutes have extensively been investigated and used. These materials include hydroxyapatite (HAP), tricalcium phosphate (TCP), titanium alloy, and tantalum alloy.5–9 However, the integration of synthetic substitutes with host bone remains insufficiently effective, causing concerns about graft loosening over the long term.10 A strategy for using scaffolds seeded with cells or bioactive components to promote bone regeneration is promising, and it may be able to promote complete bone healing.11 Recently, researchers have shown interest in designing osteogenic hydrogels to stimulate bone regeneration. Such hydrogels include collagen-based hydrogels,12 bioconjugated hydrogels grafted with bioactive groups,13 nanocomposite hydrogels incorporating bioactive nanoparticles,14 and injectable hydrogel delivery osteogenic components.15 Injectable osteogenic hydrogels have been attracting considerable attention because of their ability to fill irregular bone defects using a minimally invasive procedure.16,17 However, from a clinical perspective, the fabrication of an injectable hydrogel platform involving simple and rapid production possessing long-term osteogenic ability remains highly desirable.
Gelatin methacryloyl (GelMA) hydrogels have widely been employed in tissue engineering because of their biological compatibility and tunable physical properties.18 To introduce osteogenic properties into hydrogels, various bioactive components, such as BMP,19 osteogenic peptides,20 and bioactive nanoparticles,21 have been added. In particular, bioactive silicate nanoplatelets (SN) [Laponite: Na + 0.7[(Si8Mg5.5:Li0.3)O20(OH)4 − 0.7)] has been attracting interest because of its ability to induce osteogenic differentiation in the bone marrow and human mesenchymal stem cells (hMSCs) in the absence of extra osteoinductive factors, such as BMP-2 or dexamethasone.22,23 Owing to strong electrostatic and van der Waals forces as well as interparticle forces between disk-shaped particles, SN has been used for designing shear-thinning injectable hydrogels without the need for bioconjugation to form the chemical network.23–25 By simply mixing SN with GelMA pre-polymer, an injectable osteogenic GelMA-SN hydrogel (uncrosslinked) can be fabricated. After being injected into a defective area, the mechanical properties of the GelMA-SN hydrogel can be further modified via UV crosslinking in situ.18 The compression modulus and degradation rate can be adjusted. For MSCs growing on a hydrogel, the optimal compression modulus of the hydrogel for osteogenic differentiation is ~25 KPa.26 Ideally, the degradation speed of the hydrogel should coincide with the regeneration rate of new tissue, and the hydrogel should support the long-term release of embedded bioactive components.
To guarantee efficient bone healing, it is essential to deliver or recruit sufficient stem cells to the bone defect area.27,28 There have been numerous attempts to use scaffolds to transfer stem cells to a local area.29–31 However, these strategies remain limited by restricted cell sources, low cell viability, and controversial therapeutic effects. An alternative strategy of stimulating local stem cell homing by delivering cell recruiting growth factors to the defective area and realizing their osteogenic potential may be more valuable.32–35 Damaged tissues in critical organs, such as the heart and liver, have been regenerated by stem cell homing using the well-directed migration of stem cells.36–38 Stromal cell-derived factor 1 alpha (SDF-1α) is reportedly capable of directing bone marrow MSC homing and migration.32,33 The introduction of SDF-1α into GelMA-SN to fabricate injectable GelMA-SN-SDF-1α may be sufficient to direct MSC homing and simultaneously promote MSC osteogenic differentiation.39,40 At the nanoscale, the loaded two-dimensional SN could establish physical interactions with SDF with a high surface area and charged characteristics.23,41,42 Reportedly, SN can deliver and release active growth factors for >30 days.43 The high binding efficacy of SN did not change the protein structure, and the released proteins were able to maintain high bioactivity to MSCs at low concentrations.43 Therefore, a relatively long-term and controlled release pattern of SDF-1α could be expected from the injectable GelMA-SN-SDF-1α hydrogel. In the present research, an injectable osteogenic GelMA-SN-SDF-1α hydrogel (uncrosslinked) was fabricated by mixing GelMA pre-polymer, SN, and SDF-1α. UV radiation was applied to further modify its mechanical properties for optimal osteogenesis and controlled degradation. In vitro physical characterization and release, tests were conducted to investigate the injectability, compression modulus, swelling ratio, and degradation rates of the SN-loaded hydrogel. The release pattern of SDF-1α was analyzed and modified to achieve a long-term release profile. The viability, spreading, proliferation, and migration behavior of MSCs cultured using the GelMA-based hydrogel were assayed in vitro. The osteogenic capability of the injectable GelMA-SN-SDF-1α hydrogel was systematically validated in vitro and in vivo. In summary, we designed an injectable GelMA-SN-SDF-1α hydrogel with a simple and rapid production process. This gel released SDF-1α in a controlled pattern, stimulated MSC migration and accumulation, and promoted bone healing and regeneration.
Materials and Methods
Materials
Gelatin (Gel strength 300, Type A), methacrylic anhydride (94%), bovine serum albumin (98%), Irgacure 2959 (98%), and Alizarin Red S were obtained from Sigma-Aldrich (St. Louis, MO, USA). Laponite XLG was purchased from BYK Additives & Instruments (Wesel, Germany). Recombinant Rat CXCL12/SDF-1α (carrier-free) was purchased from Biolegend (San Diego, CA, USA). Live/Dead Viability/Cytotoxicity Kits, β-glycerol phosphate 173 (99%), L-ascorbic acid (99%), Alexa Fluor 594–phalloidin, 4′,6-diamidino-2-phenylindole (DAPI), α minimum essential medium (α-MEM), trypsin–ethylenediaminetetraacetic acid (trypsin–EDTA), L-glutamine, and penicillin/streptomycin were provided by Thermo Fisher Scientific (Waltham, MA, USA). Anti-ALP antibody (sc-271,431) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Anti-OCN antibody (MABD123), anti-OPN antibody (AB1870), and anti-RUNX2 antibody (05–1478) were provided by Millipore (Burlington, MA, USA).
Fabrication of Injectable SDF-1α-Loaded Laponite-GelMA Hydrogel
GelMA macromonomers were synthesized using gelatin from porcine skin and methacrylic anhydride, according to previously published protocols.44 Gelatin was first completely dispersed in phosphate-buffered saline (PBS, pH 7.4). Then, 10% w/v of gelatin was completely dissolved in 50 mL PBS at approximately 50°C for 20 min. Methacrylic anhydride (MA; 1.25% w/v) was added into the above solution at 50°C and incubated for 1 h under vigorous stirring. The resultant solution was diluted with 200 mL PBS and dialyzed (10 kDa cut-off dialysis membrane) against deionized (DI) water (pH ~7–7.4, 40°C) for a week to remove excess MA. The filtered solution was then transferred to 50 mL falcon tubes, placed in −80°C overnight, and lyophilized for 1 week to receive the final product presented as a white foam.
To fabricate the injectable SDF-1α-loaded laponite-GelMA hydrogel, GelMA foam was first completely dissolved in DI water. Thereafter, laponite (2% w/v) and SDF-1α (100 ng/mL) were added into the GelMA solution. Irgacure 2959 (0.5% w/v) was added into the pre-polymer as a photoinitiator, which permitted the pre-polymer to cross-link under UV radiation (6.9 mW/cm2, 360–480 nm).
Characterization of SDF-1α-Loaded Laponite-GelMA Hydrogel
The hydrogel’s injectability, internal structure and porosity, compression modulus, and swelling and degradation ability were analyzed.
Viscosity and Injectability Analysis
Rheology and stress recovery tests were performed using an ElastoSensTM Bio2 (Rheolution Instruments, Montreal, QC, Canada), according to a previously described protocol.45 The shear stresses for hydrogel samples (uncrosslinked), loaded with different SN weights (0%, 1%, and 2%), were recorded in a shear rate range of 0.1 to 100 S−1 at room temperature. The hydrogel’s (uncrosslinked) injectability was evaluated using an ElastoSensTM Bio2 to obtain the storage modulus (G′) at 37°C. The hydrogel was subjected to sequential high (100%, 10 min) and low (1%, 10 min) strain rates at 1 Hz. Injectability was assessed using syringes with 17-, 19-, 21-, 23-, 25-, and 27-G needle at room temperature. In detail, the injection force was analyzed using Instron 5940 (Instron, Norwood, MA, USA). GelMA-SN hydrogel (2%) was injected from a 1-mL syringe with 1.5-inch needles of 17, 19, 21, 23, 25, and 27 G (Figure 1D). All measurements were obtained at a flow rate of 2 mL/h. Power law model fit data were generated using the rheometer (Rheolution Instruments, Montreal, QC, Canada) at room temperature. The power law equation and above data were used to determine the power law index (n), consistency index (k), and the correlation coefficient (Corr. Coeff) values. Further, 1% and 2% GelMA-SN hydrogels were tested. Based on a previous study,46 the following power law equation was used:
o = kΫn (σ = Shear stress; k = Consistency; Ϋ = Shear rate; n = Power law index).
Observation Using Scanning Electron Microscopy (SEM)
To observe the internal microstructure of the shear-thinning hydrogel and analyze the microstructural changes after the loading of laponite, lyophilized hydrogel samples were scanned using field emission SEM (Zeiss Ultra 55).
Mechanical Analysis
To conduct mechanical analysis, cylindrical hydrogel samples (8 mm in width, 2 mm in height) were fabricated by transferring 100 μL of the pre-polymer solution into a customized polydimethylsiloxane (PDMS) mold. The hydrogel samples were then transferred to an Instron 5542 mechanical tester and compressed at a speed of 1 mm/min for 30 s. Young’s modulus was acquired by calculating the first 0–10% linear region of the stress–strain curve.
Degradation and Swelling Analysis
For the degradation assay, cylindrical hydrogel samples were prepared as above. Hydrogel samples were lyophilized and weighed to record the initial mass. After being immersed in PBS at 37°C, the hydrogel samples were extracted at pre-set time points, and the lyophilized mass was weighed. The degradation profile was then calculated.
For the swelling test, a similar cylindrical hydrogel sample was used, and it was incubated in PBS for 1 h to reach equilibrium. The mass of the swollen hydrogel was weighed as M(s). Subsequently, the hydrogel was lyophilized, and its weight was recorded as M(l). The swelling ratio was determined based on the following equation:
Swelling ratio = [M(s) − M(l)]/M(l)
SDF-1α Release Analysis
For the release assay, SDF-1α-loaded cylindrical hydrogel samples were incubated in 1 mL PBS and placed on a shaker platform at 37°C at 60 rpm. At the pre-set time points, 500 μL of PBS was retrieved and replaced with 500 μL of fresh PBS. The concentration of released SDF-1α was assayed using rat SDF-1α ELISA kits (Bluegene, Shanghai, China). The accumulated concentration was calculated.
Cell Viability, Spreading, Proliferation, and Migration Assays
Cell Viability
Rat bone marrow MSCs (Shanghai cell bank, Chinese Academy of Sciences) were cultured in αMEM medium (10% fetal bovine serum and 1% penicillin/streptomycin) at 37°C with 5% CO2. MSCs were seeded on cylindrical hydrogel samples (8 mm in diameter, 2 mm in height) in 12-well plates with 3 × 104 cells per well. The potential cellular toxicity of the released SDF-1α and laponite nanoparticles was evaluated using cell counting kit-8 (CCK-8) assays according to the manufacturer’s protocol. In addition, cell viability was analyzed using Live/Dead assay kits. Calcein acetoxymethyl and ethidium homodimer-1 were used to stain live cells and dead cells, producing red and green fluorescence, respectively. The staining results were assessed using an inverted fluorescence microscope (Brunel SP99F, Brunel Microscopes Ltd, UK), and the quantification of live cells and dead cells was conducted using Image J software (1.52 v).47
Cell Spreading
Cell spreading and morphology were observed 2 hours after cell seeding. Cells that adhered to the hydrogel surfaces were fixed with 4% paraformaldehyde and stained with Alexa Fluor 594–phalloidin and DAPI. The staining results were assessed using an inverted fluorescence microscope.
Cell Proliferation and Migration
The cell migration and chemotaxis behavior were evaluated using transwell cell migration assays. Specifically, 50 μL of the MSC suspension (1 × 104 cells/mL) was transferred to the upper chamber, and 400 μL of pure culture medium was placed into the separate lower chamber. Thereafter, hydrogel samples were transferred into the lower chamber. Chemotactic regents (SDF-1α) released from the hydrogel samples were expected to stimulate cells migrated from the upper chamber to the lower chamber via the porous chamber polyester membrane (pore size, 3 μm). After a 3-h culture, the cells that had migrated via the membrane were fixed with 4% paraformaldehyde solution for 10 min and stained with crystal violet solution (0.1%) for 10 min. After gently rinsing with PBS, the cells in randomly selected fields were imaged and counted under a microscope (Brunel SP99F, Brunel Microscopes Ltd, UK). The cell migration behavior was analyzed using scratch wound healing assays. MSC suspension (5 mL, 1 × 105 cells/mL) was first seeded on a 10-cm Petri dish. As the cells achieved ~70% confluency, a 1-mL pipette tip was used to create a ~2.0-mm-wide incision-like gap. Then, different GelMA hydrogel samples (8 mm in diameter, 2 mm in height) were placed into the Petri dish. The gap area was microscopically observed (Brunel SP99F, Brunel Microscopes Ltd, UK) at defined time points. Thereafter, the wound healing percentage was quantified and expressed as the percentage of the gap closure area.
Osteogenic Biomarker Expression Analysis
The expression of osteogenesis-related genes, including alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and osteoprotegerin (OPN), was evaluated using quantitative reverse transcription–polymerase chain reaction (qRT–PCR). The primers used in the present study are listed in Table 1. Cylindrical hydrogel samples (8 mm in diameter, 2 mm in height) were placed in 12-well plates with 3 × 104 cells/well in osteoconductive media (10 mM beta-glycerol phosphate and 50 μg/mL ascorbic acid). Cells were collected on days 3, 7, 10, and 14 and lysed using Trizol (Invitrogen). RT–PCR was conducted using a Bio-Rad MyiQ2 thermocycler with the SYBRR Green Supermix (Bio-Rad).
 |
Table 1 Primers Used for qRT–PCR |
To evaluate osteogenesis-related protein expression levels, ALP, OCN, OPN, and RUNX2 antibodies were used to label the target proteins. On days 7 and 14, cells growing on the hydrogel samples were fixed in 4% paraformaldehyde and permeabilized using 1% Triton X-100 solution. After blocking non-specific antigens with 4% normal goat serum, the cells were incubated with primary antibodies at 4°C overnight, followed by immersion in secondary antibodies at 37°C for 2 h. Cellular nuclei and actin were labeled with DAPI and Alexa Fluor 594–phalloidin. The stained images were recorded using a Brunel SP99F microscope, and the quantification of protein expression level was performed using ImageJ software.
Matrix Mineralization Analysis
The level of matrix mineralization was analyzed using alizarin red staining. Cylindrical hydrogel samples (8 mm in diameter, 2 mm in height) were placed in 12-well plates at an initial density of 3 × 104 cells/mL in osteoconductive media (10 mM beta-glycerol phosphate and 50 μg/mL ascorbic acid) and cultured for 21 days. The cells were then incubated with alizarin red (40 mM, PH = 4.2) at room temperature for 10 min. The alizarin-stained mineralized calcium nodules in five randomly chosen light microscope fields were counted. To further quantify mineralized nodule production, the alizarin-stained samples were soaked in 10% (v/v) acetic acid, which was neutralized with 10% ammonium hydroxide after 30 min. The resultant supernatant was read at 405 nm.
In vivo Bone Regeneration Analysis
Rat Calvaria Defect Model
Male Sprague Dawley (SD) rats (n = 60) with a mean weight of 120 g were randomly divided into four groups: control GelMA hydrogel group, GelMA-SN hydrogel group, GelMA-SDF-1α hydrogel group, and GelMA-SN-SDF-1α hydrogel group. Surgical procedures were conducted according to a protocol described previously.48 Rats were anesthetized with 3% pentobarbital sodium (30 mg/kg) via an intraperitoneal injection. The surgical site was shaved using electric clippers and disinfected with iodoaniline. A 1.5-cm longitudinal incision was made, and the covering periosteum was retracted to expose the calvarium. Subsequently, an 8-mm trephine was used to create a critical-sized defect. Then, the hydrogel was injected into the defect area and crosslinked in situ. The periosteum and skin were sutured and disinfected. SD rats were procured and kept in the Laboratory Animal Center of Nanfang Hospital of Southern Medical University. All experimental procedures were approved by the Animal Experimental Ethics Committee of Nanfang Hospital of Southern Medical University (NFYY-2019-81). All the animal experiments were conducted based on the Chinese National Guidelines (GB/T 35,892‐20,181) for laboratory animal welfare issued by the Technical Committee for Laboratory Animal Sciences of the Standardisation Administration of China.
Bone Regeneration Analysis
Bone defect healing and bone regeneration behavior were evaluated using micro–computed tomography (CT) analysis and histomorphometry staining. All rats were executed by overdosing with pentobarbital sodium injection 6 weeks after surgery. Thereafter, the calvarias were harvested. All samples were scanned using CT (l-CT 80 scanner, Scanco Medical, Bassersdorf, Switzerland) at 300 mS, 70 kV, and 114 mA to determine bone volume over total volume (BV/TV) and bone mineral density (BMD) values. The histomorphometric characteristics of the regenerated bone were analyzed using H&E staining and Goldner-Masson trichrome staining. For quantification, the Regions of Interest (ROI) Manager feature of ImageJ was used to select specific areas in the immunoassaying picture.49 After drawing the ROI, the Analyze–Set Measurements step was used to select the parameters. The unhealed width defect area was measured and recorded as U(a). As the original defect width was 8 mm, the healing width was calculated as 8−U(a). The bone healing percentage was calculated using the following equation:
Bone healing percentage = [8 − U(a)]/8 × 100%
Statistical Analysis
Experimental results were compared using one-way ANOVAs followed by Bonferroni’s post hoc analysis (GraphPad Prism 6.0 software). A P value of <0.05 indicated statistical significance.
Results
Material Characterization
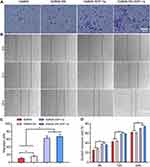
The fabrication process of the GelMA-SN-SDF-1α hydrogel (uncrosslinked) is shown in Figure 1A. The SN and SDF-1α were loaded into the GelMA hydrogel pre-polymer to construct an injectable hydrogel system, which could be further crosslinked in situ for enhanced mechanical properties. After the addition of SN (1 and 2 wt%), the pre-polymer (uncrosslinked) exhibited significantly increased viscosity (~10–300 poise) under certain shear rates, demonstrating apparent shear-thinning properties (Figure 1B).
Further, the incorporation of SN enhanced the uncrosslinked pre-polymer’s shear recovery ability (Figure 1C). Pure GelMA (uncross-linked) solution had extremely limited shear recovery ability owing to its low viscosity. Under low strain (1%), the GelMA (uncrosslinked) pre-polymer exhibited a low storage modulus (G’) of ~5 Pa. The introduction of 1% SN increased the storage modulus to ~90 Pa, whereas that of 2% SN produced a storage modulus of ~350 Pa. After being subjected to high strain (100%), the uncrosslinked pre-polymer loaded with SN recovered ~70–80% of the initial storage modulus. The injection force of 2% GelMA-SN was increased from ~0.1 to ~5 to 8.5 N when the needle size was reduced from 17 G to 23 G to 27 G, respectively (Figure 1E). The excellent injectability of the GelMA-SN hydrogel was also demonstrated by using a 1-mL syringe with various sizes of needles at room temperature (Figure S1). The power law index (n) and consistency index (k) of the 1% GelMA-SN hydrogel were ~0.24 ± 0.10 and 23 ± 2.45 and those of the 2% GelMA-SN hydrogel were 0.20 ± 0.08 and 56 ± 4.76, which were presented in Table S1.
After crosslinking using UV radiation, the GelMA-based hydrogels presented a porous internal structure with more connected pores (Figure 1F). The swelling ratio of the four GelMA-based hydrogels decreased by 30–40% after UV crosslinking (Figure 1G). The crosslinked hydrogel presented a ~5-fold increase in compression modulus (Figure 1H), and the degradation rate was reduced from 3 to 21 days after crosslinking (Figure 1I and J). However, the addition of SN and SDF-1α did not significantly change the parameters of the GelMA-based hydrogels before or after UV crosslinking.
In the SDF-1α release assay, the GelMA-SN-SDF-1α hydrogel exhibited a controlled released pattern, in which the released SDF-1α could be detected at day 21. By contrast, the GelMA-SDF-1α exhibited a burst release profile where >90% of the loaded SDF-1α was released within the first 3 days (Figure 1K).
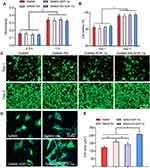
Cell Viability, Spreading, and Proliferation
Cell viability of MSCs cultured on the surface of hydrogel samples was analyzed. As shown in Figure 2A, the cell viability on different hydrogel sample groups was not significantly different at 0.5 or 1 h. Consistent with the CCK-8 assay results, the Live/Dead staining showed no viability differences (Figure 2B and C). The viabilities of the cultured cells were ~82% and ~96% at days 1 and 3, respectively. Cell spreading behavior of MSCs on different hydrogel surfaces was demonstrated by phalloidin and DAPI staining results (Figure 2D). Cells grown on SN-loaded GelMA-SN-SDF-1α hydrogels spread faster and produced a larger cell area (251 ± 22.4 μm2) than cells cultured on the other three kinds of hydrogels (Figure 2E).
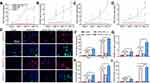
Cell Migration
On analysis of cell migration of MSCs, a 4-fold increase in migrated cells was observed when exposed to SDF-1α-loaded hydrogels (Figure 3A and C). In the scratch test, SDF- 1α-loaded hydrogel increased the wound closure rate 37.7 ± 1.34% and 36.9 ± 1.30% at 6 h and 58.2 ± 1.23% and 58.8 ± 1.49% at 12 hours, 70.8 ± 1.26% and 74.6± 1.32% at 24 hours, respectively (Figure 3B and D).
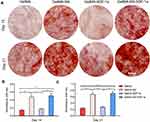
Matrix Mineralization
Alizarin red staining was used to investigate the matrix mineralization level of MSCs cultured in different hydrogel samples (Figure 4A). Typically, the mineralized matrix production of the cells was enhanced when they were cultured with SN-loaded GelMA hydrogel. The absorbance values at 405 nm indicated mineralization level were 0.48 ± 0.05 and 0.68 ± 0.05 on day 14 and 0.51 ± 0.06 and 0.71 ± 0.06 on day 21 for cells grown on GelMA-SN and GelMA- SN-SDF-1α hydrogels, respectively (Figure 4B and C). The corresponding absorbance values were 0.14 ± 0.02 and 0.24 ± 0.02 for cells grown on GelMA hydrogel without SN and 0.17 ± 0.02 and 0.27 ± 0.02 for cells grown on GelMA-SDF-1α hydrogel without SN (Figure 4B and C).
Osteogenesis-Related Biomarker Expression
The expression levels of osteogenesis-related genes at preset time points are presented in Figure 5A–D. Compared with the pure GelMA hydrogel (the control) group, ALP, RUNX2, OCN, and OPN expressions in the remaining three hydrogel groups were promoted to different extents. On day 14, the GelMA-SN-SDF-1α hydrogels promoted the highest expression of the four genes by ~17.5-, ~10.5-, ~18.9-, and ~10.5-fold (Figure 5A–D). By contrast, the GelMA-SN hydrogels stimulated an increase in the expression of the four genes by ~6.23-, ~6.93-, ~8.93-, and ~3.93-fold (Figure 5A–D). The GelMA-SDF-1α hydrogels upregulated the four genes by ~2.93-, ~1.93-, ~4.23-, and ~1.23-fold (Figure 5A–D).
Labeled osteogenesis-related proteins ALP, RUNX2, OCN, and OPN were identified using immunofluorescence microscopy (Figure 5E). Consistent with the PCR results, the quantification results of the immunofluorescence assays found that the GelMA-SN-SDF-1α hydrogel promoted the highest levels of expression of osteogenesis-related proteins compared with the GelMA hydrogel. Specifically, ~25.5-, ~10.5-, ~35.9-, and ~25.5-fold increases in the four proteins were detected at day 14 in the GelMA-SN-SDF-1α hydrogel group (Figure 5F–I). In the GelMA-SN hydrogel group, the expression of the four proteins increased by ~23.2-, ~4.93-, ~28.9-, and ~23.9-fold, respectively (Figure 5F–I). The GelMA-SDF-1α hydrogels increased ALP, RUNX2, and OCN expressions by ~3.93-, ~1.93-, and ~16.2-fold but did not alter OPN expression (Figure 5I).
In vivo Bone Healing and Regeneration
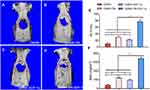
The bone healing and regeneration ability of the GelMA-SN-SDF-1α hydrogel was confirmed in vivo using a rat calvaria defect model. As shown by the CT scan results (Figure 6A–D), compared with the other three groups, rats implanted with GelMA-SN-SDF-1α hydrogel exhibited the fastest bone healing and produced more regenerated bone. Further, GelMA-SDF- 1α and GelMA-SN hydrogels promoted bone regeneration to a certain extent. The two parameters BV/TV and BMD were used for further quantification. BV/TV was ~77.5%, ~22.9%, ~26.2%, and ~10.4% for rats treated with GelMA-SN-SDF-1α hydrogel, GelMA- SDF-1α hydrogel, GelMA-SN hydrogel, and GelMA hydrogel, respectively (Figure 6E). The BMD presented a similar data trend: ~514%, ~146%, ~163%, and ~62.4% (Figure 6F).
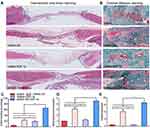
The histomorphometric characteristics of the defect areas were analyzed using H&E staining and Goldner-Masson trichrome staining to measure healing percentage (percentage of the damaged area that was healed) and osteoid formation. The H&E staining images and quantification results are presented in Figure 7A and C. GelMA-SN-SDF-1α hydrogel stimulated bone defect healing with a healing percentage of ~65.5%, which was considerably higher than the other three hydrogel samples (~18.9%, ~23.2%, and ~5.3% for GelMA- SDF-1α hydrogel, GelMA-SN hydrogel, and GelMA hydrogel, respectively). Goldner-Masson trichrome staining was applied to measure mineralized bone and osteoid formation. Bone defects injected with GelMA-SN-SDF-1α and GelMA-SN hydrogels displayed considerably more mineralization and osteoid formation after 6 weeks than bone defects filled with the other two hydrogels (Figure 7B, D, and E). Specifically, the amount of mineralized bone formation from GelMA-SN-SDF-1α, GelMA-SDF-1α, and GelMA-SN hydrogels was ~12.3-, ~3.54-, and ~4.35-fold that of the GelMA hydrogel group. The quantity of osteoid formation from GelMA-SN-SDF-1α and GelMA-SN hydrogels was 16 and 10 times that of the other two groups.
Discussion
Treating bone defects is expensive due to the significant expense incurred by the use of bone grafts for defective bone healing caused by pathologies such as injuries and tumors.1,2 Autografts that are primarily harvested from the patient’s iliac crest are considered as the gold standard in reconstructive surgery. However, the autograft strategies are severely limited by the material availability and donor site morbidity. Currently, bone ceramic materials and metallic bone substitutes (HAP, TCP, titanium alloy, and tantalum alloy) are widely used in the clinical settings owing to their good biocompatibility and excellent mechanical strength. These properties are highly favorable when treating load-bearing bone defects.50 However, these hard bone substitutes typically lack bone regenerative potential, and there is a big difference in elastic modulus between the grafts and the natural bone. Consequently, the desired integration of substitute–bone interface is barely achieved. Additionally, undesired bone resorption caused by stress shielding is typically detected.50 The insufficient bone integration and accompanied bone resorption continue to concern surgeons when using these bone grafts. Therefore, using bone tissue engineering strategies to fabricate scaffolds for bone regeneration has been attracting researchers’ attention. Ideal biofunctional scaffolds are expected to provide the desired characteristics—biocompatibility, bioactivity, osteoinductivity, biodegradability, and mechanical integrity.50 Because autograft sources are limited and there exist concerns regarding bone integration with synthetic grafts in the long term, the use of bone tissue engineering strategies to stimulate bone regeneration is of clinical importance. Several researchers have been attempting to use scaffolds for transferring stem cells and bioactive components to stimulate bone regeneration.29–31 In particular, injectable osteogenic hydrogels have been attracting attention owing to their unique ability to fill irregular bone defects using minimally invasive procedures.15–17 However, considering the complication of most synthetic processes, most fabrication strategies to date are time-consuming and expensive.51–53 Although some stem cell-loaded hydrogels have demonstrated the capability to promote bone regeneration in animal models,25,54,55 these strategies are affected by limited cell sources, low cell viability, or controversial therapeutic effects.56,57 We constructed a cell-free SDF-1α- loaded injectable osteogenic hydrogel simply by mixing SDF-1α, SN, and GelMA pre-polymer.
According to a previous study,58 the degree of GelMA hydrogel methacrylation was ~53.8% when 1.25% w/v MA was added during the synthetic process.
Consistent with previous studies,17,22,59 the composited polymer-embodied SN provided the polymer with excellent injectability. The injectability of the GelMA-SN-SDF-1α (uncrosslinked) hydrogel is primarily attributed to the shear-thinning property of SN.17,60 SN is a nano disk-shaped plate (25 nm in diameter, 0.92 nm in height) characterized by a positively charged surface and negatively charged edge.61 In dry powder form, the electrostatic interactions typically result in SN crystal stacks. However, when dissolved in water the exfoliation process promotes crystal stacks separated into individual SN crystals.62 The negatively charged edge may directly interact with the positively charged surface and form self-assembled gels.63 The viscosity of the gels is strongly thixotropic, and the gels can rapidly reform after removing the applied stress (Figure 1C and D). Therefore, the current method of using SN to fabricate injectable hydrogels offers considerable advantages compared with conventional chemical engineering approaches.
GelMA hydrogel containing nanosilicates exhibited the ability to promote osteogenesis without loading any growth factors.23 However, the above osteogenic activities were only successfully promoted with the existence of sufficient MSCs in the culture medium. From a clinical perspective, MSC accumulation cannot be guaranteed when the bone defect is caused by severe trauma, infection, and bone tumor, considering that the local bone microenvironment may be jeopardized.64 Additionally, SN itself possesses a very limited ability to recruit MSCs or stimulate MSC migration, as demonstrated in Figure 3. Therefore, a growth factor-free approach (GelMA-SN) may not guarantee sufficient MSC accumulation and efficient bone healing when used for complicated bone defects. To efficiently direct MSC migration to the bone defect area, an excellent cell chemotactic agent (SDF-1α) was loaded into the GelMA hydrogel.
After the SN-mixed hydrogel was injected in situ, the hydrogel was UV crosslinked to enhance its mechanical properties. After 30 s of UV radiation, a considerably more highly crosslinked internal network was formed, according to the SEM results. As a result, the swelling ratio was significantly decreased—a phenomenon that may be beneficial for controlling the perfusion speed of loaded bioactive components. The compression modulus showed a 5-fold increase, from ~5 to ~25 KPa, which is considered an optimal stiffness for MSC differentiation.65,66 The degradation speed was also reduced, facilitating a sustained release of the loaded SDF-1α. The GelMA-SN-SDF-1α hydrogel showed a controlled release profile as long as 21 days. By contrast, a burst release was produced by the GelMA-SDF-1α hydrogel. SN affects drug release by creating physical barriers in the polymer and can directly bind with the drugs.41,67 The long-term controlled release could be owing to SN binding because it has been suggested that various ions and molecules can be adsorbed to the surface of dispersed SN crystals in a solution.41 The underlying mechanism could be explained by van der Waals forces, hydrogen bonding, classical ion exchange, cation/water bridging, protonation, or ligand exchange at the crystal edges.41 The long duration of SDF-1α signaling is expected to benefit the bone defect healing process,42,68 which requires weeks to months. The osteoconductive medium used in this study for mimicking the local in vivo hematoma microenvironment at the fracture site typically develops a microenvironment by directing inflammatory cells to release cytokines and upregulate angiogenic and osteogenic factors.69
According to the results of the CCK-8 and Live/Dead staining assays, cells cultured on the four hydrogels showed excellent viability and proliferation behavior. No significant differences were detected between groups. GelMA is primarily composed of gelatin, which has widely been used in the clinical settings owing to its excellent biocompatibility.70 The added SN and SDF-1α are typically considered biocompatible at the relatively low dosages used in the present study. However, the spread of cultured cells on the different hydrogels was significantly different. A high number of widely spread cells were observed when they were cultured on SN-loaded GelMA hydrogel. This spread may arise because SN can stimulate cell spreading by absorbing integrins and osteogenic proteins on the cell surface.41 In general, better spreading cells are more likely to differentiate into the bone. Therefore, the ability of SN to promote cell spreading indicates its considerable potential for inducing osteogenesis.
Stem cell homing plays a crucial role in wound healing and tissue regeneration.38 The SDF-1α released from the SDF-1α-containing hydrogel evidently promoted cell migration, consistent with previous studies.32,33,71 The wound scratch and transwell migration tests indicated that SDF-1α could stimulate planar 2D cell migration and promote 3D cell invasion. It is considered that the underlying mechanism involved the specific binding of SDF-1α to the CXCR4 receptor on the plasma membrane of MSCs,72 thereby initiating the signaling pathways responsible for cell migration and stem cell homing. When applied in vivo, the SDF-1α-loaded hydrogel would chemoattract MSCs to the defect area, leading to efficient stem cell homing and bone regeneration.
The osteogenic ability of hydrogel is critical for efficient bone healing and bone regeneration. The osteogenic effects of the four hydrogels were analyzed using PCR and immunofluorescence staining. GelMA-SN-SDF-1α hydrogel produced a significant upregulation of osteogenesis-related genes and proteins, including ALP, RUNX2, OCN, and OPN. GelMA hydrogel loaded with SN and SDF-1α stimulated the highest expression of osteogenesis-related biomarkers, except for the OPN protein expression (Figure 5). OPN is considered the biomarker expressed in the late stage of osteogenesis. Therefore, a longer cell culture time may be required to prove that GelMA-SN-SDF-1α hydrogel is the best to stimulate OPN protein expression (Figure S2).
GelMA-SN and GelMA-SDF-1α produced slightly smaller increases. Promotion of cell spreading and migration are beneficial for osteogenesis. SN and SDF-1α appear to be synergistic in promoting osteogenesis-related biomarker expression. The degradation products SN-orthosilicic acid (Si(OH)4) and lithium may directly upregulate osteogenic protein expression.23,73–75 This upregulation can further explain the reason for GelMA-SN exhibiting a stronger osteogenic ability than GelMA-SDF-1α. Further, we explored the enhanced osteogenic activity of the GelMA-SN-SDF-1α hydrogel by investigating the amount of mineralization in ECMs. Calcium deposits in cells cultured with different hydrogel samples were evaluated using alizarin red staining. The two SN-loaded hydrogels induced further calcium deposition and presented a pattern of gradual increase in calcium mineralization. Presumably, the enhanced mineralization was due to large amounts of Si-OH on the surface of SN, inducing calcium ion distribution, followed by calcium phosphate nucleation and ultimately calcium deposition,41,76,77 rather than the unspecific absorption of Alizarin Red S regents (Figure S3).
The osteogenic ability of the GelMA-SN-SDF-1α hydrogel was further evaluated using a critical rat calvaria defect model (round defect with a diameter of 8 mm). The GelMA-SN-SDF- 1α hydrogel was injected into the defect via a syringe with a 17-G needle at room temperature. Hydrogel filled the defect and became a solid gel within 30 s after UV crosslinking. After 6 weeks of healing, the skull samples were retrieved and scanned using micro-CT. Compared with the other three hydrogels, GelMA-SN-SDF-1α accelerated bone regeneration in the defect area. When filled with GelMA-SN-SDF-1α, new bone regenerated from the edge as well as regrew in the center of the defect, indicating that a new ossification center was induced. The explanation could be that calcium phosphate nucleation was induced by SN and accumulated MSCs were attracted by released SDF-1α. It has been suggested that a secondary ossification center can stimulate bone healing and remodeling and completely restore a normal bone structure considerably faster.78 The BV/TV and BMD results supported this hypothesis. When treated with GelMA-SN-SDF-1α, the newly generated bone showed a significantly higher BV/TV and BMD than the remaining two hydrogels, indicating greater thickness and density of the new bone.
The regenerated bone was analyzed using H&E staining and Goldner-Masson trichrome staining to determine the histomorphometric characteristics. Consistent with the CT results, GelMA-SN-SDF-1α stimulated bone healing with a defect healing percentage of approximately 65%. This is quite faster than the percentage observed using the other three hydrogel samples, as determined by H&E staining. The Goldner-Masson trichrome staining results revealed that the GelMA-SN-SDF-1α hydrogel promoted the highest level of mineralized bone and osteoid formation (Figure 7B). On the one hand, the marked mineralized bone indicated that the GelMA- SN-SDF-1α hydrogel already induced considerably more mature bone formation. On the other hand, the labeled osteoid implied that the bones implanted with GelMA-SN-SDF-1α possessed the highest potential for new bone formation. Our analysis was qualitative at 6 weeks post-surgery. Future work will use longer observation durations to provide more evidence with respect to the complete healing of critical defects using GelMA-SN-SDF-1α hydrogel. The current injectable osteogenic GelMA-SN-SDF-1α hydrogel shows considerable potential for bone healing and bone regeneration because of its convenience of fabrication and its excellent osteogenic activity in vitro and in vivo.
Conclusion
In this study, we fabricated a cell-free injectable osteogenic GelMA-SN-SDF-1α hydrogel employing a simple and fast method, aiming at efficiently stimulating bone regeneration. SN and SDF-1α were introduced into GelMA hydrogel to render the hydrogel osteogenic and guide MSC homing. The injectable hydrogel demonstrated its excellent injectability, biocompatibility, osteogenic capacity, and bone generation ability in vitro and in vivo. The loaded SDF-1α presented a controlled long-term release pattern. This study will contribute to further studies for developing injectable hydrogels used for treating bone defects. Further, the hydrogel matrix possesses the potential to encapsulate growth factor to induce new blood vessels and nerve tissue formation, which would be beneficial for advanced tissue and organ regeneration.
Acknowledgment
This study was funded by the National Science Foundation of China (81871757).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Garcia-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112–121. doi:10.1016/j.bone.2015.07.007
2. Perez JR, Kouroupis D, Li DJ, Best TM, Kaplan L, Correa D. Tissue engineering and cell-based therapies for fractures and bone defects. Front Bioeng Biotechnol. 2018;6:105.
3. Zhang J, Liu W, Schnitzler V, Tancret F, Bouler JM. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 2014;10(3):1035–1049.
4. Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9(1):18. doi:10.1186/1749-799X-9-18
5. Gao C, Peng S, Feng P, Shuai C. Bone biomaterials and interactions with stem cells. Bone Res. 2017;5:17059.
6. Arcos D, Vallet-Regi M. Substituted hydroxyapatite coatings of bone implants. J Mater Chem B. 2020;8(9):1781–1800.
7. Maazouz Y, Montufar EB, Malbert J, Espanol M, Ginebra MP. Self-hardening and thermoresponsive alpha tricalcium phosphate/pluronic pastes. Acta Biomater. 2017;49:563–574. doi:10.1016/j.actbio.2016.11.043
8. Arsiwala A, Desai P, Patravale V. Recent advances in micro/nanoscale biomedical implants. J Control Release. 2014;189:25–45.
9. Wang H, Su K, Su L, Liang P, Ji P, Wang C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater Sci Eng C Mater Biol Appl. 2019;104:109908. doi:10.1016/j.msec.2019.109908
10. Rolvien T, Barbeck M, Wenisch S, Amling M, Krause M. Cellular mechanisms responsible for success and failure of bone substitute materials. Int J Mol Sci. 2018;19(10):2893. doi:10.3390/ijms19102893
11. Bai X, Gao M, Syed S, Zhuang J, Xu X, Zhang XQ. Bioactive hydrogels for bone regeneration. Bioact Mater. 2018;3(4):401–417.
12. Cunniffe GM, Curtin CM, Thompson EM, Dickson GR, O’Brien FJ. Content-dependent osteogenic response of nanohydroxyapatite: an in vitro and in vivo assessment within collagen-based scaffolds. ACS Appl Mater Interfaces. 2016;8(36):23477–23488. doi:10.1021/acsami.6b06596
13. Jimenez G, Venkateswaran S, Lopez-Ruiz E, et al. A soft 3D polyacrylate hydrogel recapitulates the cartilage niche and allows growth-factor free tissue engineering of human articular cartilage. Acta Biomater. 2019;90:146–156. doi:10.1016/j.actbio.2019.03.040
14. Gaharwar AK, Mukundan S, Karaca E, et al. Nanoclay-enriched poly(varepsilon- caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2014;20(15–16):2088–2101. doi:10.1089/ten.tea.2013.0281
15. Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2499–2506. doi:10.1016/j.biomaterials.2008.12.080
16. Mu Z, Chen K, Yuan S, et al. Gelatin nanoparticle-injectable platelet-rich fibrin double network hydrogels with local adaptability and bioactivity for enhanced osteogenesis. Adv Healthc Mater. 2020;9(5):e1901469. doi:10.1002/adhm.201901469
17. Piantanida E, Alonci G, Bertucci A, De Cola L. Design of nanocomposite injectable hydrogels for minimally invasive surgery. Acc Chem Res. 2019;52(8):2101–2112. doi:10.1021/acs.accounts.9b00114
18. Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi:10.1016/j.biomaterials.2015.08.045
19. Rinoldi C, Costantini M, Kijenska-Gawronska E, et al. Tendon tissue engineering: effects of mechanical and biochemical stimulation on stem cell alignment on cell- laden hydrogel yarns. Adv Healthc Mater. 2019;8(7):e1801218. doi:10.1002/adhm.201801218
20. Qiao Y, Liu X, Zhou X, et al. Gelatin templated polypeptide co-cross-linked hydrogel for bone regeneration. Adv Healthc Mater. 2020;9(1):e1901239. doi:10.1002/adhm.201901239
21. Gaharwar AK, Peppas NA, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng. 2014;111(3):441–453. doi:10.1002/bit.25160
22. Su D, Jiang L, Chen X, Dong J, Shao Z. Enhancing the gelation and bioactivity of injectable silk fibroin hydrogel with laponite nanoplatelets. ACS Appl Mater Interfaces. 2016;8(15):9619–9628. doi:10.1021/acsami.6b00891
23. Gaharwar AK, Mihaila SM, Swami A, et al. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv Mater. 2013;25(24):3329–3336. doi:10.1002/adma.201300584
24. Gaharwar AK, Avery RK, Assmann A, et al. Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS Nano. 2014;8(10):9833–9842. doi:10.1021/nn503719n
25. Alarcin E, Lee TY, Karuthedom S, et al. Injectable shear-thinning hydrogels for delivering osteogenic and angiogenic cells and growth factors. Biomater Sci. 2018;6(6):1604–1615. doi:10.1039/C8BM00293B
26. Cheng H, Chabok R, Guan X, et al. Synergistic interplay between the two major bone minerals, hydroxyapatite and whitlockite nanoparticles, for osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018;69:342–351. doi:10.1016/j.actbio.2018.01.016
27. Kumar S, Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone. 2012;50(4):1012–1018. doi:10.1016/j.bone.2012.01.027
28. Pajarinen J, Lin T, Gibon E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89.
29. Zimmermann CE, Gierloff M, Hedderich J, Acil Y, Wiltfang J, Terheyden H. Survival of transplanted rat bone marrow-derived osteogenic stem cells in vivo. Tissue Eng Part A. 2011;17(7–8):1147–1156. doi:10.1089/ten.tea.2009.0577
30. Liu G, Zhang Y, Liu B, Sun J, Li W, Cui L. Bone regeneration in a canine cranial model using allogeneic adipose derived stem cells and coral scaffold. Biomaterials. 2013;34(11):2655–2664. doi:10.1016/j.biomaterials.2013.01.004
31. Yow SZ, Quek CH, Yim EK, Lim CT, Leong KW. Collagen-based fibrous scaffold for spatial organization of encapsulated and seeded human mesenchymal stem cells. Biomaterials. 2009;30(6):1133–1142.
32. Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129(1):97–108. doi:10.1016/j.pharmthera.2010.09.011
33. Purcell BP, Elser JA, Mu A, Margulies KB, Burdick JA. Synergistic effects of SDF- 1alpha chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials. 2012;33(31):7849–7857. doi:10.1016/j.biomaterials.2012.07.005
34. Vanden Berg-Foels WS. In situ tissue regeneration: chemoattractants for endogenous stem cell recruitment. Tissue Eng Part B Rev. 2014;20(1):28–39. doi:10.1089/ten.teb.2013.0100
35. Andreas K, Sittinger M, Ringe J. Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol. 2014;32(9):483–492. doi:10.1016/j.tibtech.2014.06.008
36. Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem- cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697–703. doi:10.1016/S0140-6736(03)14232-8
37. Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32(12):3189–3209. doi:10.1016/j.biomaterials.2010.12.032
38. Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87(9 Suppl):S42–45. doi:10.1097/TP.0b013e3181a28533
39. He XT, Li X, Xia Y, et al. Building capacity for macrophage modulation and stem cell recruitment in high-stiffness hydrogels for complex periodontal regeneration: experimental studies in vitro and in rats. Acta Biomater. 2019;88:162–180. doi:10.1016/j.actbio.2019.02.004
40. Fong EL, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials. 2011;32(2):395–409. doi:10.1016/j.biomaterials.2010.08.101
41. Tomas H, Alves CS, Rodrigues J. Laponite(R): a key nanoplatform for biomedical applications? Nanomed Nanotechnol Biol Med. 2018;14(7):2407–2420. doi:10.1016/j.nano.2017.04.016
42. Zhao W, Jin K, Li J, Qiu X, Li S. Delivery of stromal cell-derived factor 1alpha for in situ tissue regeneration. J Biol Eng. 2017;11:22. doi:10.1186/s13036-017-0058-3
43. Cross LM, Carrow JK, Ding X, Singh KA, Gaharwar AK. Sustained and prolonged delivery of protein therapeutics from two-dimensional nanosilicates. ACS Appl Mater Interfaces. 2019;11(7):6741–6750. doi:10.1021/acsami.8b17733
44. Loessner D, Meinert C, Kaemmerer E, et al. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat Protoc. 2016;11(4):727.
45. Chen MH, Wang LL, Chung JJ, Kim Y-H, Atluri P, Burdick JA. Methods to assess shear- thinning hydrogels for application as injectable biomaterials. ACS Biomater Sci Eng. 2017;3(12):3146–3160. doi:10.1021/acsbiomaterials.7b00734
46. Priya MV, Kumar RA, Sivashanmugam A, Nair SV, Jayakumar R. Injectable amorphous chitin-agarose composite hydrogels for biomedical applications. J Funct Biomater. 2015;6(3):849–862. doi:10.3390/jfb6030849
47. Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev. 2015;82(7–8):518–529. doi:10.1002/mrd.22489
48. Spicer PP, Kretlow JD, Young S, Jansen JA, Kasper FK, Mikos AG. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc. 2012;7(10):1918. doi:10.1038/nprot.2012.113
49. Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken). 2013;296(3):378–381. doi:10.1002/ar.22641
50. Turnbull G, Clarke J, Picard F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3(3):278–314. doi:10.1016/j.bioactmat.2017.10.001
51. Trojani C, Weiss P, Michiels J-F, et al. Three-dimensional culture and differentiation of human osteogenic cells in an injectable hydroxypropylmethylcellulose hydrogel. Biomaterials. 2005;26(27):5509–5517. doi:10.1016/j.biomaterials.2005.02.001
52. Chun C, Lim HJ, Hong K-Y, Park K-H, Song S-C. The use of injectable, thermosensitive poly (organophosphazene)–RGD conjugates for the enhancement of mesenchymal stem cell osteogenic differentiation. Biomaterials. 2009;30(31):6295–6308. doi:10.1016/j.biomaterials.2009.08.011
53. Ressler A, Ródenas-Rochina J, Ivanković M, Ivanković H, Rogina A, Ferrer GG. Injectable chitosan-hydroxyapatite hydrogels promote the osteogenic differentiation of mesenchymal stem cells. Carbohydr Polym. 2018;197:469–477. doi:10.1016/j.carbpol.2018.06.029
54. Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64(12):1292–1309. doi:10.1016/j.addr.2012.01.016
55. Yang C, Han B, Cao C, Yang D, Qu X, Wang X. An injectable double-network hydrogel for the co-culture of vascular endothelial cells and bone marrow mesenchymal stem cells for simultaneously enhancing vascularization and osteogenesis. J Mater Chem B. 2018;6(47):7811–7821. doi:10.1039/C8TB02244E
56. Kim HJ, Park J-S. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Dev Reprod. 2017;21(1):1. doi:10.12717/DR.2017.21.1.001
57. Rusu E, Necula LG, Neagu AI, et al. Current status of stem cell therapy: opportunities and limitations. Turk J Biol. 2016;40(5):955–967.
58. Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi:10.1016/j.biomaterials.2010.03.064
59. Gaharwar AK, Rivera CP, Wu CJ, Schmidt G. Transparent, elastomeric and tough hydrogels from poly(ethylene glycol) and silicate nanoparticles. Acta Biomater. 2011;7(12):4139–4148. doi:10.1016/j.actbio.2011.07.023
60. Peak CW, Stein J, Gold KA, Gaharwar AK. Nanoengineered colloidal inks for 3D bioprinting. Langmuir. 2018;34(3):917–925. doi:10.1021/acs.langmuir.7b02540
61. Talebian S, Mehrali M, Taebnia N, et al. Self-healing hydrogels: the next paradigm shift in tissue engineering? Adv Sci. 2019;6(16):1801664.
62. Cummins HZ. Liquid, glass, gel: the phases of colloidal Laponite. J Non Cryst Solids. 2007;353(41):3891–3905. doi:10.1016/j.jnoncrysol.2007.02.066
63. Xu P, Lan Y, Xing Z, Eiser E. Liquid crystalline behaviour of self-assembled LAPONITE(R)/PLL-PEG nanocomposites. Soft Matter. 2018;14(15):2782–2788.
64. Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi:10.1016/j.bone.2016.02.020
65. Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104.
66. Cipitria A, Boettcher K, Schoenhals S, et al. In-situ tissue regeneration through SDF- 1alpha driven cell recruitment and stiffness-mediated bone regeneration in a critical- sized segmental femoral defect. Acta Biomater. 2017;60:50–63. doi:10.1016/j.actbio.2017.07.032
67. Dawson JI, Oreffo RO. Clay: new opportunities for tissue regeneration and biomaterial design. Adv Mater. 2013;25(30):4069–4086. doi:10.1002/adma.201301034
68. Li X, He XT, Yin Y, Wu RX, Tian BM, Chen FM. Administration of signalling molecules dictates stem cell homing for in situ regeneration. J Cell Mol Med. 2017;21(12):3162–3177.
69. Schell H, Duda GN, Peters A, Tsitsilonis S, Johnson KA, Schmidt-Bleek K. The haematoma and its role in bone healing. J Exp Orthop. 2017;4(1):5.
70. Klotz BJ, Gawlitta D, Rosenberg A, Malda J, Melchels FPW. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016;34(5):394–407. doi:10.1016/j.tibtech.2016.01.002
71. Yu JR, Janssen M, Liang BJ, Huang HC, Fisher JP. A liposome/gelatin methacrylate nanocomposite hydrogel system for delivery of stromal cell-derived factor-1alpha and stimulation of cell migration. Acta Biomater. 2020;108:67–76. doi:10.1016/j.actbio.2020.03.015
72. Liu X, Duan B, Cheng Z, et al. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2(10):845–854. doi:10.1007/s13238-011-1097-z
73. Cheng H, Yue K, Kazemzadeh-Narbat M, et al. Mussel-inspired multifunctional hydrogel coating for prevention of infections and enhanced osteogenesis. ACS Appl Mater Interfaces. 2017;9(13):11428–11439. doi:10.1021/acsami.6b16779
74. Ganesh N, Jayakumar R, Koyakutty M, Mony U, Nair SV. Embedded silica nanoparticles in poly(caprolactone) nanofibrous scaffolds enhanced osteogenic potential for bone tissue engineering. Tissue Eng Part A. 2012;18(17–18):1867–1881. doi:10.1089/ten.tea.2012.0167
75. Gaharwar AK, Kishore V, Rivera C, et al. Physically crosslinked nanocomposites from silicate-crosslinked PEO: mechanical properties and osteogenic differentiation of human mesenchymal stem cells. Macromol Biosci. 2012;12(6):779–793. doi:10.1002/mabi.201100508
76. Toworfe GK, Composto RJ, Shapiro IM, Ducheyne P. Nucleation and growth of calcium phosphate on amine-, carboxyl- and hydroxyl-silane self-assembled monolayers. Biomaterials. 2006;27(4):631–642. doi:10.1016/j.biomaterials.2005.06.017
77. Jatav S, Joshi YM. Chemical stability of Laponite in aqueous media. Appl Clay Sci. 2014;97–98:72–77.
78. Nyary T, Scammell BE. Principles of bone and joint injuries and their healing. Surgery (Oxford). 2018;36(1):7–14. doi:10.1016/j.mpsur.2017.10.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.