Back to Journals » International Journal of Nanomedicine » Volume 15
Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications
Authors Chen Y, Lu Y, Lee RJ, Xiang G
Received 30 January 2020
Accepted for publication 7 April 2020
Published 1 May 2020 Volume 2020:15 Pages 3099—3120
DOI https://doi.org/10.2147/IJN.S210320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Yan Chen,1 Yao Lu,1 Robert J Lee,2 Guangya Xiang1
1School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Division of Pharmaceutics and Pharmacology, The Ohio State University, Columbus, OH, USA
Correspondence: Guangya Xiang
School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Hangkong Road 13, Wuhan, Hubei 430030, People’s Republic of China
Tel +86 27 83692793
Email [email protected]
Abstract: Curcumin, a yellow-colored polyphenol extracted from the rhizome of turmeric root, is commonly used as a spice and nutritional supplement. It exhibits many pharmacological activities such as anti-inflammatory, anti-bacterial, anti-cancer, anti-Alzheimer, and anti-fungal. However, the therapeutic application of curcumin is limited by its extremely low solubility in aqueous buffer, instability in body fluids, and rapid metabolism. Nano delivery system has shown excellent potential to improve the solubility, biocompatibility and therapeutic effect of curcumin. In this review, we focus on the recent development of nano encapsulated curcumin and its potential for biomedical applications.
Keywords: curcumin, nanoformulations, bioavailability, nanoparticles, therapy
Introduction
Curcumin (Cur), 1,7-bis(4-hydroxy-3-methoxy-phenyl)-1,6-heptadiene-3,5-dione,1 is a yellow-colored polyphenol extracted from the rhizome of turmeric root (Curcuma longa).2,3 Commercial curcuminoid powder is a mixture of curcumin, demethoxycurcumin and bisdemethoxycurcumin in a ratio of 77:17:3 (Figure 1). It has been used as a spice and dietary supplement as well as a component of many traditional medicines in the oriental world for many centuries.4,5 In recent decades, curcumin is shown to exhibit anti-inflammatory, anti-bacterial, anti-cancer, anti-Alzheimer, anti-fungus and so on.6–12
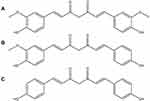 |
Figure 1 Chemical structures of (A) curcumin, (B) demethoxycurcumin and (C) bisdemethoxycurcumin. |
However, its poor bioavailability, extremely low solubility in aqueous buffer, instability in body fluids, and rapid metabolism have jeopardized the therapeutic applications of curcumin in clinic.13,14 Its water solubility is only at 0.0004 mg/mL at pH 7.3.15 Besides this, soluble curcumin molecules are extremely sensitive at physiological conditions owing to rapid hydrolytic degradation.16 Numerous preclinical and clinical studies in mice and humans showed a low bioavailability of curcumin.17,18 Although 12 g of curcumin administered orally in humans was still in the safe range, the final curcumin level existed in serum was approximately only 50 ng/mL, this reflected a minimum availability of curcumin in the systemic circulation to achieve its therapeutic effects.2,19
To circumvent these obstacles, researchers encapsulate curcumin into nanocarriers, such as liposomes, polymeric micelles, polymeric nanoparticles, mesoporous silica nanoparticles, protein-based nanocarriers, solid lipid nanoparticles, cyclodextrins, nanogels, nanocrystals etc.20,21 What are nanocarriers in pharmaceutical materials? Nanocarriers generally refer to pharmaceutical materials and products of approximately 1 nm to 100 nm;22 however, the US Food and Drug Administration (FDA) often considers 1000 nm as an upper limit.23 Why researchers encapsulate drugs into nanocarriers? Because of the high surface area to volume ratio offered by nanocarriers, both the solubility and dissolution rate of the drugs can be increased. Furthermore, the small particle size can prolong the drug’s maintenance in the systemic circulation, modify drug distribution, and permit drug targeting and transport across barriers. Due to the aforementioned reasons, curcumin encapsulated in nanoformulations can effectively improve its solubility and bioavailability.24,25
In this review, we will retrospect the different nano encapsulated curcumin formulations and their potential for biomedical applications.
Nano Formulations
Liposomes
Liposomes are the first nanocarriers approved by FDA for clinical application.26 As a spherical bilayer vesicle with an aqueous core, a liposome is composed of phospholipid bilayer, and the particle size is generally between 25−1000 nm.27 Hydrophilic drugs can be loaded in the aqueous cores and lipophilic drugs can be incorporated into liposome bilayers. According to the structure, liposomes can be divided into single unilamellar vesicles (SUVs), large unilamellar vesicles (LUVs) and multilamellar vesicles (MLVs).28 The preparation methods of liposome include thin-film dispersion method, ultrasonic dispersion method, injection method, reverse phase evaporation method etc.29 Since their compositions are the same as human cell membranes, liposomes have incomparable advantages as targeted drug carriers. Owing to their properties of non-toxic, non-immunogenicity, assorted carrying capacity and easy preparation, numerous liposomal formulations have been developed and some liposomal drugs have been launched.26,30,31 However, the shortcomings of low encapsulation efficiency, easy fusion and leakage are potential obstacles for further application.32
In recent years, plenty of Cur-loaded liposomal formulations have been developed. Shi et al prepared curcumin-loaded liposomes (Lipo-cur) composed of lecithin and cholesterol to inhibit radiation pneumonitis and sensitize lung carcinoma to radiation by thin-film dispersion method.33 Lipo-cur had an average particle size of 114.9 nm, a zeta potential of −2.62 mV, a drug loading of 5% and an encapsulation efficiency of 90.1%, respectively. Liposomal formulation significantly increased the water solubility and reduced the potential long-term toxicity of curcumin. Radiation pneumonitis (RP) is a major dose-limiting side effect during thoracic radiotherapy.34 It has been reported that curcumin was a radioprotective agent and could reduce the mortality rate in the irradiated mice.35 In this study, it was observed that Lipo-cur inhibited nuclear factor-kappaB (NF-κB) pathway and downregulated relevant inflammatory factors induced by thoracic irradiation, including transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and IL-8. In addition, the combined treatment of Lipo-cur and radiotherapy exhibited enhanced anti-cancer efficiency in LL/2 Lewis lung carcinoma cell model. Increased intratumoral apoptosis and microvessel responses to irradiation were also reported. In another study, curcumin-loaded cationic liposomes were produced with the addition of didodecyldimethylammonium bromide (DDAB).36 Compared to DDAB-free liposomes, DDAB-containing liposomes improved cell uptake and cytotoxicity of curcumin, likely due to the increased cell internalization capability of cationic liposomes. Dhule et al prepared curcumin-loaded 2-hydroxypropyl-γ-cyclodextrin liposomal nanoparticles (HPγCD-curcumin liposomes) for the treatment of osteosarcoma.37 HPγCD-curcumin system was first made to improve the solubility of Cur and then was encapsulated in the aqueous core of liposomes. The results of the cytotoxicity study showed that HPγCD-curcumin liposomes exhibited increased anticancer effect against KHOS osteosarcoma cells and MCF-7 breast cancer cells. In addition, more apoptotic were observed in the tumors treated with HPγCD-curcumin liposomes in vivo.
In order to enhance the antitumor efficiency of liposomal formulations, some new functions, such as long-circulating, ligand-mediated targeting, and responsive drug release were introduced into the design of liposomes.38,39 Mahmud et al prepared different types of long-circulating Cur-loaded liposomes by thin-film hydration followed by extrusion technique.40 1,2-distearoyl-sn-glycero-phosphoethanolamine-N-(poly [ethylene glycol]2000) (DSPE-PEG2000) was used as the long-circulating lipid material. By comparing the parameters of particle size, zeta potential, shape and stability, it was found that PEGylated, cholesterol-free liposomes based on hydrogenated soy phosphatidylcholine (HSPC) at a drug-to-lipid molar ratio of 1:19 was the best formulation. This formulation had a low drug release in plasma and high stability in vitro. Furthermore, Cur-loaded long-circulating liposomes significantly induced the reactive oxygen species (ROS) level and upregulated the expression of caspase 3/7, leading to higher cytotoxic activity on AsPC-1 and BxPC-3 pancreatic cancer cells. Folate receptor (FR)-targeted liposomal Cur (F-CUR-L) was reported in our previous research (Figure 2).41 F-CUR-L composed of cholesterol, Egg phosphatidylcholine (Egg PC), DSPE-PEG2000, folate-poly [ethylene glycol]3350-cholesteryl hemisuccinate (folate-PEG3350-CHEMS) was prepared by thin-film dispersion. In a cellular uptake experiment, the mean fluorescence intensity of F-CUR-L was higher than that of non-targeted CUR-L both in KB and Hela cells, which overexpress FRs. However, in A549 cells that lack FRs, the uptake amounts of F-CUR-L, CUR and F-CUR-L+1 mM folic acid were all extremely low and nearly the same. Moreover, F-CUR-L exhibited enhanced antitumor activity than non-targeted CUR-L, demonstrating the increased tumor targeting by a FR-mediated endocytosis pathway. Ju et al fabricated a novel pH-sensitive liposomes using N-(3- Aminopropyl) imidazole-cholesterol (IM-Chol) and phosphatidylcholine (PC).42 Imidazolyl group has an electron lone pair on the unsaturated nitrogen and a pKa of about 6.0. It was easily protonated under the slightly acidic environment of a tumor and destroyed the structure of liposomes, thus giving liposomes acid-sensitive property (Figure 3). In a drug release study, 72.5% of Cur in liposomes was released at pH 5.0 while only 35.25% of the drug was released at pH 7.4 after incubation of 24 h.
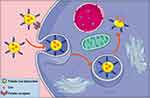 |
Figure 2 The uptake of F-CUR-L through folate receptor-mediated endocytosis pathway. |
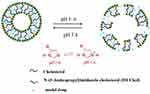 |
Figure 3 Schematic representation of pH-triggered release of IM-Chol liposomes. Reprinted from Int J Pharm. 518. Ju L, Cailin F, Wenlan W, et al. Preparation and properties evaluation of a novel pH-sensitive liposomes based on imidazole-modified cholesterol derivatives, 213–219, Copyright 2017, with permission from Elsevier.42 |
Liposomes are superior nanocarriers for combined drug delivery due to the ability of co-encapsulating both hydrophilic and lipophilic drugs. Ruttala et al developed PEGylated liposomes co-loading Cur and Paclitaxel (PTX) for enhanced synergistic antitumor efficacy.43 In this research, PTX-loaded albumin nanoparticles (APN) were encapsulated in the aqueous core and Cur was loaded in the liposome bilayers. Also, the ratio of PTX and Cur at 1:1 was chosen to prepare co-loaded liposomes (CL-APN) because of the significant inhibition of cell proliferation compared to other ratios. CL-APN had an average particle size of 250 nm and a zeta potential of 7 mV. In an MTT assay, CL-APN showed more cytotoxicity than individual drug-loaded liposomes in MCF-7 and B16F10 cells. It was believed that Cur downregulated the expression of NF-κB and protein kinase B (Akt), resulting in an increased therapeutic efficacy of PTX. Moreover, more early and late apoptosis, stronger G2/M arrest, and enhanced subG1-cell population were observed upon the treatment of co-loaded liposomes. In our previous work, Cur and cisplatin co-loaded liposomes were prepared and showed a synergistic effect against hepatocellular carcinoma (HCC).44 Furthermore, enhanced intracellular ROS was also detected during the treatment of liposomes.
Polymeric Micelles
Polymeric micelles were used as nanoscale drug delivery system (nano-DDS) in the late 1980s.45 Micelles are thermodynamically stable colloidal solutions, formed by self-assembly of amphiphiles in water above the critical micelle concentration (CMC).46 Structurally, the micelles are composed of hydrophilic shell and hydrophobic core which can load hydrophobic drugs. Various amphiphilic polymers, such as diblock, triblock and graft copolymers have been synthesized to prepare micelles.47–49 Among these, common hydrophilic blocks included poly (ethylene glycol) (PEG), chitosan and polyvinyl pyrrolidone (PVP). In addition, hydrophobic materials such as distearoyl phosphoethanolamine (DSPE), poly (lactic acid) (PLA), dioleoyl phosphatidylethanolamine (DOPE), poly (ε-caprolactone) (PCL), poly (lactide-co-glycolide) (PLGA), cholesteryl hemisuccinate (CHEMS) and vitamin E were used to form the cores of micelles. The size of micelles is generally ranging between 20 and 100 nm, which allows micelles overflow from tumor vessel walls and enter cancer cells.50 Meanwhile, with the incorporation of an outer hydrophilic shell, micelles are able to escape the clearance of reticuloendothelial system (RES) and increase systemic circulation time of drugs.
As shown in Table 1, numerous Cur-loaded polymeric micelles have been developed. Liu et al synthesized monomethyl poly (ethylene glycol)-poly (ε-caprolactone) copolymer (MPEG-PCL) and prepared Cur-loaded micelles (Cur-M) by one-step solid dispersion method.51 Cur-M had a small particle size (28.2 nm) with good dispersity, whereas the drug loading capacity was 14.84% and the encapsulation efficiency was 98.91%, respectively. Compared to free curcumin, Cur-M showed a sustained release behavior at pH 7.4 in vitro and ~ 60% of drugs were released in one week. Moreover, IC50 value for Cur-M was a little lower than that for free drugs on 4T1 cells since the enhanced cellular uptake of micelles. The similar result was also reported by Manjili et al using the MPEG-PCL micelles.52 Furthermore, enhanced chemotherapeutic efficacy, improved anti-metastasis activity and prolonged survival were observed in 4T1 xenograft tumor-bearing nude mice. Another Cur-loaded polymeric micelle consisted of PLGA-PEG-PLGA copolymer was prepared by a solvent-dialysis method and the CMC of PLGA-PEG-PLGA was at a lower concentration (2.82×10−5 g/mL) at room temperature which indicated the strong hydrophobicity of copolymer.53 The average particle size, zeta potential, drug loading capacity and encapsulated efficiency were 26.29 nm, −0.71 mV, 70% and 6.4%, respectively. In pharmacokinetics analysis, the plasma parameters including distribution half-life (t1/2α), elimination half-life (t1/2β), area under the curve (AUC0–∞) and mean residence time (MRT0–∞) of Cur micelles were 2.48, 4.54, 1.31, and 2.67-times than that of free Cur solution. The biodistribution study showed that Cur-loaded micelles reduced drug distribution in liver and spleen, and increased distribution in lung and kidney. Moreover, enhanced accumulation of Cur in the brain was detected and it may be related to the breakthrough of blood-brain barrier (BBB) because of the smaller diameter and the nearly neutrally charged property of Cur-loaded polymeric micelles. Manjili et al prepared poly (caprolactone)–poly (ethylene glycol)-poly (caprolactone) (PCL-PEG-PCL) nanoparticles with a nano-precipitation method.54 The results showed that CUR-loaded nanoparticles significantly inhibited cell proliferation, induced apoptosis and enhanced anti-tumor immunity stimulation. Phan et al synthesized PLA-PEG copolymers at the weight ratio of PLA/PEG in 3:1, 2:1, 1:1, 1:2 and 1:3, and then loaded curcumin in micelles by emulsification/solvent evaporation method.55 It was reported that decreasing the amount of PLA (from 3: 1 to 1: 3) resulted in the reduction of drug loading capacity. However, a higher loading capacity of PLA-PEG micelles did not lead to greater cytotoxicity of the samples. Cur/PLA-PEG micelles achieved the most effective inhibition on HepG2 cells at the PLA/PEG ratio of 1:3. It may be attributed to the smaller size, higher release rate and better biocompatibility.
 |
Table 1 Different Types of Cur-Loaded Polymeric Micelles |
In order to increase the intracellular uptake and improve therapeutic efficacy, the surface of micelles was modified with various targeted ligands to recognize tumor cell surface-over-expressed receptors.56,57 Jin et al have developed an epidermal growth factor receptor (EGFR)-targeted polymeric micelle to deliver curcumin for breast cancer therapy.58 Cur-loaded PLGA-PEG nanoparticles (Cur-NPs) were synthesized by the emulsification solvent evaporation method. Then EGFR-targeted GE11 peptides were chemically conjugated to Cur-NPs to prepare GE11-modified Cur-loaded PLGA-PEG nanoparticles (GE11-Cur-NPs). The release profiles of Cur-NPs and GE11-Cur-NPs at pH 7.4 PBS were similar for approximately 24 hours. Intracellular uptake test showed that GE11-Cur-NPs delivered more drugs to EGFR-overexpressing MCF-7 cells compared with EGFR-low-expression MCF-10A cells. Furthermore, GE11-Cur-NPs exhibited higher intracellular fluorescence intensity than Cur-NPs after MCF-7 cells were incubated with both drug formulations for 2, 6 and 12 h. In the studies of pharmacodynamics, loading Cur into targeted PLGA-PEG NPs significantly enhanced the anti-cancer effect both in vitro and in vivo. Yang et al investigated the antitumor activity of FR-targeted micelles loaded with curcumin.13 In this study, Folate-PEG3000-PLA2000, a type of FR-targeted material was first synthesized. Then two kinds of micelles were prepared with mPEG2000-PLA2000 (Cur-PPs) or mPEG2000-PLA2000/Folate-PEG3000-PLA2000 (Cur-FPPs) through the thin film-hydration method. In an in vitro cellular uptake study, coumarin 6 was used as a model drug. Results showed that modification with folate on the surface of micelles could increase cellular uptake both on MCF-7 or HepG2 cells through folate mediated targeting. Meanwhile, enhanced uptake of Cur resulted in increased anti-cancer activity. The IC50 value of Cur-FPPs was 42.9 μg/mL while that of Cur-PPs was 52.9 μg/mL on MCF-7 cells, and the IC50 values were 43.3 and 54.3 μg/mL for Cur-FPPs and Cur-PPs on HepG2 cells, respectively.
In recent years, tumor microenvironment-responsive polymeric micelles have been widely investigated to deliver Cur and other drugs.59 Zhang et al developed pH-sensitive micelles prepared from D-a-tocopheryl polyethylene glycol 1000-block-poly (β-amino ester) (TPGS-PAE) polymers for combined delivery of Dox and Cur.60 TPGS-PAE was synthesized by the Michael addition polymerization method, and Dox/Cur co-loaded NPs (D + C/NPs) were prepared by a one-step solvent evaporation method. D + C/NPs could rapidly release entrapped drugs under acidic environment (Figure 4). After incubation of 192 h, 59.37% of Dox and 66.63% of Cur in NPs were released at pH 7.4, whereas over 90% of drugs were released at pH 5.8.60 In another study, redox-responsive polymeric micelles, composed by monomethoxy-poly (ethylene glycol)-chitosan-S-S-hexadecyl (C16-SS-CS-mPEG) were designed for enhanced anti-cancer efficacy.61 Moreover, monomethoxy-poly (ethylene glycol)-chitosan-C-C-hexadecyl (C16-CC-CS-mPEG) without a redox-responsive disulfide bond was synthesized as a control using the same method (Figure 5). In the presence of reducing agents in vitro, it was observed that more drugs were released from C16-SS-CS-mPEG@Cur micelles compared with C16-CC-CS-mPEG@Cur micelles. The similar results were found in the intracellular drug release study. Furthermore, C16-SS-CS-mPEG@Cur efficiently enhanced cellular uptake of Cur, leading to improved bioactivity both in vitro and in vivo.
 |
Figure 4 In vitro release profiles of (A) Dox and (B) Cur from D + C/NPs at pH 7.4 or 5.8. Reprinted from Acta Biomater. 58. Zhang J, Li J, Shi Z, et al. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities, 349–364, Copyright 2017, with permission from Elsevier.60 |
 |
Figure 5 Chemical structures of (A) C16-SS-CS-mPEG and (B) C16-CC-CS-mPEG. |
Polymeric Nanoparticles
Polymeric nanoparticles are solid colloidal particles formed from polymer materials. Owing to their good biodegradability, excellent biocompatibility, controllable structure, and easy industrial production, polymeric nanoparticles have attracted widespread attention and research.62–64 In recent years, numerous natural and synthetic polymers have been used to prepare polymeric nanoparticles, for example, PLGA, poly(butyl cyanoacrylate) (PBCA), N-isopropylacrylamide (NIPAAM), polyvinyl alcohol (PVA), polyamidoamine (PAMAM)65 and chitosan (CS).
PLGA
PLGA is polymerized from lactic acid and glycolic acid. It is an organic polymer with good biocompatibility and biodegradability. In the United States, PLGA passed FDA certification and was officially included in the United States Pharmacopoeia as a pharmaceutical excipient.66
The improved oral bioavailability of Cur encapsulated by PLGA nanoparticles was reported. Xie et al produced Cur-loaded PLGA nanoparticles (CUR-PLGA-NPs) with a particle size of about 200 nm by a solvent evaporation method.67 The drug loading capacity and encapsulation efficiency were 5.75% and 91.96%, respectively. In addition, the solubility of CUR-PLGA-NPs was 4.35 mg/mL which was much higher than that of free Cur (6.79 μg/mL). In a pharmacokinetic study in vivo, rats were orally administrated of CUR-PLGA-NPs and free Cur at a dose of 100 mg/kg. The results indicated that PLGA NPs improved the oral bioavailability of Cur at 5.6-fold and had a longer half-life compared to free Cur. The authors thought that the improved oral bioavailability was associated with increased water solubility, higher release rate, improved absorption, inhibition of P-glycoprotein-mediated efflux and increased residence time in the intestinal cavity. Meanwhile, a similar result of improved oral bioavailability of Cur-loaded PLGA NPs was reported by Shaikh et al.68 The relative bioavailability of Cur entrapped nanoparticles was 9-fold compared with that of curcumin administered with piperine as an absorption enhancer.
PLGA nanoparticles are widely used in cancer therapy due to their good biocompatibility and stability.69–71 Yallapu et al prepared Cur-loaded PLGA nanoparticles using PVA and poly (L-lysine) (PLL) as stabilizers by a nano-precipitation technique.72 The particle size of Cur-loaded PLGA nanoparticles was decreased from 560.4 nm to 95.6 nm with the increased concentration of PVA from 0 to 1% (w/v). Moreover, the addition of PLL further reduced the size of nanoparticles (76.2 nm). The zeta potential values of all nanoformulations were close to 0 mV, indicating a better stability.73 Furthermore, higher drug encapsulation, increased Cur solubility, sustained drug release and enhanced intracellular uptake in A2780CP and MDA-MB-231 cells were observed when Cur-loaded nanoparticles were prepared with 1% PVA in the presence of PLL (Nano-CUR6). Thus, Nano-CUR6 formulation was chosen for further pharmacodynamics studies. In an in vitro cytotoxicity study, IC50 values for nano-CUR6 and free Cur were found to be 13.9 and 15.2 μM in A2780CP cells, and 9.1 and 16.4 μM in MDA-MB-231 cell, respectively. In addition, nano-CUR6 significantly inhibited colony formation, suppressed pro-survival genes and enhanced apoptosis of cancer cells. These data clearly indicated the superior efficacy of Cur-loaded PLGA nanoparticles for cancer therapy.
NIPAAM
NIPAAM is one of the most commonly used temperature-sensitive polymers with a low critical solution temperature (LCST) at 32 °C74 It is hydrophobic when the temperature is higher than LCST while it is hydrophilic when the temperature is lower than LCST. Owing to this reversible sensitivity to environmental temperature stimuli, NIPAAM copolymers are widely used in drug delivery, chromatographic analysis, and other fields.75–77
Nanoparticles made from NIPAAM were widely investigated. Lim et al synthesized nanocurcumin using NIPAAM, vinylpyrrolidone (VP) and acrylic acid (AA).78 MTT assays showed that curcumin nanoparticles significantly inhibited the growth of DAOY, D283Med and HSR-GBM1 brain tumor cells due to apoptotic induction and G2/M cell cycle arrest. In addition, nanocurcumin reduced the clonogenicity of DAOY, D283Med, U87, and HSR-GBM1 brain tumor cells, and the CD133-positive stem-like fraction in JHH-GBM14 and HSR-GBM1 glioblastoma cells. Downregulation of IGF, STAT3, Akt and Hh were also observed, indicating that Cur-loaded NPs could modulate the signaling pathways affecting both survival and neoplastic stem cell phenotype. Zeighamian et al prepared Cur-loaded nanoparticles from N-isopropylacrylamide-methacrylic acid (NIPAAm-MAA) using a post-polymerization method.79 NPs showed higher cytotoxicity against MCF-7 breast cancer cells. After 72h incubation, the IC50 values of free Cur and Cur-loaded NIPAAm-MAA NPs were 13.7 and 7.3μM, respectively.
PBCA
PBCA nanoparticles were widely used in targeted drug delivery systems due to their good biocompatibility, biodegradability, and non-immunogenicity.80,81 The commonly used preparation methods of PBCA nanoparticles are emulsion polymerization method and interfacial polycondensation method. Drugs are loaded in the porous structure of nanoparticles.
To enhance the transport of curcumin to the brain, Sun et al prepared Cur-loaded PBCA nanoparticles (CUR-PBCN) using an anionic polymerization method.82 CUR-PBCN had a core-shell structure with an average particle size of 152.0 nm and a drug loading of 21.1%. Moreover, CUR-PBCN slowly released 75% of drugs without a burst within 24 h, indicating a sustained-release property of nanoparticles. In an in vivo pharmacokinetic and biodistribution study, Kunming mice were given curcumin solution at a dose of 10 mg/kg or CUR-PBCN at a dose of 5 mg/kg by tail intravenous injection. The results showed that the mean residence time of CUR-PBCN was 14-fold that of free Cur in plasma. Furthermore, nanoparticles improved AUC0–∞ of free Cur solution at 2.53-fold in the brain. In conclusion, the research demonstrated that PBCA nanoparticles could enhance the transport of Cur to the brain and have the potential to cross BBB. A novel PBCA nanoparticles coated with chitosan was synthesized through the emulsion polymerization method for the treatment of hepatocellular carcinoma.83 In vitro, the results showed that curcumin nanoparticles could suppress the expression of COX-2 and VEGF, leading to increased anti-angiogenic activity. In vivo, Cur-loaded PBCA nanoparticles inhibited the growth of hepatocellular carcinoma in mice xenograft models and suppressed tumor angiogenesis.
Chitosan
Chitosan, a semi-synthetic polysaccharide is a linear polymer derived from chitin. Due to its good biodegradability, biocompatibility, non-immunogenicity, non-irritating, non-pyrogenic and non-toxicity, chitosan can be used as an ideal carrier material for drug delivery.84 Recently, chitosan-based nanoparticles have been widely reported for pharmaceutical and biomedical applications.85,86
Chuah et al produced curcumin-containing chitosan nanoparticles with a mean particle size of 340 nm, polydispersity index of 0.352 and zeta potential of 43.7 mV.87 In a cytotoxicity assay, the IC50 was about 10 μM for free curcumin and less than 10 μM for CUR-CS-NP after 72 h incubation on HT-29 cells. In addition, increased cellular uptake and induced cell apoptosis were also observed upon the treatment of nanoparticles, indicating the potential of CUR-CS-NP for colorectal cancer therapy. In a recent study, Cur was encapsulated into nanoparticles based on folate modified chitosan for FR-targeting.88 The results of the cytotoxicity test showed that curcumin loaded folate-modified-chitosan-nanoparticles are potential carriers for targeted therapy of breast cancer.
Mesoporous Silica Nanoparticles
In recent years, porous materials, such as mesoporous silica nanoparticles (MSNs), metal-organic frameworks89 and iron oxide-gold nanoparticles90 have been widely used in drug delivery. Kresge et al first used surfactants as structure directing agents to prepare highly ordered MSNs with uniform pore sizes in 1992.91 In the past decade, MSNs have been immensely explored due to their unique properties such as good biocompatibility, large specific surface area, tunable pore sizes and volumes and easily surface modification. MSNs were firstly introduced as promising carriers for drug delivery by Vallet-Regi et al in the early 2000s.92 In addition, they have been projected to be a type of nontoxic candidate for biocatalysis, bioimaging and biosensor.93,94 Microwave synthesis, template synthesis, hydrothermal synthesis and sol-gel method are the most common method to prepare MSNs.95 Among these, template synthesis method using surfactants is the most commonly used method due to the mild conditions and convenience.
The improved solubility and enhanced cell cytotoxicity of Cur encapsulated by mesoporous silica nanoparticles (MSNs) were reported. Jambhrunkar et al prepared CUR-loaded mesoporous silica nanoparticles (MCM-41-CUR) with a mean size of 190 nm through a rotary evaporator technique.96 The adsorption of curcumin in the nanopores of MSNs was achieved via hydrogen-bond interaction (Figure 6). MCM-41-CUR improved the curcumin solubility and increased the in vitro release compared with free Cur and MCM-41-CUR PM, the physical mixture of MCM-41. Consequently, Cur loaded MCM-41 nanoparticles significantly enhanced the cell cytotoxicity in SCC-25 cells due to the activation of caspase-3 and the inhibition of PcG proteins. Moreover, the amino ligands modificated mesoporous silica nanoparticles were also investigated.97,98 The drug loading capacity of MSNs functionalized with polyamino ligands (MSU-2-CP-TR-Curc and MCM-41-CP-TR-Curc) was 4.3% and 3.6% respectively, which was higher than that of MSNs functionalized with monoamino ligands (MSU-2-AP-CUR: 3.5%; MCM-41-AP-CUR:3.5%). The results indicated that modification of polyamino ligands on the surface of MSNs increased the drug loading capacity. Thus, they proposed that curcumin was encapsulated in MSNs by the interactions between the amino groups of silica materials and the polar groups of curcumin. Chen et al constructed a novel type of folate-receptor targeted mesoporous silica nanoparticles loaded curcumin via pH-sensitive schiff base reactions (FA-MSN-N=C-Cur).99 It was found that the average size of the nanoparticles increased from 136 nm to 165 nm with the modification of 3-aminopropyl trimethoxysilane (APTES), Cur and folate. In a study of pH-responsive release in vitro, the released amount of curcumin from nanoparticles at pH 7.4, 6.5 and 5.0 was 11.7%, 32.5% and 58.6%, indicating the pH-responsive release property of FA-MSN-N=C-Cur. In addition, FA-MSN-N=C-Cur could effectively target to FA receptor over-expressed MCF-7 cells. The MTT assay showed that FA-MSN-N=C-Cur increased the cell cytotoxicity in MCF-7 cells through FA-mediated endocytosis and tumor acidic environment-responsive release.
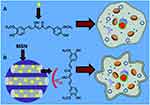 |
Figure 6 Schematic representation comparing cytotoxic effect of (A) pure curcumin and (B) curcumin loaded MCM-41on SCC-25 cells. MCM-41-CUR was achieved via hydrogen-bond interaction and showed higher cytotoxicity. Reprinted from RSC Adv. 4. Jambhrunkar S, Karmakar S, Popat A, et al. Mesoporous silica nanoparticles enhance the cytotoxicity of curcumin, 709–712, Copyright 2014, with permission from The Royal Society of Chemistry.96 |
In a recent study, curcumin was loaded into MSN grafted of polyethyleneimine (PEI) and folic acid (FA) for targeted drug delivery.100 The enhanced cellular uptake and increased cytotoxicity of MSN-PEI-FA confirmed the significance of nanocarriers conjugated with folic acid. Similarly, in another study, Li et al developed MSN modified with PEI-FA (MSN-PEI-FA) and hyaluronic acid (MSN-HA) via disulphide bonds to achieve FA receptor- or CD44-targeting.101
Surface modification of mesoporous silica nanoparticles with polymers plays an effective role in improving biocompatibility, providing controlled drug release, enhancing therapeutic effect and decreasing systemic toxicity. Elbialy et al prepared Cur-loaded PEGylated MSNs (PEG-MSNPs-Cur) with an average particle size of 184.6 nm, zeta potential of 20.8 mV and loading efficiency of 92.4%.102 The nanoparticles released about 52% of loaded drugs within 192 h at pH 5.5 while only 1% of Cur was released at pH 7.4. In addition, IC50 values for PEG-MSNPs-Cur after 24 h incubation were 20 and 28 μg/mL for HepG2 and HeLa cells, which were much lower than free Cur. In another study, Mamani et al produced guanidine functionalized PEGylated MSNs for the treatment of breast cancer.103 The characteristics of the nanoparticles, including high loading efficiency, biocompatibility, pH-sensitive, and penetrability made it a promising system for delivery of curcumin. Lipid bilayer and chitosan were also used to modify MSNs for enhanced transport of curcumin.104,105 Nasab et al developed the surface-modified MSNs with chitosan (CS-MCM-41) for glioblastoma treatment.106 In this study, CS-MCM-41 slowly released about 42.72% of their content at pH 5.5 in 96 h due to covering the porous surface of MCM-41. Moreover, this nanocarrier increased the anti-cancer efficiency of curcumin against U87MG cells. The IC50 of CS-MCM-41 and free curcumin were 5.21 and 15.20 μg/mL, respectively.
Protein-Based Nanocarriers
As a natural biomaterial with unique advantages, protein has excellent non-immunogenicity, biocompatibility, biodegradability and low cost. Protein-based nanocarriers have been widely used in the field of biomedicine.107,108 In recent years, many forms of protein nanoparticles for Cur delivery were developed, such as bovine serum albumin (BSA), human serum albumin (HSA), ovalbumin (OVA), zein, casein and silk fibroin (SF) nanoparticles.
BSA
Albumin nanoparticle has a lot of superior properties that make it an attractive nanocarrier system. Albumin is biocompatible, nontoxic, non-immunogenic, biodegradable, easy to purify and soluble in water. The preparation of albumin nanoparticles can combine the advantages of both nanoparticles and albumin. Also, a noteworthy amount of drugs can be embedded in the hydrophobic pocket of albumin through hydrophobic interaction. BSA has a molecular weight of 69,323 Da and an isoelectric point of 4.7 in water at 25 °C.109 BSA has been widely concerned and applied for drug delivery due to its low cost, easy access and ease of purification.
It was reported that polyphenols-BSA bindings occurs by hydrophilic and hydrophobic interactions.110 The binding constants of genistein, resveratrol and curcumin were 1.26 ×104 M−1, 2.52×104 M−1 and 3.33 × 104 M−1, respectively. These results indicated that the affinity of curcumin to BSA was stronger. The desolvation method was widely used to prepare BSA nanoparticles. Sadeghi et al investigated the effect of different desolvating agents on Cur-loaded BSA nanoparticles.111 They found that using ethanol could produce more uniform spherical nanoparticles and acetone resulted in higher encapsulation efficiency. Salehiabar et al synthesized Cur-loaded BSA nanoparticles (BSA@CUR NPs) with a mean size of about 99.78 nm, a zeta potential of −9.19 mV, an entrapment efficiency of 78.12% and a loading efficiency of 2.61%.112 The in vitro release behavior of BSA@CUR NPs was time and pH dependent due to the degradation of BSA in acidic medium. Moreover, an in vitro hemolysis assay on the human red blood cells (HRBCs) showed the biocompatibility of the nanoparticles. The same research group also prepared bovine serum albumin-coated magnetic nanoparticles to delivery curcumin (F@BSA@CUR NPs).113 The results of MTT assay revealed the significant cytotoxicity of nanoparticles on MCF-7 cells. In a recent study of Zhang et al, curcumin/BSA nanoparticles were used to suppress the toxicity of CuO.114 Nanoparticles were prepared by co-precipitation of BSA and curcumin and followed by glutaraldehyde cross-linking. Results showed that particles could reduce cytotoxicity and genotoxicity of CuO nanoparticles by decreasing the ROS level and Cu2+ concentration. In addition, in vivo instillation and inflammation assessment further demonstrated the inhibitory effect of CUR/BSA NPs on the toxicity of metal-based nanomaterials.
BSA-saccharide conjugates were produced to achieve excellent stability or targeted ability.115 Huang et al prepared Cur-loaded galactosylated BSA nanoparticles (Gal-BSA-Cur NPs) to target asialoglycoprotein receptor (ASGPR) overexpressed on HepG2 cells.116 BSA-galactose conjugate was first synthesized using the reductive amination method and then Gal-BSA-Cur NPs were prepared by the desolvation method. The intracellular uptake of Gal-BSA-Cur NPs, BSA-Cur NPs and free Cur in HepG2 cells were 240.0 ± 18.2, 133.6 ± 7.9 and 46.8 ± 14.8 ng/105 cells, respectively, indicating the importance of galactose-mediated endocytosis. Fan et al produced Cur-loaded BSA-dextran nanoparticles with a particle size of 115 nm.117 BSA-dextran nanoparticles significantly induced the cellular antioxidant activity of Cur because of the increased uptake.
HSA
In order to avoid a possible immunologic response, BSA can be replaced with HSA, the most abundant protein in human serum. HSA has a molecular weight of 66,500 Da and is consisted of 585 amino acid residues.109 HSA nanoparticles have attracted great intention in the field of drug delivery.
Kim et al prepared curcumin-loaded HSA nanoparticles (CCM-HSA-NPs) with a mean size of 135.5nm using albumin bound technology.118 The solubility of CCM-HSA-NPs in water was 851.416 μg/mL while that of free curcumin was only 2.792 μg/mL. At all-time points of biodistribution study, tumor accumulations of CCM-HSA-NPs were remarkably higher than that of free Cur, probably due to the EPR effects and enhanced penetration of albumin. Moreover, the in vivo antitumor activity of nanoparticles was investigated in HCT116 and MiaPaCa2 xenograft tumor-bearing nude mice. CCM-HSA-NPs had a greater therapeutic effect than free drug without observable toxicity. In a recent study, folate-conjugated HSA nanoparticles loaded with curcumin were also reported.119 HSA NPs significantly improved the antitumor activity of free drug both in vitro and in vivo. Saleh et al fabricated Cur-loaded, redox-responsive HSA nanoparticles (CCM/HSA NPs) by using a modified desolvation method.120 GSH, a reducing agent was used to break down the disulfide bonds of HSA and curcumin was entrapped in the hydrophobic cavity to form HSA-CCM nanoparticles. The release rate of curcumin in CCM/HSA NPs was promptly increased when it was incubated with 10 mM GSH, indicating the property of reductive disintegration.
OVA
Ovalbumin, a highly functional food protein is usually obtained from egg white.121 It is consisted of 385 amino acid residues with a molecular weight of 47,000 Da and isoelectric point of 4.8.109 Recently, the application of ovalbumin for drug delivery has gained interest owing to its inexpensive and biocompatible properties. Moreover, OVA is a promising carrier for controlled drug release because of its temperature- and pH- sensitive properties.
Liu et al prepared OVA/curcumin complex powder by freeze-drying.122 The mean particle size of OVA/curcumin complex in aqueous solution was 103.57 nm by dynamic light scattering (DLS) measurement. The authors reported that the complex of OVA and Cur resulted in the secondary structure change of OVA and the crystal structure disappear of Cur, leading to more prone to pyrolyze. Compared with free curcumin, OVA/curcumin complex showed a higher antioxidant activity, representing the potential applications of OVA/curcumin complex in functional food. Recent study reported that OVA nanoparticles could improve the oral bioavailability of curcumin.123 In an in vitro gastrointestinal tract (GIT) model, the transformation of curcumin in ovalbumin-dextran nanogels was significantly higher than that in ovalbumin nanoparticles.
Zein
Zein, an alcohol-soluble protein is obtained from corn and has a molecular weight of 22 to 27 kDa.124 The major fractions of zein include α-, β-, γ- and δ-zein, and hydrophobic amino acid residues account for more than 50%. Zein is one of the few proteins that can be solubilized in 60–90% ethanol solutions but not in pure water.125 Due to its hydrophobicity, biodegradability and biocompatibility, zein has been widely investigated to encapsulate the hydrophobic bioactive compounds. Moreover, zein can easily form self-assembled nanoparticles by the method of antisolvent co-precipitation.
In the present work, Cur was successfully incorporated into zein fibers by electrospinning technique.126 The encapsulation efficiency of CUR-zein fibers was close to one hundred percent. In an antibacterial test, Escherichia coli and Staphylococcus aureus were used as Gram-positive and Gram-negative model bacteria, respectively. The results showed that CUR-zein fibers have good antibacterial activity towards both two kinds of bacterias, indicating the potential to inhibit bacterial growth and propagation in food packaging. In addition, PEG, shellac, chitosan and pectin were used to coat and stabilize zein nanoparticles.125,127-129 Sun et al produced curcumin-loaded zein-shellac nanoparticles with a higher encapsulation efficiency than pure zein particles.130 Also, the nanoparticles showed a sustaining drug release in PBS and simulated gastrointestinal fluids.
Casein
Casein, an amphiphilic protein with an isoelectric point of 4.8 is the major protein of milk.131 It is composed of αs1-, αs2-, β- and κ-casein. Casein can spontaneously forms micelles and the average particle size is 150 nm.132 Esmaili et al used beta casein-micelle as a nanocarrier for hydrophobic drugs.133 When Cur was loaded into the micellar nanostructures, its solubility enhanced at least 2500 fold. The IC50 values for B-CN encapsulated and free Cur were 17.7 and 26.5 μmol/L for K562 cells, respectively. Antioxidant activity of Cur was also improved via the process of drug loading into beta casein-micelle. Similarly, in another study, curcumin-loaded casein nanoparticles not only increase the solubility and bioactivity, but also improve the dispersibility of pristine Cur.134
SF
Silk, an insoluble natural polymeric biomaterial, is obtained from Bombyx mori silkworm.135 Silk consists of an inner core of silk fibroin and an outer layer of silk sericin. Because of the biocompatibility, controllable degradability, processibility and excellent mechanical properties, SF has been engineered for sustained and controlled drug delivery systems. Several curcumin-loaded SF nanoparticles have been reported.136,137 For example, Xie et al prepared Curcumin-silk fibroin nanoparticles (CM–SF NPs) using a solution-enhanced dispersion by supercritical CO2 process.138 The size distribution, particle size, drug loading and encapsulation efficiency were influenced by various parameters, including the ratio of Cur and SK, pressure, and final concentration of NPs. Gupta et al simultaneously fabricated pure SF and silk fibroin-chitosan (SFCS) nanoparticles to encapsulate curcumin using a capillary-microdot technique.139 The encapsulation efficiency of SF-coated curcumin nanoparticles was higher than that of SFCS-coated nanoparticles. This may be because curcumin is a hydrophobic drug, so the presence of CS, a hydrophilic polymer reduces the encapsulation efficiency of curcumin by SK. In addition, Cur-loaded SF nanoparticles showed higher intracellular uptake and cytotoxicity against breast cancer cells.
Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) are novel colloidal drug delivery system composed of biodegradable solid lipids.140 The drugs are wrapped or embedded in the lipid core consisted of the phospholipid hydrophobic chains and high melting fat matrix. The particle sizes are usually between 50 and 1000 nm.141 Previous studies have reported that SLNs exhibited unique advantages, such as high biocompatibility, physical stability, drug targeting and controlled drug release.
The encapsulation of Cur in SLNs have been widely investigated. Wang et al synthesized SLN-curcumin by sol-gel method for non-small cell lung cancer treatment.142 It was found that SLN-curcumin increased the antitumor effect both in vitro and in vivo. Similarly, in another study, Sun et al prepared Cur-loaded SLNs by high-pressure homogenization to improve the dispersibility, stability and bioavailability of curcumin, and enhance its cellular uptake and anti-cancer efficacy.14
The burst release of drugs from solid lipid nanoparticles in acidic environment limits the usage as an oral delivery system. In order to inhibit the rapid release of curcumin from SLNs in acidic environment, N-carboxymethyl chitosan (NCC) coated curcumin-loaded SLN (NCC-SLN) was prepared.143 NCC-SLN (182.0 ± 6.7 nm) has a larger particle size than curcumin-loaded SLN (C-SLN) (166.5 ± 5.4 nm) due to the successful surface modification. In an in vitro release study, 40.4% of encapsulated Cur in C-SLN was released while only 1.5% Cur was released from NCC-SLN in simulated gastric fluid (SGF) within 2 h. In addition, NCC-SLN improved the lymphatic uptake of free Cur at 6.3-fold and increased the oral bioavailability at 9.5-fold. These results indicated that NCC-SLN could be a promising oral delivery carrier for Cur.
Cyclodextrins
Cyclodextrins (CDs) are cyclic oligosaccharides with a structure of hollow truncated cone.144 Due to its hydrophilic outer surface and lipophilic cavity, CDs are able to solubilize hydrophobic drugs. β-CD, γ-CD, hydroxypropyl-β-CD and methyl-β-CD are the most frequently used compounds (Figure 7). Yallapu et al developed β-cyclodextrin- curcumin (CD-CUR) inclusion complexes by a solvent evaporation method.145 The IC50 values of CD30 and free Cur were 16.8 and 19.6 μM for C4-2 prostate cancer cells, and 17.6 and 18.4 μM for DU145 prostate cancer cells, respectively. In another study, CUR was loaded into hydroxypropyl-β-CD (HP-β-CD) in the presence of different stabilizers.146 It was found that PVA was the most suitable stabilizer, resulting in the highest encapsulation efficiency (~ 60%) with lower nanoparticle size (40 nm). In addition, the results indicated that prepared HP-β-CD-curcumin nanoparticles could effectively overcome the multidrug resistance of DOX in COLO205 colorectal adenocarcinoma cells.
 |
Figure 7 Chemical structures of most frequently used cyclodextrins. |
Nanogels
As a type of promising drug delivery carriers, nanogels have attracted a lot of attention.147–149 They are usually formed from amphiphilic or hydrophilic polymer networks by physical self-assembly or chemical crosslinking. The preparation of nanogels combines the advantages of both hydrogels and nanoparticles. Similar to other nanoparticles, nanogels can easily pass through tumor vessel walls and be taken up by cancer cells. Owing to their high loading capability, biocompatibility and responsive drug release property, nanogels are ideal candidates for drug delivery. In addition, the presence of large surface area and functional groups for further modification with targeted agents or other biomolecules is another advantage.
Mangalathillam et al developed Cur-loaded chitin nanogels (CCNGs) for skin cancer therapy.150 The values of particle size, zeta potential and entrapment efficiency were 70–80 nm, 49.34 mV and 95%, respectively. The swelling studies showed that Cur conjugation decreased the swelling ratio of chitin nanogels both at alkaline, acidic and neutral conditions due to the reduction of free reactive functional groups. When exposed to fresh human blood, the hemolytic ratio of CCNGs was 2.45% which is far below 5%, the critical safe hemolytic ratio for biomaterials according to ISO/TR 7406,151 indicating the biocompatibility and biological safety of nanogels. In skin permeation studies in vivo, CCNGs increased the penetration of Cur. By histopathological evaluations, it was observed that the keratin was comparatively thin and stratum corneum was fragmented upon the treatment of nanogels. All these data indicated that CCNGs offered an effective transdermal delivery for the treatment of skin cancer. Wu et al designed and produced core-shell nanogels, which were consisted of Ag/Au nanoparticles as core and polystyrene-poly (ethylene glycol) gels as shell.16 The Ag/Au core exhibited strong absorption in the near-infrared region (NIR) for photothermal conversion and fluorescence for cellular imaging. Cur was loaded in the hydrophobic polystyrene chain networks through hydrophobic interactions. Temperature rise or NIR irradiation can accelerate the release of curcumin from nanogels. Furthermore, Cur-loaded hybrid nanogels showed higher therapeutic efficacy upon combined chemo-photothermal therapy, indicating the potential for cancer treatment. In a recent study, Cur was conjugated to cholesteryl-hyaluronic acid (CHA) nanogel for targeted delivery to CD44-expressing cancer cells.152 Curcumin and CHA-CUR significantly down-regulated the expression of COX-2, TNF-α, and NF-κB. In a tumor growth inhibition study using MiaPaCa-2 xenograft model, CHA-CUR resulted in 5-fold tumor suppression than free drug, making this Cur-loaded nanogel a potential candidate for cancer therapy.
Environment-responsive nanogels were also prepared for anti-cancer drug delivery. A novel interpenetrating polymeric network nanogels (IPN-NGs) composed of gelatin (GL) acrylamidoglycolic acid (AGA) were produced by emulsion polymerization.153 The IPN-NGs showed pH-sensitive properties due to dual crosslinking and the hydrogen bonds between polymer chains. Luckanagul et al developed thermo-responsive nanogels from chitosan-grafted poly-(N-isopropylacrylamide) (CS-g-pN) by sonication method.154 The authors mentioned that pNIPAM has a low critical solution temperature of 32 °C. When the surrounding temperature is higher than LCST, drugs will release from the pNIPAM nanogels. However, no data regarding responsive drug release property were reported in this study.
Nanocrystals
Nanocrystals are carrier-free nanoparticles composed of pure drug crystals and minimum stabilizer.155 Nanocrystals have small particle size and high drug loading capacity. Owing to the properties of increased surface area and saturation solubility, nanocrystals can solve the solubility and dissolution problems of most hydrophobic drugs, resulting in increased bioavailability and improved biodistribution. Nanocrystals were initially investigated to increase the bioavailability of poorly soluble oral medication. Nowadays, nanocrystals were widely developed for intravenous injection, transdermal delivery, ocular delivery, and pulmonary administration.156–159 The preparation methods of nanocrystals include bottom-up and top-down approaches160 (Table 2). The former is a method in which nanoparticles are precipitated or crystallized through the combination of molecules in solution, mainly including precipitation method and emulsification method. The latter is a method of dispersing large drug particles into small particles, mainly including grinding method and high-pressure homogenization method. In addition, nanocrystals are highly dispersed heterogeneous systems with a large specific surface area and require stabilizers to maintain stability.161,162 Surfactants and polymers, such as Tween 80, cetyltrimethylammonium bromide (CTAB), TPGS, PVA and pluronic were usually used to stabilize curcumin molecules.
 |
Table 2 Preparation Methods of Cur-Loaded Nanocrystals |
Rachmawati et al prepared curcumin nanocrystals with different stabilizers by high-pressure homogenization (HPH).163 It was found that PVP is the most efficient polymer to stabilize curcumin nanocrystal. The grinding method was also used to develop curcumin nanocrystals.164 There appeared to be a significant improvement in oral bioavailability. In a recent study, novel curcumin nanocrystals were produced for dermal penetration enhancement by a smart crystal technology, which is a combination of grinding and HPH.165 The average particle size and zeta potential were about 180 nm and −30 mV, respectively. The skin penetration profile was performed for curcumin nanocrystal with decreasing concentrations. The results showed that curcumin nanocrystals at 2%, 0.2% and 0.02% (w/w) revealed strong fluorescence intensity in the epidermis while at 0.002% revealed a very faint fluorescence.
In a study of Moorthi et al, the nanoprecipitation method with the assistance of sonication was used to prepared curcumin nanocrystals.166 Sodium lauryl sulfate and poloxamer 188 were used as the stabilizers. Sonication could inhibit the growth of crystal at the initial stage and stabilizers inhibited further growth. In addition, curcumin nanocrystal stabilized by sodium lauryl sulfate produced a smaller particle size, higher surface area and higher negative potential than that stabilized by poloxamer 188. The authors considered that the anionic property of sodium lauryl sulfate overcame van der Waals force and gravitational force, resulting in the prevention of nanocrystal aggregation. Recently, curcumin nanocrystals coated by hyaluronic acid (HA@Cur-NCs) has been developed for the targeted drug delivery.167 The curcumin nanocrystals (Cur-NCs) were first synthesized by thin-film dispersion and then coated with HA using a nanoprecipitation method. It was observed from transmission electron microscopy (TEM) images that HA@Cur-NCs showed a more regular structure after surface modification with HA (Figure 8). DLS analysis showed that the average particle size and zeta potential of HA@Cur-NCs were 161.9 ±1.7 nm and −25.0 ± 0.8 mV, respectively. Compared to Cur-NCs, HA@Cur-NCs exhibited increased cellular uptake and cytotoxicity in breast cancer cells. It was also observed that HA@Cur-NCs showed better anticancer effects compared with Cur-NCs and free Cur in an in vivo animal experiments, demonstrating the necessity and importance of HA targeting.
 |
Figure 8 Characterization of formulations. (A) Appearances of free Cur, Cur-NCs, and HA@Cur-NCs. TEM images of (B) Cur-NCs and (C) HA@Cur-NCs. Reprinted from Biomater Sci. Ji P, Wang L, Chen Y, et al. Hyaluronic acid hydrophilic surface rehabilitating curcumin nanocrystals for targeted breast cancer treatment with prolonged biodistribution, 462-472. Copyright 2020, with permission from The Royal Society of Chemistry.167 |
Biomedical Applications
As described above, plenty of studies reported that different Cur-loaded nanoformulations showed anti-tumor effects for many types of cancer. Furthermore, curcumin nanoformulations exhibited the potential applications for the prevention and treatment of other diseases, such as brain injury,168,169 wound healing,170,171 malaria,172,173 asthma,174 psoriasis,175 arteriosclerosis,176 leishmaniasis,177 bacterial infection8 (Table 3), etc.
 |
Table 3 Examples of Cur-Loaded Nanoformulations for Treatment of Different Diseases |
Due to its promising therapeutic effect, several curcumin formulations based on free form and nanoparticles have been under investigation in clinical trials.178–181 In recent years, some clinical trials were performed to evaluate the improved bioavailability of curcumin nanoformulations. In a human pharmacokinetic study, healthy subjects were divided into three groups and then were given single oral medication of curcuminoids, micronized powder and Cur-loaded micelles at a dose of 500 mg, respectively.182 The results showed that the AUC of micronized curcumin was 14-, 5-, and 9-fold than curcumin in women, men and all subjects. Furthermore, curcumin micelles improved the oral bioavailability of Cur at 277-, 114- and 185-fold, respectively. In another study, Kanai et al evaluated the safety and pharmacokinetics of THERACURMIN, a novel nanoparticle curcumin.183 THERACURMIN increased the curcumin concentration in plasma in a dose-dependent manner. In addition, no toxicities of curcumin nanoformulations were observed in this study.
Although some preclinical researches showed potent anticancer effects of curcumin nanoformulations, clinical findings have not been conclusive. But several clinical trials were conducted to investigate the reduced adverse effects caused by curcumin nanoformulations in cancer treatment. Meriva®, a nano delivery system composed of curcuminoids and phosphatidylcholine, has exhibited a boosted pharmacokinetic profile in both rats and human.184 In a clinical trial, patients with histologically documented solid tumors were randomized to receive either Meriva® or placebo for 8 weeks. It was found that Meriva® significantly improved the health-related quality of life (QoL) compared with the placebo. Moreover, some markers associated with systemic inflammation including TNF-α, TGF-β, IL-6, substance P, calcitonin gene-related peptide (CGRP), high-sensitivity C-reactive protein (hs-CRP) and monocyte chemotactic protein-1 (MCP-1) were significantly decreased upon the treatment of curcumin nanoformulations. The results indicated that adjuvant therapy with curcumin nanoformulations can improve quality of life and suppress systemic inflammation in patients with solid tumors who are under treatment with standard chemotherapy protocols. Furthermore, a similar study showed that Meriva® could decrease the side effects after chemo- and radiotherapy.185 In another clinical trial, nano-curcumin was combined with ω-3 fatty acids to treat migraines.186 Eighty patients were divided into four groups and given the treatment of different drug formulations. It has been reported that TNF-α plays an important role in neuroimmunity pathogenesis of migraine. The combination of nano-curcumin and ω-3 fatty acids significantly decreased the expression of TNF-α in serum while nano-curcumin or ω-3 fatty acids alone did not exhibit a significant reduction in the levels of TNF-α. This combinational therapy represented a promising approach for migraine management.
Conclusions and Future Perspectives
Curcumin is a natural polyphenolic compound that has been widely used in traditional Chinese medicine. The main pharmacological activities of CUR are anti-inflammatory, anti-oxidation, anti-tumor, anti-bacterial, liver protection, etc. However, the poor solubility and low bioavailability of Curcumin result in poor intestinal absorption, rapid metabolism in plasma and liver, and fast systemic clearance. Thus, the in vivo activities of Cur are limited. Nanoparticles have shown many advantages as drug carriers, including increased drug stability, slowed drug degradation, enhanced drug solubility, and improved pharmacokinetics. Several types of Cur-loaded nanoparticles have been developed for the treatment of different diseases. As we discussed in this review, nanoformulations not only increase the solubility and bioavailability but also improve the therapeutic effect of curcumin. As a research focus, the anti-tumor efficacy of CUR has been widely studied. The preclinical and clinical trials of curcumin-loaded nanoformulations that have been carried out indicated that curcumin nanoparticles might act as enhanced chemotherapeutic and chemopreventive agents. Moreover, by down-regulating the expression of P-glycoprotein (P-gp), multidrug resistance protein (MRP), breast cancer resistance protein (BCRP) and other drug efflux transporter proteins, CUR can reverse the resistance of common chemotherapeutic drugs. Therefore, combination of curcumin and other anticancer drugs using nano delivery systems is a promising therapeutic strategy.
However, there are still some problems that need to be solved. The foremost is the toxicity of nanocarriers. Although in most cases materials used to prepare the carriers are biodegradable, biocompatible and non-toxic, some reagents added during the preparation process, such as solvents and surfactants, may cause some damage to the human body. Moreover, the lack of tissue specificity is also a big problem. In tumor treatment, nanoparticles not only deliver drugs to cancer cells, but also to normal tissues. Therefore, targeted nanoparticles should be investigated. Additionally, small and large cohorts, as well as patients in Phase I/II clinical trials are still needed to evaluate the pharmacological and toxicological effects of Cur-loaded nanoparticles. Various curcumin nanoformulations are still at the stage of laboratory research, and there are some problems in large-scale preparation. Since the preparation of most nanoparticles is relatively complicated and difficult to scale up, we also need to make breakthrough progress in processes and technologies of large-scale production, stability, and quality control of drug loading.
In summary, nanoformulations improve the solubility and biocompatibility, increase the therapeutic effect, and lay the foundation for further clinical research and applications of curcumin.
Acknowledgments
This work was supported by the Programs of the National Natural Science Foundation of China (Grant Nos. 81673368, 81603046, 81703446 and 81973257).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Sun M, Su X, Ding B, et al. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine. 2012;7:1085–1100. doi:10.2217/nnm.12.80
2. Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17:71–80. doi:10.1016/j.drudis.2011.09.009
3. Duvoix A, Blasius R, Delhalle S, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–190. doi:10.1016/j.canlet.2004.09.041
4. Esatbeyoglu T, Huebbe P, Ernst IM, et al. Curcumin–from molecule to biological function. Angew Chem Int Ed Engl. 2012;51:5308–5332. doi:10.1002/anie.201107724
5. Huang Y-S, Hsieh T-J, Lu C-Y. Simple analytical strategy for MALDI-TOF-MS and nanoUPLC–MS/MS: quantitating curcumin in food condiments and dietary supplements and screening of acrylamide-induced ROS protein indicators reduced by curcumin. Food Chem. 2015;174:571–576. doi:10.1016/j.foodchem.2014.11.115
6. Kant V, Gopal A, Pathak NN, et al. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int Immunopharmacol. 2014;20:322–330. doi:10.1016/j.intimp.2014.03.009
7. Wang J, Wang H, Zhu R, et al. Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1β transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials. 2015;53:475–483. doi:10.1016/j.biomaterials.2015.02.116
8. Peng K-T, Chiang Y-C, Huang T-Y, et al. Curcumin nanoparticles are a promising anti-bacterial and anti-inflammatory agent for treating periprosthetic joint infections. Int J Nanomed. 2019;14:469–481. doi:10.2147/IJN.S191504
9. Yavarpour-Bali H, Ghasemi-Kasman M, Pirzadeh M. Curcumin-loaded nanoparticles: a novel therapeutic strategy in treatment of central nervous system disorders. Int J Nanomed. 2019;14:4449–4460. doi:10.2147/IJN.S208332
10. Chen Y, Chen C, Zhang X, et al. Platinum complexes of curcumin delivered by dual-responsive polymeric nanoparticles improve chemotherapeutic efficacy based on the enhanced anti-metastasis activity and reduce side effects. Acta Pharm Sin B. 2019. doi:10.1016/j.apsb.2019.10.011
11. Hamaguchi T, Ono K, Yamada M. Curcumin and Alzheimer’s disease. CNS Neurosci Ther. 2010;16:285–297. doi:10.1111/j.1755-5949.2010.00147.x
12. Zhang L, Yang Z, Wei J, et al. Essential oil composition and bioactivity variation in wild-growing populations of curcuma phaeocaulis valeton collected from China. Ind Crops Prod. 2017;103:274–282. doi:10.1016/j.indcrop.2017.04.019
13. Yang C, Chen H, Zhao J, et al. Development of a folate-modified curcumin loaded micelle delivery system for cancer targeting. Colloid Surf B. 2014;121:206–213. doi:10.1016/j.colsurfb.2014.05.005
14. Sun J, Bi C, Chan HM, et al. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloid Surf B. 2013;111:367–375. doi:10.1016/j.colsurfb.2013.06.032
15. Li L, Xiang D, Shigdar S, et al. Epithelial cell adhesion molecule aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted drug delivery to human colorectal adenocarcinoma cells. Int J Nanomed. 2014;9:1083–1096.
16. Wu W, Shen J, Banerjee P, et al. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials. 2011;32:598–609. doi:10.1016/j.biomaterials.2010.08.112
17. Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi:10.1021/mp700113r
18. Siviero A, Gallo E, Maggini V, et al. Curcumin, a golden spice with a low bioavailability. J Herb Med. 2015;5:57–70. doi:10.1016/j.hermed.2015.03.001
19. Lao CD, Ruffin M, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complem Altern M. 2006;6:10. doi:10.1186/1472-6882-6-10
20. Naksuriya O, Okonogi S, Schiffelers RM, et al. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383. doi:10.1016/j.biomaterials.2013.12.090
21. Wong KE, Ngai SC, Chan KG, et al. Curcumin nanoformulations for colorectal cancer: a review. Front Pharmacol. 2019;10:152. doi:10.1021/cm0011559
22. Saxena A, Tripathi R, Singh R. Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig J Nanomater Bios. 2010;5:427–432.
23. Parthasarathi S, Muthukumar SP, Anandharamakrishnan C. The influence of droplet size on the stability, in vivo digestion, and oral bioavailability of vitamin E emulsions. Food Funct. 2016;7:2294–2302. doi:10.1039/C5FO01517K
24. Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597–6611. doi:10.1016/j.biomaterials.2010.04.062
25. Peng S, Li Z, Zou L, et al. Improving curcumin solubility and bioavailability by encapsulation in saponin-coated curcumin nanoparticles prepared using a simple pH-driven loading method. Food Funct. 2018;9:1829–1839. doi:10.1039/C7FO01814B
26. Barenholz YC. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi:10.1016/j.jconrel.2012.03.020
27. Zhang W, Ma W, Zhang J, et al. The immunoregulatory activities of astragalus polysaccharide liposome on macrophages and dendritic cells. Int J Biol Macromol. 2017;105:852–861. doi:10.1016/j.ijbiomac.2017.07.108
28. Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release. 2014;185:22–36. doi:10.1016/j.jconrel.2014.04.015
29. Feng T, Wei Y, Lee RJ, et al. Liposomal curcumin and its application in cancer. Int J Nanomed. 2017;12:6027–6044. doi:10.2147/IJN.S132434
30. Š K, Turánek J. Liposomal paclitaxel formulations. J Control Release. 2012;163:322–334. doi:10.1016/j.jconrel.2012.09.006
31. He C, Zhang X, Yan R, et al. Enhancement of cisplatin efficacy by lipid-CaO2 nanocarrier-mediated comprehensive modulation of the tumor microenvironment. Biomater Sci. 2019;7:4260–4272. doi:10.1039/C9BM00797K
32. Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154:123–140. doi:10.1016/S0378-5173(97)00135-X
33. Shi HS, Gao X, Li D, et al. A systemic administration of liposomal curcumin inhibits radiation pneumonitis and sensitizes lung carcinoma to radiation. Int J Nanomed. 2012;7:2601–2611.
34. Baker R, Han G, Sarangkasiri S, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol. 2013;85:190–195. doi:10.1016/j.ijrobp.2012.03.041
35. Sadeghi R, Razzaghdoust A, Bakhshandeh M, et al. Nanocurcumin as a radioprotective agent against radiation-induced mortality in mice. Nanomed J. 2019;6:43–49.
36. Saengkrit N, Saesoo S, Srinuanchai W, et al. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloid Surf B. 2014;114:349–356. doi:10.1016/j.colsurfb.2013.10.005
37. Dhule SS, Penfornis P, Frazier T, et al. Curcumin-loaded gamma-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8:440–451. doi:10.1016/j.nano.2011.07.011
38. Chen Y, Cheng Y, Zhao P, et al. Co-delivery of doxorubicin and imatinib by pH sensitive cleavable PEGylated nanoliposomes with folate-mediated targeting to overcome multidrug resistance. Int J Pharm. 2018;542:266–279. doi:10.1016/j.ijpharm.2018.03.024
39. Barattin M, Mattarei A, Balasso A, et al. pH-controlled liposomes for enhanced cell penetration in tumor environment. ACS Appl Mater Inter. 2018;10:17646–17661. doi:10.1021/acsami.8b03469
40. Mahmud M, Piwoni A, Filipczak N, et al. Long-circulating curcumin-loaded liposome formulations with high incorporation efficiency, stability and anticancer activity towards pancreatic adenocarcinoma cell lines in vitro. PLoS One. 2016;11:e0167787. doi:10.1371/journal.pone.0167787
41. Lu Y, Ding N, Yang C, et al. Preparation and in vitro evaluation of a folate-linked liposomal curcumin formulation. J Liposome Res. 2012;22:110–119. doi:10.3109/08982104.2011.627514
42. Ju L, Cailin F, Wenlan W, Pinghua Y, Jiayu G, Junbo L. Preparation and properties evaluation of a novel pH-sensitive liposomes based on imidazole-modified cholesterol derivatives. Int J Pharm. 2017;518:213–219. doi:10.1016/j.ijpharm.2016.11.044
43. Ruttala HB, Ko YT. Liposomal co-delivery of curcumin and albumin/paclitaxel nanoparticle for enhanced synergistic antitumor efficacy. Colloid Surf B. 2015;128:419–426. doi:10.1016/j.colsurfb.2015.02.040
44. Cheng Y, Zhao P, Wu S, et al. Cisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinoma. Int J Pharm. 2018;545:261–273. doi:10.1016/j.ijpharm.2018.05.007
45. Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release. 2014;190:465–476. doi:10.1016/j.jconrel.2014.06.042
46. Duhem N, Danhier F, Préat V. Vitamin E-based nanomedicines for anti-cancer drug delivery. J Control Release. 2014;182:33–44. doi:10.1016/j.jconrel.2014.03.009
47. Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliver Rev. 2012;64:37–48. doi:10.1016/j.addr.2012.09.013
48. Gong J, Chen M, Zheng Y, et al. Polymeric micelles drug delivery system in oncology. J Control Release. 2012;159:312–323. doi:10.1016/j.jconrel.2011.12.012
49. Kedar U, Phutane P, Shidhaye S, et al. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed-Nanotechnol. 2010;6:714–729. doi:10.1016/j.nano.2010.05.005
50. Chen S, Yang K, Tuguntaev RG, et al. Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomedicine. 2016;12:269–286. doi:10.1016/j.nano.2015.10.020
51. Liu L, Sun L, Wu Q, et al. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int J Pharm. 2013;443:175–182. doi:10.1016/j.ijpharm.2012.12.032
52. Kheiri Manjili H, Ghasemi P, Malvandi H, et al. Pharmacokinetics and in vivo delivery of curcumin by copolymeric mPEG-PCL micelles. Eur J Pharm Biopharm. 2017;116:17–30. doi:10.1016/j.ejpb.2016.10.003
53. Song Z, Feng R, Sun M, et al. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: preparation, pharmacokinetics and distribution in vivo. J Colloid Interf Sci. 2011;354:116–123. doi:10.1016/j.jcis.2010.10.024
54. Manjili HK, Sharafi A, Danafar H, et al. Poly(caprolactone)–poly(ethylene glycol)–poly(caprolactone) (PCL–PEG–PCL) nanoparticles: a valuable and efficient system for in vitro and in vivo delivery of curcumin. RSC Adv. 2016;6:14403–14415. doi:10.1039/C5RA24942B
55. Phan QT, Le MH, Le TT, et al. Characteristics and cytotoxicity of folate-modified curcumin-loaded PLA-PEG micellar nano systems with various PLA:PEG ratios. Int J Pharm. 2016;507:32–40. doi:10.1016/j.ijpharm.2016.05.003
56. Guan J, Zhou Z-Q, Chen M-H, et al. Folate-conjugated and pH-responsive polymeric micelles for target-cell-specific anticancer drug delivery. Acta Biomater. 2017;60:244–255. doi:10.1016/j.actbio.2017.07.018
57. Zhong Y, Goltsche K, Cheng L, et al. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials. 2016;84:250–261. doi:10.1016/j.biomaterials.2016.01.049
58. Jin H, Pi J, Zhao Y, et al. EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale. 2017;9:16365–16374. doi:10.1039/C7NR06898K
59. Zhao D, Zhang H, Yang S, et al. Redox-sensitive mPEG-SS-PTX/TPGS mixed micelles: an efficient drug delivery system for overcoming multidrug resistance. Int J Pharm. 2016;515:281–292. doi:10.1016/j.ijpharm.2016.10.029
60. Zhang J, Li J, Shi Z, et al. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017;58:349–364. doi:10.1016/j.actbio.2017.04.029
61. Zhao S, Ma L, Cao C, et al. Curcumin-loaded redox response of self-assembled micelles for enhanced antitumor and anti-inflammation efficacy. Int J Nanomed. 2017;12:2489–2504. doi:10.2147/IJN.S123190
62. Wu W, Chen M, Luo T, et al. ROS and GSH-responsive S-nitrosoglutathione functionalized polymeric nanoparticles to overcome multidrug resistance in cancer. Acta Biomater. 2020;103:259–271. doi:10.1016/j.actbio.2019.12.016
63. Kamaly N, Yameen B, Wu J, et al. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116:2602–2663. doi:10.1021/acs.chemrev.5b00346
64. Khalid M, El-Sawy HS. Polymeric nanoparticles: promising platform for drug delivery. Int J Pharm. 2017;528:675–691. doi:10.1016/j.ijpharm.2017.06.052
65. Nosrati H, Adibtabar M, Sharafi A, et al. PAMAM-modified citric acid-coated magnetic nanoparticles as pH sensitive biocompatible carrier against human breast cancer cells. Drug Dev Ind Pharm. 2018;44:1377–1384. doi:10.1080/03639045.2018.1451881
66. Pan Q, Li W, Yuan X, et al. Chondrogenic effect of cell-based scaffold of self-assembling peptides/PLGA-PLL loading the hTGFβ3 plasmid DNA. J Mater Sci-Mater M. 2016;27:19. doi:10.1007/s10856-015-5631-z
67. Xie X, Tao Q, Zou Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agr Food Chem. 2011;59:9280–9289. doi:10.1021/jf202135j
68. Shaikh J, Ankola DD, Beniwal V, et al. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223–230. doi:10.1016/j.ejps.2009.02.019
69. Bowerman CJ, Byrne JD, Chu KS, et al. Docetaxel-loaded PLGA nanoparticles improve efficacy in taxane-resistant triple-negative breast cancer. Nano Lett. 2017;17:242–248. doi:10.1021/acs.nanolett.6b03971
70. Kennedy PJ, Sousa F, Ferreira D, et al. Fab-conjugated PLGA nanoparticles effectively target cancer cells expressing human CD44v6. Acta Biomater. 2018;81:208–218. doi:10.1016/j.actbio.2018.09.043
71. Reardon PJ, Parhizkar M, Harker AH, et al. Electrohydrodynamic fabrication of core–shell PLGA nanoparticles with controlled release of cisplatin for enhanced cancer treatment. Int J Nanomed. 2017;12:3913–3926. doi:10.2147/IJN.S134833
72. Yallapu MM, Gupta BK, Jaggi M, et al. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interf Sci. 2010;351:19–29. doi:10.1016/j.jcis.2010.05.022
73. Sourabhan S, Kaladhar K, Sharma CP. Method to enhance the encapsulation of biologically active molecules in PLGA nanoparticles. Trends Biomater Artif Organs. 2009;22:211–215.
74. Wang Y, Yang N, Wang D, et al. Poly (MAH-β-cyclodextrin-co-NIPAAm) hydrogels with drug hosting and thermo/pH-sensitive for controlled drug release. Polym Degrad Stabil. 2018;147:123–131. doi:10.1016/j.polymdegradstab.2017.11.023
75. Nishio T, Ayano E, Suzuki Y, et al. Separation of phosphorylated peptides utilizing dual pH-and temperature-responsive chromatography. J Chromatogr A. 2011;1218:2079–2084. doi:10.1016/j.chroma.2010.10.076
76. Wu D-Q, Zhu J, Han H, et al. Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: in vitro and in vivo study. Acta Biomater. 2018;65:305–316. doi:10.1016/j.actbio.2017.08.048
77. Aguilar LE, GhavamiNejad A, Park CH, et al. On-demand drug release and hyperthermia therapy applications of thermoresponsive poly-(NIPAAm-co-HMAAm)/polyurethane core-shell nanofiber mat on non-vascular nitinol stents. Nanomed-Nanotechnol. 2017;13:527–538. doi:10.1016/j.nano.2016.12.012
78. Lim KJ, Bisht S, Bar EE, et al. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther. 2011;11:464–473. doi:10.4161/cbt.11.5.14410
79. Zeighamian V, Darabi M, Akbarzadeh A, et al. PNIPAAm-MAA nanoparticles as delivery vehicles for curcumin against MCF-7 breast cancer cells. Artif Cell, Nanomed B. 2016;44:735–742. doi:10.3109/21691401.2014.982803
80. Patel T, Zhou J, Piepmeier JM, et al. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliver Rev. 2012;64:701–705. doi:10.1016/j.addr.2011.12.006
81. Tian X-H, Lin X-N, Wei F, et al. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int J Nanomed. 2011;6:445–452.
82. Sun M, Gao Y, Guo C, et al. Enhancement of transport of curcumin to brain in mice by poly(n-butylcyanoacrylate) nanoparticle. J Nanopart Res. 2010;12:3111–3122. doi:10.1007/s11051-010-9907-4
83. Duan J, Zhang Y, Han S, et al. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm. 2010;400:211–220. doi:10.1016/j.ijpharm.2010.08.033
84. Zhang X, He C, Yan R, et al. HIF-1 dependent reversal of cisplatin resistance via anti-oxidative nano selenium for effective cancer therapy. Chem Eng J. 2020;380:122540. doi:10.1016/j.cej.2019.122540
85. Wang JJ, Zeng ZW, Xiao RZ, et al. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomed. 2011;6:765–774.
86. He Z, Santos JL, Tian H, et al. Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomaterials. 2017;130:28–41. doi:10.1016/j.biomaterials.2017.03.028
87. Chuah LH, Roberts CJ, Billa N, et al. Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloid Surf B. 2014;116:228–236. doi:10.1016/j.colsurfb.2014.01.007
88. Esfandiarpour-Boroujeni S, Bagheri-Khoulenjani S, Mirzadeh H, et al. Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy application. Carbohyd Polym. 2017;168:14–21. doi:10.1016/j.carbpol.2017.03.031
89. Molavi H, Zamani M, Aghajanzadeh M, et al. Evaluation of UiO-66 metal organic framework as an effective sorbent for Curcumin’s overdose. Appl Organomet Chem. 2018;32:e4221. doi:10.1002/aoc.4221
90. Danafar H, Sharafi A, Askarlou S, et al. Preparation and characterization of PEGylated iron oxide-gold nanoparticles for delivery of sulforaphane and curcumin. Drug Res. 2017;67:698–704. doi:10.1055/s-0043-115905
91. Kresge C, Leonowicz M, Roth WJ, et al. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–712. doi:10.1038/359710a0
92. Vallet-Regi M, Ramila A, Del Real R, et al. A new property of MCM-41: drug delivery system. Chem Mater. 2001;13:308–311.
93. Li Z, Barnes JC, Bosoy A, et al. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41:2590–2605. doi:10.1039/c1cs15246g
94. Slowing II, Trewyn BG, Giri S, et al. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater. 2007;17:1225–1236. doi:10.1002/adfm.200601191
95. Wu S-H, Mou C-Y, Lin H-P. Synthesis of mesoporous silica nanoparticles. Chem Soc Rev. 2013;42:3862–3875. doi:10.1039/c3cs35405a
96. Jambhrunkar S, Karmakar S, Popat A, et al. Mesoporous silica nanoparticles enhance the cytotoxicity of curcumin. RSC Adv. 2014;4:709–712. doi:10.1039/C3RA44257H
97. Bollu VS, Barui AK, Mondal SK, et al. Curcumin-loaded silica-based mesoporous materials: synthesis, characterization and cytotoxic properties against cancer cells. Mat Sci Eng C-Mater. 2016;63:393–410. doi:10.1016/j.msec.2016.03.011
98. Kotcherlakota R, Barui AK, Prashar S, et al. Curcumin loaded mesoporous silica: an effective drug delivery system for cancer treatment. Biomater Sci. 2016;4:448–459. doi:10.1039/C5BM00552C
99. Chen C, Sun W, Wang X, et al. Rational design of curcumin loaded multifunctional mesoporous silica nanoparticles to enhance the cytotoxicity for targeted and controlled drug release. Mat Sci Eng C-Mater. 2018;85:88–96. doi:10.1016/j.msec.2017.12.007
100. Sun X, Wang N, Yang L-Y, et al. Folic acid and PEI modified mesoporous silica for targeted delivery of curcumin. Pharmaceutics. 2019;11:430. doi:10.3390/pharmaceutics11090430
101. Li N, Wang Z, Zhang Y, et al. Curcumin-loaded redox-responsive mesoporous silica nanoparticles for targeted breast cancer therapy. Artif Cell, Nanomed B. 2018;46:921–935. doi:10.1080/21691401.2018.1473412
102. Elbialy NS, Aboushoushah SF, Sofi BF, et al. Multifunctional curcumin-loaded mesoporous silica nanoparticles for cancer chemoprevention and therapy. Micropor Mesopor Mat. 2020;291:109540. doi:10.1016/j.micromeso.2019.06.002
103. Ma’mani L, Nikzad S, Kheiri-Manjili H, et al. Curcumin-loaded guanidine functionalized PEGylated I3ad mesoporous silica nanoparticles KIT-6: practical strategy for the breast cancer therapy. Eur J Med Chem. 2014;83:646–654. doi:10.1016/j.ejmech.2014.06.069
104. Kong ZL, Kuo HP, Johnson A, et al. Curcumin-loaded mesoporous silica nanoparticles markedly enhanced cytotoxicity in hepatocellular carcinoma cells. Int J Mol Sci. 2019;20:2918. doi:10.3390/ijms20122918
105. Datz S, Engelke H, Schirnding C, et al. Lipid bilayer-coated curcumin-based mesoporous organosilica nanoparticles for cellular delivery. Micropor Mesopor Mat. 2016;225:371–377. doi:10.1016/j.micromeso.2015.12.006
106. Ahmadi Nasab N, Hassani Kumleh H, Beygzadeh M, et al. Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif Cell, Nanomed B. 2018;46:75–81. doi:10.1080/21691401.2017.1290648
107. Karimi M, Bahrami S, Ravari SB, et al. Albumin nanostructures as advanced drug delivery systems. Expert Opin Drug Del. 2016;13:1609–1623. doi:10.1080/17425247.2016.1193149
108. Kundu B, Rajkhowa R, Kundu SC, et al. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliver Rev. 2013;65:457–470. doi:10.1016/j.addr.2012.09.043
109. Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157:168–182. doi:10.1016/j.jconrel.2011.07.031
110. Bourassa P, Kanakis C, Tarantilis P, et al. Resveratrol, genistein, and curcumin bind bovine serum albumin. J Phys Chem B. 2010;114:3348–3354. doi:10.1021/jp9115996
111. Sadeghi R, Moosavi-Movahedi AA, Emam-jomeh Z, et al. The effect of different desolvating agents on BSA nanoparticle properties and encapsulation of curcumin. J Nanopart Res. 2014;16:2565. doi:10.1007/s11051-014-2565-1
112. Salehiabar M, Nosrati H, Javani E, et al. Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery. Int J Biol Macromol. 2018;115:83–89. doi:10.1016/j.ijbiomac.2018.04.043
113. Nosrati H, Sefidi N, Sharafi A, et al. Bovine Serum Albumin (BSA) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorg Chem. 2018;76:501–509. doi:10.1016/j.bioorg.2017.12.033
114. Zhang W, Jiang P, Chen Y, et al. Suppressing the cytotoxicity of CuO nanoparticles by uptake of curcumin/BSA particles. Nanoscale. 2016;8:9572–9582. doi:10.1039/C6NR02181F
115. Wang C, Liu Z, Xu G, et al. BSA-dextran emulsion for protection and oral delivery of curcumin. Food Hydrocolloid. 2016;61:11–19. doi:10.1016/j.foodhyd.2016.04.037
116. Huang Y, Hu L, Huang S, et al. Curcumin-loaded galactosylated BSA nanoparticles as targeted drug delivery carriers inhibit hepatocellular carcinoma cell proliferation and migration. Int J Nanomed. 2018;13:8309–8323. doi:10.2147/IJN.S184379
117. Fan Y, Yi J, Zhang Y, et al. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018;239:1210–1218. doi:10.1016/j.foodchem.2017.07.075
118. Kim TH, Jiang HH, Youn YS, et al. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm. 2011;403:285–291. doi:10.1016/j.ijpharm.2010.10.041
119. Song Z, Lu Y, Zhang X, et al. Novel curcumin-loaded human serum albumin nanoparticles surface functionalized with folate: characterization and in vitro/vivo evaluation. Drug Des Dev Ther. 2016;10:2643–2649. doi:10.2147/DDDT.S112039
120. Saleh T, Soudi T, Shojaosadati SA. Redox responsive curcumin-loaded human serum albumin nanoparticles: preparation, characterization and in vitro evaluation. Int J Biol Macromol. 2018;114:759–766. doi:10.1016/j.ijbiomac.2018.03.085
121. Aniesrani Delfiya DS, Thangavel K, Amirtham D. Preparation of curcumin loaded egg albumin nanoparticles using acetone and optimization of desolvation process. Protein J. 2016;35:124–135. doi:10.1007/s10930-016-9652-3
122. Liu Y, Ying D, Cai Y, et al. Improved antioxidant activity and physicochemical properties of curcumin by adding ovalbumin and its structural characterization. Food Hydrocolloid. 2017;72:304–311. doi:10.1016/j.foodhyd.2017.06.007
123. Feng J, Wu S, Wang H, et al. Improved bioavailability of curcumin in ovalbumin-dextran nanogels prepared by Maillard reaction. J Funct Foods. 2016;27:55–68. doi:10.1016/j.jff.2016.09.002
124. Shukla R, Cheryan M. Zein: the industrial protein from corn. Ind Crops Prod. 2001;13:171–192. doi:10.1016/S0926-6690(00)00064-9
125. Hu K, Huang X, Gao Y, et al. Core-shell biopolymer nanoparticle delivery systems: synthesis and characterization of curcumin fortified zein-pectin nanoparticles. Food Chem. 2015;182:275–281. doi:10.1016/j.foodchem.2015.03.009
126. Wang H, Hao L, Wang P, et al. Release kinetics and antibacterial activity of curcumin loaded zein fibers. Food Hydrocolloid. 2017;63:437–446. doi:10.1016/j.foodhyd.2016.09.028
127. Seok HY, Sanoj Rejinold N, Lekshmi KM, et al. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: in vitro and in vivo evaluation. J Control Release. 2018;280:20–30. doi:10.1016/j.jconrel.2018.04.050
128. Liang H, Zhou B, He L, et al. Fabrication of zein/quaternized chitosan nanoparticles for the encapsulation and protection of curcumin. RSC Adv. 2015;5:13891–13900. doi:10.1039/C4RA14270E
129. Podaralla S, Averineni R, Alqahtani M, et al. Synthesis of novel biodegradable methoxy poly(ethylene glycol)-zein micelles for effective delivery of curcumin. Mol Pharm. 2012;9:2778–2786. doi:10.1021/mp2006455
130. Sun C, Xu C, Mao L, et al. Preparation, characterization and stability of curcumin-loaded zein-shellac composite colloidal particles. Food Chem. 2017;228:656–667. doi:10.1016/j.foodchem.2017.02.001
131. Tian S, Wu Z, Liu W, et al. Effective recovery of casein from its highly diluted solution by using a technology of foam fractionation coupled with isoelectric precipitation. J Food Eng. 2018;216:72–80. doi:10.1016/j.jfoodeng.2017.07.004
132. Ghayour N, Hosseini SMH, Eskandari MH, et al. Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocolloid. 2019;87:394–403. doi:10.1016/j.foodhyd.2018.08.031
133. Esmaili M, Ghaffari SM, Moosavi-Movahedi Z, et al. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT-Food Sci Technol. 2011;44:2166–2172. doi:10.1016/j.lwt.2011.05.023
134. Pan K, Zhong Q, Baek SJ. Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules. J Agr Food Chem. 2013;61:6036–6043. doi:10.1021/jf400752a
135. Mottaghitalab F, Farokhi M, Shokrgozar MA, et al. Silk fibroin nanoparticle as a novel drug delivery system. J Control Release. 2015;206:161–176. doi:10.1016/j.jconrel.2015.03.020
136. Li C, Luo T, Zheng Z, et al. Curcumin-functionalized silk materials for enhancing adipogenic differentiation of bone marrow-derived human mesenchymal stem cells. Acta Biomater. 2015;11:222–232. doi:10.1016/j.actbio.2014.08.009
137. Montalban MG, Coburn JM, Lozano-Perez AA, et al. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials. 2018;8:126. doi:10.3390/nano8020126
138. Xie M-B, Li Y, Zhao Z, et al. Solubility enhancement of curcumin via supercritical CO2 based silk fibroin carrier. J Supercrit Fluid. 2015;103:1–9. doi:10.1016/j.supflu.2015.04.021
139. Gupta V, Aseh A, Ríos CN, et al. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomed. 2009;4:115–122. doi:10.2147/IJN.S5581
140. Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliver Rev. 2012;64:83–101. doi:10.1016/j.addr.2012.09.021
141. Ekambaram P, Sathali AAH, Priyanka K. Solid lipid nanoparticles: a review. Sci Rev Chem Commun. 2012;2:80–102.
142. Wang P, Zhang L, Peng H, et al. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mat Sci Eng C-Mater. 2013;33:4802–4808. doi:10.1016/j.msec.2013.07.047
143. Baek JS, Cho CW. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur J Pharm Biopharm. 2017;117:132–140. doi:10.1016/j.ejpb.2017.04.013
144. He H, Chen S, Zhou J, et al. Cyclodextrin-derived pH-responsive nanoparticles for delivery of paclitaxel. Biomaterials. 2013;34:5344–5358. doi:10.1016/j.biomaterials.2013.03.068
145. Yallapu MM, Jaggi M, Chauhan SC. beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloid Surf B. 2010;79:113–125. doi:10.1016/j.colsurfb.2010.03.039
146. Dash TK, Konkimalla VSB. Selection and optimization of nano‐formulation of P‐glycoprotein inhibitor for reversal of doxorubicin resistance in COLO 205 cells. J Pharm Pharmacol. 2017;69:834–843. doi:10.1111/jphp.12722
147. Chacko RT, Ventura J, Zhuang J, et al. Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv Drug Deliver Rev. 2012;64:836–851. doi:10.1016/j.addr.2012.02.002
148. Li Y, Maciel D, Rodrigues J, et al. Biodegradable polymer nanogels for drug/nucleic acid delivery. Chem Rev. 2015;115:8564–8608. doi:10.1021/cr500131f
149. Jiang Y, Chen J, Deng C, et al. Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35:4969–4985. doi:10.1016/j.biomaterials.2014.03.001
150. Mangalathillam S, Rejinold NS, Nair A, et al. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale. 2012;4:239–250. doi:10.1039/C1NR11271F
151. Yu S-R, Zhang X-P, He Z-M, et al. Effects of Ce on the short-term biocompatibility of Ti–Fe–Mo–Mn–Nb–Zr alloy for dental materials. J Mater Sci-Mater M. 2004;15:687–691. doi:10.1023/B:JMSM.0000030210.83891.d4
152. Wei X, Senanayake TH, Bohling A, et al. Targeted nanogel conjugate for improved stability and cellular permeability of curcumin: synthesis, pharmacokinetics, and tumor growth inhibition. Mol Pharm. 2014;11:3112–3122. doi:10.1021/mp500290f
153. Madhusudana Rao K, Krishna Rao KS, Ramanjaneyulu G, et al. Curcumin encapsulated pH sensitive gelatin based interpenetrating polymeric network nanogels for anti cancer drug delivery. Int J Pharm. 2015;478:788–795. doi:10.1016/j.ijpharm.2014.12.001
154. Luckanagul JA, Pitakchatwong C Ratnatilaka Na Bhuket P, et al. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohyd Polym. 2018;181:1119–1127. doi:10.1016/j.carbpol.2017.11.027
155. Junghanns J-UA, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomed. 2008;3:295–310.
156. Gu L, Fang RH, Sailor MJ, et al. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano. 2012;6:4947–4954. doi:10.1021/nn300456z
157. Ali HS, Hanafy AF. Glibenclamide nanocrystals in a biodegradable chitosan patch for transdermal delivery: engineering, formulation, and evaluation. J Pharm Sci. 2017;106:402–410. doi:10.1016/j.xphs.2016.10.010
158. Romero GB, Keck CM, Müller RH, et al. Development of cationic nanocrystals for ocular delivery. Eur J Pharm Biopharm. 2016;107:215–222. doi:10.1016/j.ejpb.2016.07.005
159. Lu Y, Qi J, Dong X, et al. The in vivo fate of nanocrystals. Drug Discov Today. 2017;22:744–750. doi:10.1016/j.drudis.2017.01.003
160. Salazar J, Ghanem A, Müller RH, et al. Nanocrystals: comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur J Pharm Biopharm. 2012;81:82–90. doi:10.1016/j.ejpb.2011.12.015
161. Zhu Z. Flash nanoprecipitation: prediction and enhancement of particle stability via drug structure. Mol Pharm. 2014;11:776–786. doi:10.1021/mp500025e
162. Chow SF, Wan KY, Cheng KK, et al. Development of highly stabilized curcumin nanoparticles by flash nanoprecipitation and lyophilization. Eur J Pharm Biopharm. 2015;94:436–449. doi:10.1016/j.ejpb.2015.06.022
163. Rachmawati H, Al Shaal L, Muller RH, et al. Development of curcumin nanocrystal: physical aspects. J Pharm Sci. 2013;102:204–214. doi:10.1002/jps.23335
164. Onoue S, Takahashi H, Kawabata Y, et al. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J Pharm Sci. 2010;99:1871–1881. doi:10.1002/jps.21964
165. Vidlarova L, Romero GB, Hanus J, et al. Nanocrystals for dermal penetration enhancement - effect of concentration and underlying mechanisms using curcumin as model. Eur J Pharm Biopharm. 2016;104:216–225. doi:10.1016/j.ejpb.2016.05.004
166. Moorthi C, Kathiresan K. Fabrication of highly stable sonication assisted curcumin nanocrystals by nanoprecipitation method. Drug Invent Today. 2013;5:66–69. doi:10.1016/j.dit.2013.02.003
167. Ji P, Wang L, Chen Y, et al. Hyaluronic acid hydrophilic surface rehabilitating curcumin nanocrystals for targeted breast cancer treatment with prolonged biodistribution. Biomater Sci. 2020;8:462–472. doi:10.1039/C9BM01605H
168. Chang CZ, Wu SC, Lin CL, et al. Curcumin, encapsulated in nano-sized PLGA, down-regulates nuclear factor kappaB (p65) and subarachnoid hemorrhage induced early brain injury in a rat model. Brain Res. 2015;1608:215–224. doi:10.1016/j.brainres.2015.02.039
169. Zhang ZY, Jiang M, Fang J, et al. Enhanced therapeutic potential of nano-curcumin against subarachnoid hemorrhage-induced blood-brain barrier disruption through inhibition of inflammatory response and oxidative stress. Mol Neurobiol. 2017;54:1–14. doi:10.1007/s12035-015-9635-y
170. Alibolandi M, Mohammadi M, Taghdisi SM, et al. Synthesis and preparation of biodegradable hybrid dextran hydrogel incorporated with biodegradable curcumin nanomicelles for full thickness wound healing. Int J Pharm. 2017;532:466–477. doi:10.1016/j.ijpharm.2017.09.042
171. Niranjan R, Kaushik M, Prakash J, et al. Enhanced wound healing by PVA/Chitosan/Curcumin patches: in vitro and in vivo study. Colloid Surf B. 2019;182:110339. doi:10.1016/j.colsurfb.2019.06.068
172. Dende C, Meena J, Nagarajan P, et al. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci Rep. 2017;7:10062. doi:10.1038/s41598-017-10672-9
173. Busari ZA, Dauda KA, Morenikeji OA, et al. Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticles. Front Pharmacol. 2017;8:622.
174. Wang W, Zhu R, Xie Q, et al. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomed. 2012;7:3667–3677. doi:10.2147/IJN.S30428
175. Sun L, Liu Z, Wang L, et al. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J Control Release. 2017;254:44–54. doi:10.1016/j.jconrel.2017.03.385
176. Meng N, Gong Y, Zhang J, et al. A novel curcumin-loaded nanoparticle restricts atherosclerosis development and promotes plaques stability in apolipoprotein E deficient mice. J Biomater Appl. 2019;33:946–954. doi:10.1177/0885328218815328
177. Chaubey P, Patel RR, Mishra B. Development and optimization of curcumin-loaded mannosylated chitosan nanoparticles using response surface methodology in the treatment of visceral leishmaniasis. Expert Opin Drug Del. 2014;11:1163–1181. doi:10.1517/17425247.2014.917076
178. Henrotin Y, Gharbi M, Dierckxsens Y, et al. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complem Altern M. 2014;14:159. doi:10.1186/1472-6882-14-159
179. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis-A randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145. doi:10.4103/0973-029X.164524
180. Salehi B, Stojanovic-Radic Z, Matejic J, et al. The therapeutic potential of curcumin: a review of clinical trials. Eur J Med Chem. 2019;163:527–545. doi:10.1016/j.ejmech.2018.12.016
181. Storka A, Vcelar B, Klickovic U, et al. Safety, tolerability and pharmacokinetics of liposomal curcumin in healthy humans. Int J Clin Pharm Th. 2015;53:54–65. doi:10.5414/CP202076
182. Schiborr C, Kocher A, Behnam D, et al. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol Nutr Food Res. 2014;58:516–527. doi:10.1002/mnfr.201300724
183. Kanai M, Imaizumi A, Otsuka Y, et al. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemoth Pharm. 2012;69:65–70. doi:10.1007/s00280-011-1673-1
184. Panahi Y, Saadat A, Beiraghdar F, et al. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytother Res. 2014;28:1461–1467. doi:10.1002/ptr.5149
185. Belcaro G, Hosoi M, Pellegrini L, et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytother Res. 2014;28:444–450. doi:10.1002/ptr.5014
186. Abdolahi M, Tafakhori A, Togha M, et al. The synergistic effects of ω-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-α gene expression and serum level in migraine patients. Immunogenetics. 2017;69:371–378. doi:10.1007/s00251-017-0992-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
