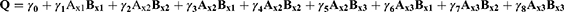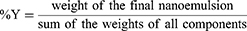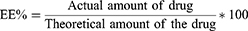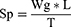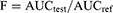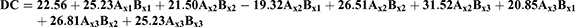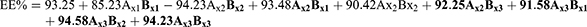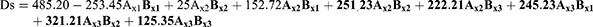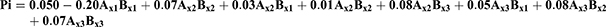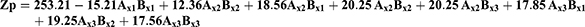Back to Journals » Drug Design, Development and Therapy » Volume 17
Nano-Emulsion Based Gel for Topical Delivery of an Anti-Inflammatory Drug: In vitro and in vivo Evaluation
Authors Gaber DA, Alsubaiyel AM, Alabdulrahim AK, Alharbi HZ, Aldubaikhy RM, Alharbi RS, Albishr WK, Mohamed HA
Received 6 February 2023
Accepted for publication 5 May 2023
Published 15 May 2023 Volume 2023:17 Pages 1435—1451
DOI https://doi.org/10.2147/DDDT.S407475
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Dalia A Gaber,1 Amal M Alsubaiyel,1 Alanoud K Alabdulrahim,2 Hanan Z Alharbi,2 Rama M Aldubaikhy,2 Rawan S Alharbi,2 Wades K Albishr,2 Heba A Mohamed3
1Department of Pharmaceutics, College of Pharmacy, Qassim University, Buraidah, 52571, Saudi Arabia; 2College of Pharmacy, Qassim University, Buraidah, 52571, Saudi Arabia; 3Department of Organic Chemistry, National Research Center, Giza, Egypt
Correspondence: Dalia A Gaber, Email [email protected]
Introduction: Arthritic disorder is a common disease in elderly patients and the most common cause of joint dysfunction. This study aims to design Piroxicam-loaded nanoemulsion (PXM-NE) formulations to enhance the analgesic and anti-inflammatory activity of the drug for topical use.
Methods: The nanoemulsion preparations were designed based on a high-pressure homogenization technique and were characterized for particle size (PS), poly dispersity index (Pi), zeta potential (ZP), drug content, and the selected formula was investigated for its topical analgesic activity and pharmacokinetic parameters.
Results: The characterizations showed that the PS was 310.20± 19.84 nm, Pi was 0.15± 0.02, and ZP was − 15.74± 1.6 mV for the selected formula. A morphology study showed that the PXM-NE droplets were spherical with a uniform size distribution. The in vitro release study showed a biphasic release pattern with a rapid release within the first 2 hours followed by a sustained release pattern. The analgesic activity for optimal formula was 1.66 times higher than the commercial gel with a double duration of analgesic activity. The Cmax was 45.73± 9.95 and 28.48± 6.44 ng/mL for the gel form of the selected formula and the commercial gel respectively. The relevant bioavailability of the selected formula was 2.41 higher than the commercial gel.
Conclusion: The results showed good physicochemical properties, higher bioavailability, and a longer analgesic effect of PXM from nanoemulsion gel, as compared to the commercial product.
Keywords: analgesics, in vivo study, nanocarrier, drug efficacy, anti-inflammatory activity
Introduction
Arthritic disease is one of the most common disorders and a main cause of joint dysfunction and disability in the elderly patient.1,2 The most predominant type of arthritis is rheumatoid arthritis, which is caused by the activation of pro-inflammatory mediators such as IL-1β and TNF-α which is characterized by peripheral joint pain, swelling, and stiffness.2 Rheumatoid arthritis treatment is depending mainly on the use of analgesics, non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, monoclonal antibodies, and specific moderator inhibitors.2–4 NSAIDs are widely used in the management and treatment of rheumatoid arthritis.5 Oral use of NSAIDs is an effective way, but its clinical use is strongly limited by its significant side effects on the gastrointestinal tract that include irritation, ulceration, and bleeding.6 Due to these side effects, many studies are aimed to develop an efficient delivery of NSAIDs via topical administration in order to increase local concentration and reduce systemic side effects.7–9 Dermal route offers many advantages over the conventional route such as allowing smooth and continuous delivery of the drug to the site of action without systemic side effects.8,9 Piroxicam (Figure 1), is a non-steroidal anti-inflammatory drug belonging to the oxicam class which is used usually to relieve the symptoms of painful inflammatory conditions like arthritis.10 It plays an important role in preventing the transformation of arachidonic acid to prostaglandin precursors and hence, it keeps potent analgesic and anti-inflammatory actions.11–13 Additionally, the oral dosage forms of piroxicam have applications in the field of sports such as body building, land injuries, boxing, etc.10,11 However, the long term oral use of the drug leads to many side effects such as gastric upset, gastric ulcers, gastric bleeding, renal failure, and many cardiovascular complications.12 The best choice to come across these side effects is to use the topical dosage forms that allow the permeation of the drug across the skin layers.13 Piroxicam showed low aqueous solubility in addition to the lipid layers barrier of the skin which brings further limitations for its topical application.10 A lipid nanoemulsion is an emulsion with a particle size in the nano range scale.14 Currently, lipid nanoemulsions are extensively used as drug carriers to increase the delivery of many drugs.15–17 Lipid nanoemulsion is a nanocarrier system formed from a mixture of two or more immiscible liquids in which the dispersed phase droplets are of average size range 20–500 nm.16 The system seems to be transparent and the sign of instability is the formation of turbidity.17 Nanoemulsion systems are highly susceptible to stability problems, primarily due to the Ostwald ripening phenomena; that the formula showed differences in solubility due to the differences in the size.16,18 It occurs usually due to the movement of small droplets and their agglomeration into larger droplets.19 To achieve stable formulation, a proper preparation method with a suitable selection of oils and surfactants is essential.20 Many techniques are used to formulate stable nanoemulsions that are classified into high energy and low energy methods.18 The preparation of nanoemulsions using high energy methods is based on the use of strong pressure force to break the course emulsion of large droplets into small particles in the nanosize scale.21 One of these methods is high pressure homogenizer, in which the coarse particle is minimized into nanosize ia high pressure force.22,23 Lipid base emulsions are a type of nanoemulsions that are able to dissolve hydrophobic drugs and protects them from dermal hydrolysis and enzymatic degradation in addition to increasing the drug retention and bioavailability.23,24 Nanoemulsions have been successfully formed using high pressure homogenizers in the laboratory and large scale.24 Many drugs showed poor efficacy while applied topically, and its incorporation in the oil core of nanoemulsion formulation helped in improving its permeability via passive diffusion through skin lipid layers.25 Moreover, the side effects of drugs were minimized by decreasing both the frequency and the total dosage of the drug during the therapy period.26 Furthermore, the large surface area of nanoemulsions improves drug transport, delivery, and targeting to specific sites.26 The presented study aimed to prepare piroxicam as nanoemulsion lipid formulations, the solubility of piroxicam in different oils were investigated. Piroxicam-loaded nanoemulsions (PM-NEs) were also estimated for their physical stability, particle size, and entrapment efficiency (EE). The selected formula was further tested for its topical analgesic activity and pharmacokinetic parameters in comparison to commercial gel.
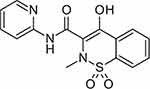 |
Figure 1 Chemical structure of piroxicam. |
Materials and Method
Materials
Piroxicam (PXM) was a free gift from “El-Nile Company for Pharmaceutical & Chemical Industries”, Egypt. Castor oil, Cremophor EL, arachis oil, soybean oil, coconut oil, corn oil, olive oil, and sesame oil were purchased from Sigma Aldrich Co. (St Louis, MO, USA). Lecithin Lipoid S100 was also purchased from Lipoid Kosmetik AG (Steinhausen, Switzerland); polyoxyethylene sorbitan monooleate (Tween 80) was obtained from Merck (Hohenbrunn, Germany), Propylene glycol monocaprylate was donated from Gattefosse (Gennevilliers, France). Glycerol and propylene glycol were obtained from Sigma-Aldrich (Darmstadt, Germany). Acetonitrile and dimethyl formamide were purchased from Merck, Mumbai, India. Tenoxicam was purchased from Sigma-Aldrich, Darmstadt, Germany. Ethanol and Methanol were HPLC grade. Double-distilled water was used during the experiment.
Methods
Solubility Study
The solubility test of piroxicam in different oils was performed to select the most proper oil phase for the development of piroxicam nanoemulsion formulations. The solubility of piroxicam was determined in eight oils namely; castor oil, Cremophor EL, Arachis oil, soybean oil, coconut oil, corn oil, olive oil, and sesame oil. Piroxicam was added to the selected oils at a ratio of 1:1 in the presence of lecithin. The solution was stirred using magnetic stirring (ADAM®, UK) for 12 hours to reach equilibrium. The samples were then centrifuged (Hettich Zentrifugen, Tuttlingen, Germany) at 4000 rpm for 15 minutes. The supernatant was filtered, and the filtrates were diluted with appropriate volume of methanol. The solubility of piroxicam was measured at 335 nm by using UV spectrophotometric method (Jenway, UK). All estimations were done in triplicates. Based on the results of the experiment three oils were selected to be used in the preparation of nanoemulsion formulations.19
Design of Experiment
Factorial design method was used for designing the experiment. Two factors raised to the power three were used for enhancing the drug bioavailability. The first factor was the oil type, that three oils were tested namely Soybean oil, Cremophor EL, and Arachis oil. The second factor was the surfactant type; Lecithin Lipoid S100, polyoxyethylene sorbitan monooleate, and Propylene glycol monocaprylate were tested. The correlation between the processing factors (PFs) and basic formula characteristics (BCs) were studied. The BCs considered were the yield, the drug content, the entrapment efficiency percentage, the particle size, and the zeta potential. The processing factors (PFs) were symbolled as (Ax) for oil type and (Bx) for surfactant type, they were studied at three levels mentioned in Table 1.27 Nine formulae were prepared each with 3 runs for each, following the design and described for five characteristics namely; C1: the yield, C2: the drug content, C3: the entrapment efficiency percentage, C4: Droplet size (Ds), and C5: zeta potential (ZP).
 |
Table 1 Full Factorial Design Levels (32) and Independent Variables |
The regression first order equation was presented as follows:
Where:
Q is the dependent variable (BCs)
Ax1, Ax2, Ax3, Bx1, Bx2, Bx3 are the independent variables (PFs).
γ0 is the arithmetic mean of the nine formula.
γ1, γ2 are the linear coefficients.
γ 4, γ 5, γ 6, γ 7, γ 8 are the coefficients of interaction between the two PFs.
Based on the equation results, optimal mixtures of independent variables was determined. Formulations with accepted properties were spray dried and incorporated in a gel preparation, the gels were evaluated for organoleptic features, drug content, viscosity, and in vitro release parameters. Finally, analgesic activity, and pharmacokinetics parameters were assessed in rats for the optimized formula.
Preparation of Piroxicam Nanoemulations (PXM-NE) Using High Pressure Homogenizer
PM-NEs were formulated using high pressure homogenization technique on two steps.28 The first step was to prepare the course emulsion, that PXM (0.4%) was dispersed into the oil phase (10 mL) and mixed at 5000 rpm using overhead stirrer, the surfactant and co-surfactant were added to the mixture and the mixture vortexed at 300 rpm for 10 minutes. The oil phase was added dropwise via 0.2 µm syringe orifice at rate 10 mL/minute into a magnetic stirred aqueous phase that contained glycerol (1%, w/w) and propylene glycol (0.5%, w/w). This solution was continuously stirred using an overhead stirrer at 500rpm for one hour. The prepared emulsions (course) were then subjected to high pressure homogenizer (FJ200-SH Lab Model, AIK Ins. Ltd., India) for five cycles at one thousand bar. The formulations components are presented in Table 2.
 |
Table 2 Formulations Code, Content, and Nanoemulsion Formulations Characteristics |
Characterizations of PXM-NE Formulations
PXM-NE formulations were kept at 8°C and characterized with respect to yield, percentage drug content, encapsulation efficiency percentage, particle size (Ps), polydispersity index (Pi), zeta potential (Zp), viscosity, and thermodynamic stability.
Percentage Yield (% Y) of PXM-NE Formulations
The percentage yield of nanoemulsion formulations was determined by dividing the weight of the final formula by the initial weight of the formula components, ie weight of drug, oil, surfactants and co-surfactants used in the preparation.29 The percentage yield was calculated as following:
The Percentage of PXM Content (%DC)
The actual content of PXM was determined by suspending 5 mg of dry PXM-NE formulations in 10 mL of methanol and vortex the mixture for 30 minutes, then stirring for 2 hours. The obtained suspension was then filtered through 0.45 lm Millipore filter papers to remove any insoluble particles, if any; 0.1 mL of the solution was pipetted and diluted up to 10 mL with methanol and measured spectrophotometrically at 335 nm. Blank nanoemulsion samples were taken and were treated in the same manner to revoke any interference from the polymers.30
The Percentage Encapsulation Efficiency Percentage (EE%)
Briefly, 0.5 mL of PXM- Ne formulations was dissolved in 5 mL of methanol, centrifuged at 3500 rpm for 15 min, the supernatant was withdrawn, diluted and analyzed spectrophotometrically at a wavelength of 335 nm (Jenway, UK). The encapsulation efficiency was calculated from the following equation:
Particle size (Ps), Poly dispersity index (Pi), and Zeta potential (Zp) measurements
Ps, Pi, and Zp were measured using zetasizer (DTS Ver. 4.11, Malvern Instruments, UK) that based on dynamic light scattering at 25°C. One mL sample was suspended in deionized water for testing. All samples were assessed triplicate.
Evaluation of PXM-NEs Samples Viscosity
All samples were tested for its viscosity using a Brookfield viscometer (Sofast, Sofraser, France). The instrument spindle was completely immersed into samples maintained at 25°C at 1.7 rad/sec. All samples were measured in triplicate.
Thermodynamic Stability Studies
All formulation were tested for their stability under stress conditions using the following tests.
Heating/Cooling Cycles
The effect of temperature changes on the stability of nanoemulsion were observed through heating/cooling cycles. Six cycles of temperature changes between 8 and 40 °C were completed with storage 48 hours at each temperature.
Centrifugation test
The nanoemulsion formulations were centrifuged for 15 min at 1500 rpm and then evaluated for any instability signs for example: creaming, phase separation, cracking, precipitation and/or phase inversion.
Freeze/Thaw Cycles
All formulations were subjected to four freeze-thaw cycles at temperatures between −20°C and 25 °C and were stored for 48 hours at each temperature.
Characterization Tests for Optimal Formula of PXM-NE
Identification Tests for Selected Nanoemulsion Formulations
Staining Test
On a glass slide Sudan Red IV (oil soluble dye), was added to 20 µL nanoemulsion, and the sample was observed under the optical microscope.
Dilution Test
The test was applied to assure the suitability of the used surfactant and co-surfactant to achieve stable nanoemulsion. The nanoemulsion was diluted in ratios 1:10 and 1:100 with distilled water and visually observed for any turbidity, cracking, and/or phase separation.
Electrical Conductivity Test
Nanoemulsion conductivity (σ) was tested to confirm whether it is O/W or W/O nanoemulsion. The conductivity meter (Thermoscientific 113, Ltd, India) composed of platinized electrodes was used. All the mentioned parameters were tested in triplicate.
Microscopic Study of PXM Nanoemulsion
Uranyl acetate stain (1% w/v) was used for microscopic examination of nanoemulsion using transmission electron microscope (TEM) (Metler Toledo, Tokyo, Japan). One drop of the selected PXM –NE formula was added to Formvar carbon-coated copper grid, and stained with 50 μL uranyl acetate stain. The sample was left to dry at room temperature for 15 minutes and observed through TEM.
Preparation of PXM-NE Gel for Selected Formulations
Nanoemulsion hydrogel for selected formulations was prepared by adding one gram of carbopol 934 in the 100 milliliters distilled water and stirred using magnetic stirrer for 30 minutes then 0.5 mL triethanolamine was added, followed by 0.1 mL of DMSO. After complete formation of gel, PXM nanoemulsion formulations (1% w/w) were incorporated in the gel with continuous stirring till uniform distribution. The gel was kept in a well closed plastic jar until further investigation.
Evaluation of PXM-NE Gel
Organoleptic Properties
The prepared gels were evaluated for physical appearance, homogeneity, presence of particles and/or aggregates, and for its color by visual observation.
Determination of the Drug Content
One gram of nanoemulsion gel formulation was added to 10 mL of methanol for extracting the drug, centrifuged for 15 minutes at 3500 rpm, the supernatant was taken, diluted and the PXM content was assessed by UV spectrophotometer at 335 nm.
Transparency Test for Optimal Formula Gel
The Optimal formula of PXM- NE formulation was inspected for optical transparency by measuring percentage transmission (% T). One mL of the formulation was diluted ten times with methanol and analyzed spectrophotometrically at 335 nm.
Evaluation of the Gel Viscosity
The viscosity of the PXM-nanoemulsion gel formulations was measured at 25°C using a Brookfield viscometer at 5–20 rpm (Sofast, Sofraser, France).
Determination of the Gel pH
The pH of the gels was determined using a digital pH meter (Orion 3 star, thermos-scientific) at room temperature. An accurately weighed 2 grams of the gel was dispersed in 20 mL of distilled water and pH was recorded.
Evaluation of Gel Spread-Ability
A weighed amount of the gel was put on a glass slide with dimensions 5*5 cm and another similar glass slide was fallen from a distance of 3 cm. The spread-ability was measured using the equation:
Where,
SP = Spread-ability of the tested gel,
M = Weight of the gel tied to upper glass slide
L = Length of glass slide
T = Time need to separate the two slides completely from each other.
In vitro Dissolution Study
The in vitro release study of PXM from NE, NE gel formulations, and drug suspension (Equivalent amount of the drug suspended in 5 mL distilled water) was carried out using Franz diffusion cells of five milliliters capacity. The membrane utilized for diffusion was dialysis membrane (Synthetic cellulose ester membrane, 2 cm*2 cm). The dialysis membrane was mounted on the diffusion cell, 200 mg of NE/ NE-gel or drug suspension was packed on it, and the membrane was closed and clamped securely. The receptor medium contained phosphate buffers pH 7.4 and maintained at temperature of 37 ± 0.5°C. The samples (0.2 mL) were withdrawn at predetermined time intervals (0, 0.5, 1, 2, 3, 4, 8, and 24 hours) and replaced with fresh buffer solution, to keep sink condition. The samples were analyzed using UV-Vis spectrophotometer at 335 nm.
Mathematical Modeling for in vitro Release Data
The in vitro release data were fitted into four mathematical models to determine the best mathematical model that fit the release data. Both diffusion exponent (n) and regression co-efficient (R) parameters were identified to best express the release mechanisms. The model showed the highest regression coefficient value was deliberated as the fit model. If Korsmeyer- Peppas equation showed “n” value ˂ 0.45, the drug release will be considered as Fickian diffusion mechanism, while if “n” value was > 0.5, it will be considered as non- Fickian diffusion or relaxation controlled release mechanism.
Analgesic Effect Study Using Tail Flick Model
The analgesic activity of the selected formula was investigated using tail flick method. Three groups of Swiss-albino mice weighing between 30 and 40 g were used; each group consisted of six mice. The first group “Group I” was used as a –ve control, which did not received any medication. Group II, 0.5 gm of hydrogel formula was applied and spread uniformly over the distal part (5 cm) of mice tail fifteen minutes prior to the begining of the experiment. Group III (+ ve control) was treated with 0.5 gm of commercial hydrogel of PXM, the gel was applied on the mice tail in the same manner as group II. All mice were kept at room temperature with free access to standard laboratory diet and tap water during the experiment. All the experiments were carried out according to the ethical guidelines established and approved by the research ethics committee in Qassim University. Each mouse was placed on tail flick apparatus (analgesia meter) (MK-350 D, Japan) using simple restrain mean to fix the animal during the test. The animal tail was put into the sensing groove and a photo-sensor was placed under the groove. The radiant heat stimulus was focused on the area of the distal part of the tail and the radiant heat lamp strength was kept constant. The time in seconds taken by each mouse to withdraw its tail was measured and considered as the reaction time for the analgesic effect. The reaction time for the analgesic activity was measured at 0.25, 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 h.7
In vivo Pharmacokinetic Study in Rats
The plasma concentration of PXM after topical application of the gel of selected formula commercial gel was determined. The in vivo studies were conducted using twelve rats divided randomly into two groups. The study was in agreement with the stated principles of animal care published by the European center for the validation of alternative methods (NIH publication No. 8023, revised 1987), and was approved by the committee of research ethics, Al-Qassim University (number: 22–15-10). Prior to topical application of gels, 10 cm2 area of animal back was shaved, cleaned with cotton, and 0.5 gm of PXM-NE7 gels was applied to the saved skin to group I, and 0.5 gm of commercial gel was applied to group II, the skin was then covered with clean gauze during the study. Blood samples (0.2 mL) were withdrawn in heparinized tubes from the tail vein at 0, 0.5, 1, 2, 3, 4, 6, 8, and 12 hours after topical application of the gels. Blood samples were then centrifuged at 3500 rpm for fifteen minutes and were frozen at −20°C until further analysis. The PXM concentrations in plasma samples were assayed by means of HPLC technique.28
HPLC Chromatographic Analysis of PXM in Plasma
Determination of PXM concentration in the withdrawn plasma samples was prepared and analyzed using the reported method by Burcea-Dragomiroiu et al.29 The analysis was done using Waters Acquity HPLC™ (Waters Corp., Milford, MA, USA) with UV detector and the samples were detected at 330 nm. Tenoxicam was used as an internal standard (IS) during the analysis. Trifluoroacetic acid and acetonitrile mixture (60:40 v/v) was used as a mobile phase supplied isocratically under a flow rate of 1.1 mL/minute. The plasma concentrations versus time curve was constructed form data and the pharmacokinetic parameters were calculated. Plasma concentrations of PXM are calculated as the mean ± SE. The extent of absorption (AUC0–t) was calculated using linear trapezoidal rules. The relative bioavailability (F) with the pure drug was calculated using the following equation:
Statistical analysis of data was done using IBM SPSS Statistics 20 program (Armonk, NY, USA) and one-way ANOVA with extended LSD post hoc test for determination of pharmacokinetic parameters, P-value <0.01 was considered significant.
Results and Discussion
Solubility Study of Piroxicam in Different Oils
Determination of the proper oil was the first step to prepare a good nanoemulsion formulation, and the high solubility of drug is the key step for oil selection. In nanoemulsion formulation, the solubility of the drug in the oil is an important point to achieve optimal drug encapsulation and to avoid the drug precipitation after emulsification. The solubility of piroxicam in different oils was measured and the solubility results are presented in Figure 2. Among the tested oils, piroxicam showed the highest solubility ≥ 120 µg/mL in the following oils: arachis, soybean and cremophor El oil. While it shows low solubility did not exceed 80 µg/mL in other oils. Based on the previous results the three oils (arachis, soybean and cremophor El) were selected as a core phase for the preparation of PXM-NE formulations. Soybean oil, arachis and cremophor El are considered a reliable source of essential fatty acid in the form of linoleic acid and α-linolenic acid. It assumed that the high amount of linoleic acid will increase the drug penetration through skin fatty layers by simple diffusion method.30
 |
Figure 2 Solubility of piroxicam in different oils. |
Characterization of PXM-NE Formulations
The results of BCs for the prepared nanoemulsion formulations are shown in Table 2. The correlation between the processing factors (PFs) and each of the following: the yield, the drug content, the entrapment efficiency percentage, the particle size, and the zeta potential was determined using the polynomial equations. The positive sign indicates an increase in the value while the negative one refers to the decrease (Figure 3).
 |
Figure 3 Surface plot showed the effect of oil, and surfactant on drug content in PMN-NE formulations. |
Yield Percentage (% Yield) of PXM Nanoemulsion
Nine formulations of PXM nanoemulsion were prepared using three different oils in addition to three emulsifiers using 32 factorial design, all formulations showed an accepted yield ranged between 62.21±3.5% and 79.22±1.3% for PXM-NE1 and PXM-NE7 respectively. The highest yield among the oils tested was observed with Arachis oil which could be due to the chemical composition of arachic oil. Arachis oil is a natural oil with molecular weight ranging between 15K −25 K Da characterized by high triacylglycerols content covered by a monolayer of phospholipid, that composed of three structural domains, amphipathic N-terminal, central hydrophobic domain, and amphipathic C- terminal which has an alpha helical structure locating at the lipid water interface that help in stabilizing the nanoemulsion structure.31 The results were in agreement with Anuchapreeda and Wang et al, who have reported relevant results for the preparation of lipid nanoemulsions of curcumin and nalbuphine respectively.32,33
The Percentage Drug Content (%DC) of PXM Nanoemulsions
The percentage of PXM content for all formulations was calculated and expressed in Table 2, and Figure 3. The results display that the %DC ranged between 73.42 ± 2.1 and 90.01 ± 2.3% for PXM-NE2 and PXM-NE7 respectively. The results of drug content showed an appropriate selection of both the oil and emulsifier. Khurana et al, and Hussain et al, mention results in a good agreement with our work for meloxicam and amphotericin B respectively.14,19
The polynomial equation express %DC parameter was as following:
F = 71:04, p<:001, R2 = 0:9551
The equation reveals that both the oil and emulsifier have a significant effect on drug content. Pople et al, reported relevant results for retinol.20
% Encapsulation Efficiency
The model used to express the factors was as following:
F = 92.22, p<0.001, and adjusted R2 = 0.9910
The model showed different encapsulation efficiency with different oils and surfactants. The highest encapsulation was for Arachis oil formulations, that %EE ranged between 93.3±2.3 and 91.3±2.0 For PXM-NE7 and PXM-NE9 respectively. While the lowest encapsulation was for soybeans formulation that ranged between 83.1±2.3 and 85.2±2.1 For PXM-NE1 and PXM-NE3.
Droplet Size (Ds), Polydispersity Index (Pi), and Zeta Potential (Zp) Assessment
Ds, Pi, and Zp have a great influence on stability, release, absorption, diffusion, and the bioavailability of the formula. The polynomial equations expressing these BCs are:
F = 130.22, p<0.001, and adjusted R2 = 0.9780
F = 102.05, p<0.001, and adjusted R2 = 0.9880
F = 125.85, p<0.001, and adjusted R2 = 0.9780
The three parameters (Ds, Pi, and Zp) were measured using Malvern Zetasizer. The mean globule size of the nanoemulsion is a critical factor in emulsification development as it affects not only the rate and extent of drug release but also for absorption. Fresh PXM-NE formulations showed average particle size ranged between 523.58±28.4 and 310.20±19.84 for PXM-NE2 and PXM-NE7 respectively (Figure 4). Ps equation shows that both the oil and emulsifier have a significant effect on the Particle size. The results showed that Arachis oil formulations showed the smallest size followed by Cermophor EL formulations, and the largest size was for soybean oil formulations. The results of Ds may be interpreted as smaller particles was formed with low molecular weight oil while larger particle obtained with oils with large molecular weight. A small Pi less than one indicates a narrow and homogenous particle size distribution and thus more stability. All formulations showed Pi values ranged between 0.15 ± 0.03 and 0.24 ± 0.04; which shows low Pi values and a uniform particle size distribution. Zeta potential (ZP) is one of the essential parameters that is used for describing the stability of nano size particles, that it measures the charges on the particles. So it gives an idea about the repulsion between particles with similar charges and the possibility of particles aggregation. Generally, the system stability increases with the increase in electric repulsion between the particles and decreases with the decrease in electric charge, which will lead to particles aggregation.34,35 Table 2 shows that all the nanoemulsion formulations have negative values ranged between 11.0 ± 1.25 mv and 18.87 ± 2.05 mv. Results disclose an accepted repulsion between sample particles and a low possibility of aggregation. Results show that PXM-NE5 prepared from Cr-Le oil and PSO emulsifier showed the highest ZP value (18.87 ± 2.05 mv). The negative charge of PXM-NE formulations may be due to fatty acid contents of the soybean oil, Cermopher EL, and arachis oils and the referral phospholipid of the surfactants. The negatively charged phospholipid increased the repulsion between nano-molecules and hence decreased flocculation, which improve emulsion stability.36
 |
Figure 4 Surface plot shows the effect of oil type (AX) and surfactant type (BX) on the particle size (Y). |
Determination of Nanoemulsion Viscosity
Viscosity measurement is an important parameter reflecting the flow characteristic of nanoemulsion system; thus, for good delivery of the product, the formulation should have good spreading and diffusion ability. Overall, low viscosity of the formulations was detected, which is likely with nanoemulsions (Table 2). Previous studies on nanoemulsion preparations reported that the acceptable viscosity for topical application ranged between 4–7 mPa. The viscosity value of PXM-NE formulations was found to be in the range of 4.52–5.02 mPa, and these values were within the specification for topical preparations. Non-significant difference at level p˂0.1 was observed between formulations which may be due to the relative similarities in the densities of used oils. The low viscosity value of PXM-NE formulations reflects the stability of the formulations in addition to good application characteristics. Relevant results were reported by Lalatsa et al.37
Thermodynamic Stability Tests of PXM Loaded Nanoemulsion
In order to minimize the probability of metastable formulations, stress testing is an important step to confirm the stability of nanoemulsion formulations. All formulations were subjected to three thermodynamic stability tests namely; heating/ cooling cycle, centrifugation, and freeze/thaw cycles.37 Results of thermodynamic stability tests were shown in Table 3. Results reveal that only PXM-NE6, PXM-NE7, and PXM-NE8 formulations are pass all the three stability tests without separation, creaming or cracking signs for that theses formulations will be selected for further studies. The results were in a good accordance with the results reported by Pineros et al38–40
 |
Table 3 Thermodynamic Stability of PXM –NE Formulations |
Identification Tests of Optimized PXM-NE Formulations
Staining Test
The test was performed using oil soluble stain. The optimized formulations (PXM-NE6, PXM-NE7, and PXM-NE8) was found to be o/w type of nano-emulsion. The result shows colorless background with red distributed globules.35
Dilution Test
Samples were diluted to ten times and hundred times its volume and observed visually for any separation or cracking, the test showed that the system was observed transparent and clear without any signs of phase separation or cracking.35
Electrical Conductivity
Electrical conductivity test is proportional to the water percentage. The conductivity (σ) of PXM-NE formulations was found to be 408.23±12.12 μS/cm. This indicates that the nanoemulsion formulation was o/w type of emulsion. The o/w nano-emulsion showed conductivity due to water in the external phase while, w/o nano-emulsion did not show such conductivity.35
Study of Surface Morphology of PXM Loaded Nanoemulsion
TEM analysis was conducted using uranyl acetate as a negative stain. Staining helps in identification of the nanoemulsion droplets and its surrounded hydrated shells, which are stabilized by the surfactant. As shown in Figure 5, PXM loaded nanoemulsion was spherical droplets with smooth surface, flexible boundary, without visible aggregations and uniformly well distributed. The TEM study proves that the implement method was suitable to achieve a stabilized nanosize preparation without aggregations. The reported results were in a good agreement with the result obtained by other nano-delivery studies.41–43
 |
Figure 5 Morphology of PXM loaded nanoemulsion examined using Transmission electron microscopy (TEM) voltage of 15 kV (Magnification ×18,000 and 2500, 000). |
Evaluation of PXM-NE Gel
The prepared gel formulations were transparent with smooth, elegant appearance without any aggregations or particles could be observed. The percentage drug content was ranged between 87.13%±2.46 and 89.23%±3.39 for PXM-NE8 and PXM-NE7 respectively indicating that there was no loss of the drug during the gelling process of the nanoemulsions. This result reflects the efficiency of processing procedure for encapsulation PXM in a successful topical preparation. Percentage transmittance of the nanoemulsion gel was found to be 96.08 ±0.18, 95.18 ±0.28, and 96.08 ±0.18% for PXM-NE 6, PXM-NE7, and PXM-NE8 respectively which prove the clarity and the transparency of the gel preparations. The pH value of the formulations was measured to assure its suitability for topical application. It was found to be 5.72±0.12, 5.58±0.16 for PXM-NE8 and PXM-NE7, and 5.80±0.13 indicating slightly acidic value and hence it would be nonirritant and compatible with the skin. The viscosity of PXM-NE gel was 4.75±0.08, 4.82±0.05, and 4.85±0.08 mp at 5 rpm. In addition it was observed that the viscosity of the gel was decreased with the increase in rpm without any disruption in the internal structure of the gels. The gel displayed pseudoplastic flow behavior but the thixotropy characteristics could not identified. To calculate the spread ability of the gel, the spread ability model was applied to measure the properties of the gel formulation. The spread ability of PXM- NE gels was ranged between 0.512± 0.09 and 0.602± 0.06 confirming the shear thinning behavior of the gels and their suitability for smooth application.44
In vitro Release Profile of PXM-NE & PXM-NE Gels
In vitro release study is an indirect assessment of drug availability, especially in primary stages of formulations design. In vitro release figure express fundamental evidence on the behavior of the formulation in vivo, and their impact on the rate and mechanism of drug release. For topical nanoemulsion preparations, the entrapped drug should be released from nanoemulsion droplets after spreading on the skin to assure that it reached the targeted site of action. Franz cell method was implemented to study the release of drug from the PXM-NE and its gel formulations. In this study, a sink condition was maintained by adding PBS (phosphate buffer pH 7) with emulsion samples onto the donor compartment by 1:2 ratios. The in vitro release profile of PXM is illustrated in Figure 6. Percentage of cumulative release of PXM from the suspension was slow and not more than 45.2±3.51% was released within 8 hours and the drug had reached the steady-state with a non-significant increase in the rate of PXM released after 8 h. Both PXM-NEs and PXM-NE gels exhibited a biphasic release pattern, as shown by an initial rapid release of the drug during the first 2 hours, followed by a slower rate of release for the next 24 hours that it reached a total drug release of 97.20%±3.31 (Figure 6). PXM showed a similar release profile for both the emulsion and the gel with a nonsignificant difference over 24 hours for the three formulations. PXM-NE 7 gel showed the most rapid initial release and the highest cumulative percent (87.20%±4.21) within 4 hours compared with the other formulation which could be explained based on the particle size, poly dispersity index, and encapsulation efficiency. This result was supported by Araújo et al, for thalidomide and Lu et al for clarithromycin who reported the effect of encapsulation efficiency, particle size, and encapsulation efficiency on drug release behavior from nanoemulsion systems.26,45 The release profile data was fitted to zero-o, first-order, Higuchi and Korsmeyer Peppas models to estimate the release pattern from nanocarrier systems and the mechanism of drug release. The values of R “correlation coefficient” of Korsmeyer–Peppas model for release data were greater than 0.97 in nanoemulsion preparations. The drug release pattern from gel revealed zero order release pattern with R2 value 0.985, 0.988 and 0.981, for PXM-NE7 gel, PXM-NE8 gel, and PXM-NE9 gel respectively, while for PXM suspension the drug release pattern showed first order release kinetics with R2 =0.977 during the first 2 hours.
 |
Figure 6 In vitro cumulative amount of PXM release from PXM suspension and selected formulation as nanoemulsion and gel through dialysis membrane. |
Analgesic Activity Study
Based on previous results The PXM nanoemulsion formula (PXM-NE7) composed of Arc oil and LL S100 surfactants in a gel preparation was selected to evaluate the analgesic activity of PXM in the comparison with control group. Tail flick method was performed to evaluate the analgesic activity of PXM in presence of external pain stimuli. Tail removal time “time taken until mice start to withdraw its tail from the radiant source” was reserved as the end point. Group I did not receive any medication, and used as a –ve control for the evaluation of analgesic activity in Group II, and III (Tables 4). The experiment showed that the tail removal time did not exceed 1.8±0.60 sec in the – ve control group while the use of PXM-NE hydrogel and commercial gel induced significant (p <0.05) analgesic effect compared with the – ve control group, that the tail withdraw time in group II was 4.3 ±0.10 sec after 0. 5 h, and the analgesic activity was detected up to 8 hours with a maximum analgesic effect was observed after 4 hours 7.2 ±0.80 sec. Table 4, showed that the tail withdrawal time for Group III was 3.9 ±0.15 sec after 0.5 h with maximum effect was observed after 2 hours followed by rapid decline in analgesic activity. Results reveal that the analgesic activity was 1.66 times higher than the commercial gel with a double duration of analgesic activity. This finding could be attributed based on the higher ability of PXM to be absorbed, delivered, penetrated, and diffused through skin layers from the nano emulsion dosage form.7 Many lipophilic drugs showed high mobilization with respect to leaving the oily phase and penetrating through skin lipid layers. For example, cyclosporine A and paclitaxel although its high lipophilicity it exhibits high exchange between different lipid layers therefore, it is assumed that highly lipophilic drugs have a good ability to pass through membranes especially high phospholipid layers that the main transmitting mechanism is the impact between the lipid vesicles.46 This observation was in agreement with the in vitro release results which revealed a rapid and complete release of PXM from nanogel.
 |
Table 4 Analgesic Activity Time (Mean ± SE) of PXM-NE Gel and PXM Commercial Gel in Mice Using Tail-Flick Method |
In vivo Pharmacokinetic Study
Plasma levels data of PXM after topical administration of PXM-NE7 gel and commercial gel showed that nanoemulsion gel showed higher bioavailability compared with the control gel. Table 5 and Figure 7 show that the peak plasma concentrations of PXM (Cmax) were 45.73± 9.95 and 28.48 ± 6.44 ng/mL for PXM-NE7 gel and commercial gel, respectively. Results showed that a 1.6 fold increase in Cmax was observed for the selected formula compared with the commercial gel. The enhancement in penetration of PXM (lipophilic drug) could be expressed based on the higher solubilization of the drug in the lipophilic area of the subcutaneous region because of solubilization of drug in an oil followed by its incorporation in a nanoemulsion formulation. Also, there was a significant increase at p˂0.01 in the area under the curve of plasma concentration vs time plot of PXM after topical administration of PXM-NE7 gel (168.58 ± 28.70 ng/mL h) and the control gel (69.70 ± 23.47 ng/mL h). The time to achieve the maximum concentration was 1.71 ± 0.21 h for PXM-NE7 gel while it was 1.66 ± 0.11 h for commercial gel. The study showed a 2.4 fold enhancement in the bioavailability of PXM after topical administration of PXM-NE7 gel. Formulation of PXM in a nonemulsion form helps in increasing the surface area and hence improve its absorption and bioavailability.46,47 The results in a good agreement with Shakeel et al who prepared nanoemulsion of celecoxib for improving its bioavailability and anti-inflammatory activity.23,48
 |
Table 5 Pharmacokinetic Parameters of PXM After Topical Administration of PXM-NE7 Gel and Commercial Gel to Rats |
 |
Figure 7 Plasma concentration time curve of PXM after topical administrations of PXM-NE7 Gel and marketed gel in rats (mean ± SE). |
Conclusion
Piroxicam is one of the most effectively used NSAIDs for the treatment of arthritis. Present research study shows the design of a new formulation through topical administration which aimed to minimize the side effects of PXM. Soybean, Cremophor EL, and arachis oils were used for incorporating PXM in a successful nanoemulsion formula using LL S100: PS, and PGC as emulsifiers. The optimized formulation revealed improved stability, acceptable particle size and in vitro release properties, enhanced analgesic activity, and 2.4 fold increase in bioavailability. Hence the research work has confirmed that PXM-NE gel is a promising alternative in the treatment of rheumatoid arthritis complications through topical application.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Ramadan G, El-Menshawy O. Protective effects of ginger-turmeric rhizomes mixture on joint inflammation, atherogenesis, kidney dysfunction and other complications in a rat model of human rheumatoid arthritis, Int. J Rheum Dis. 2013;16:219–229. doi:10.1111/1756-185X.12054
2. Mediavilla VG, Crespo I, Collado PS, et al. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C- protein, and down-regulation of the nuclear factor kappa- B pathway in Chang Liver cells. Eur J Pharmacol. 2007;557:221–229. doi:10.1016/j.ejphar.2006.11.014
3. Cipriani P, Ruscitti P, Carubbi F, Liakouli V, Giacomelli R. Methotrexate in rheumatoid arthritis: optimizing therapy among different formulations. Current and emerging paradigms. Clin Ther. 2014;36(3):427–435. doi:10.1016/j.clinthera.2014.01.014
4. Gilani SJ, Bin-Jumah MN, Imam SS, Alshehri S, Jahangir MA, Zafar A. Formulation and optimization of nano lipid based oral delivery systems for arthritis. Coatings. 2021;11:548. doi:10.3390/coatings11050548
5. Anita C, Munira M, Mural Q, Shaily L. Topical nanocarriers for management of rheumatoid arthritis: a review. Biomed Pharmacoth. 2021;141:111880.
6. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284(10):1247–1255. doi:10.1001/jama.284.10.1247
7. Ibrahim M, Amin M, Fetih G, Abou Ela A. Formulation and evaluation of ketorolac tromethamine-Eudragit solid dispersions of potential sustained release properties. J Pharm Partiques. 2010;20:189–200.
8. Borgia SL, Regehly M, Sivaramakrishnan R, et al. Lipid nanoparticles for skin penetration enhancement-correlation to drug localization within the particle matrix as determined by fluorescence and parelectric spectroscopy. J Contr Rel. 2005;110:151–163. doi:10.1016/j.jconrel.2005.09.045
9. Bowser PA, White RJ. Isolation, barrier properties and lipid analysis of stratum compactum, a discrete region of the stratum corneum. Br J Dermatol. 1985;112:1–14. doi:10.1111/j.1365-2133.1985.tb02285.x
10. Prabhu S, Ortega M, Ma C. Novel lipid-based formulations enhancing the in vitro dissolution and permeability characteristics of a poorly water –soluble model drug, piroxicam. Int J Pharm. 2005;301:209–216. doi:10.1016/j.ijpharm.2005.05.032
11. Wu K, Li J, Wang W, Winstead DA. Formation and characterization of solid dispersions of piroxicam and polyvinylpyrrolidone using spray drying and precipitation with compressed antisolvent. J Pharm Sci. 2009;98:2422–2431. doi:10.1002/jps.21598
12. Gangadharappa HV, Prasad SM, Singh RP. Chandra Prasad, Rudra Pratap Singh. Formulation, in vitro and in vivo evaluation of celecoxib nanosponge hydrogels for topical application. J Drug Deliv Sci Technol. 2017;41:488–501. doi:10.1016/j.jddst.2017.09.004
13. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–1080. doi:10.1056/NEJMoa050405
14. Khurana S, Jain NK, Bedi PMS. Nanoemulsion based gel for transdermal delivery of meloxicam: physico-chemical, mechanistic investigation. Life Sci. 2013;92:383–392. doi:10.1016/j.lfs.2013.01.005
15. Morsi N, Ibrahim M, Refai H, Sorogy HE. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of Acetazolamide. Eur J Pharm Sci. 2017;104:302–314. doi:10.1016/j.ejps.2017.04.013
16. Rachmawati H, Budiputra DK, Mauludin R. Curcumin nanoemulsion for transdermal application: formulation and evaluation. Drug Dev Ind Pharm. 2015;41:560–566. doi:10.3109/03639045.2014.884127
17. Sarkar A, Shimu IJ, Tuhin MRH, Raju AA. Nanoemulsion: an excellent mode for delivery of poorly soluble drug through different routes. J Chem Pharm Res. 2015;7:966–976.
18. Bali V, Ali M, Ali J. Study of surfactant combinations and development of a novel nanoemulsion for minimizing variations in bioavailability of ezetimibe. Colloids Surf B. 2010;76:410–420. doi:10.1016/j.colsurfb.2009.11.021
19. Hussain A, Samad A, Singh SK, et al. Nano-emulsion gel-based topical delivery of an antifungal drug: in vitro activity and in vivo evaluation. Drug Deliv. 2014;23:642–657. doi:10.3109/10717544.2014.933284
20. Pople PV, Singh KK. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS PharmSciTech. 2006;7:E63–E69. doi:10.1208/pt070491
21. Date AA, Nagarsenker MS. Parenteral microemulsions: an overview. Int J Pharm. 2008;355:19–30. doi:10.1016/j.ijpharm.2008.01.004
22. Jeyadevi R, Sivasudha T, Rameshkumara A, et al. Enhancement of antiarthritic effect of quercetin using thioglycolic acid-capped cadmium telluride quantum dots as nanocarrier in adjuvant induced arthritic Wistar rats. Colloids Surf B. 2013;112:255–263. doi:10.1016/j.colsurfb.2013.07.065
23. Baboota S, Shakeel F, Ahuja A, Ali J, Shafiq S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007;57:315–332.
24. Soliman SM, Abdel Malak NS, El-Gazayerly ON, Abdel Rehim AA. Formulation of microemulsion gel systems for transdermal delivery of celecoxib: in vitro permeation, anti-inflammatory activity and skin irritation tests. Drug Discov Ther. 2010;4:459–471.
25. Prabhakar K, Afzal SM, Surender G, Kishan V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharm Sin B. 2013;3(5):345–353. doi:10.1016/j.apsb.2013.08.001
26. Araújo FA, Kelmann RG, Araújo BV, Finatto RB, Teixeira HF, Koester LS. European journal of pharmaceutical sciences development and characterization of parenteral nanoemulsions containing thalidomide. Eur J Pharm Sci. 2011;42:238–245. doi:10.1016/j.ejps.2010.11.014
27. Abrol S, Trehan A, Katare OP. Comparative study of different silymarin formulations: formulation, characterization and in vitro/in vivo evaluation. Curr Drug Deliv. 2005;2:45–51. doi:10.2174/1567201052772870
28. Schuh RS, Bruxel F, Teixeira HF. Physicochemical properties of lecithin-based nanoemulsions obtained by spontaneous emulsification or high-pressure homogenization. Quim Nova. 2014;37(7):1193–1198.
29. Burcea-Dragomiroiu G, Cimpoiesu A, Ginghina O, et al. The development and validation of a rapid HPLC method for determination of piroxicam. Farmacia. 2015;63:123–131.
30. Peng J, Dong WJ, Li L, et al. Effect of high-pressure homogenization preparation on mean globule size and large-diameter tail of oil-in-water injectable emulsions. J Food Drug Anal. 2015;23(4):828–835. doi:10.1016/j.jfda.2015.04.004
31. Kawakami K, Yoshikawa T, Moroto Y, et al. Microemulsion formulation for enhanced absorption of poorly soluble drugs II. In vivo study. J Control Rel. 2002;81:75–82. doi:10.1016/S0168-3659(02)00050-0
32. Anuchapreeda S, Fukumori Y, Okonogi S, Ichikawa H. Preparation of lipid nanoemulsions incorporating curcumin for cancer therapy. J Nanotechnol. 2012;2012:11. doi:10.1155/2012/270383
33. Wang JJ, Sung KC, Hu OYP, Yeh CH, Fang JY. Submicron lipid emulsion as a drug delivery system for nalbuphine and its prodrugs. J Control Release. 2006;115(2):140–149. doi:10.1016/j.jconrel.2006.07.023
34. Shaiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of Ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66(2):227–243. doi:10.1016/j.ejpb.2006.10.014
35. Sintov AC, Botner S. Transdermal drug delivery using microemulsion and aqueous systems: influence of skin storage conditions on the in vitro permeability of diclofenac from aqueous vehicle systems. Int J Pharm. 2006;311:55–62. doi:10.1016/j.ijpharm.2005.12.019
36. Van Balen GP, Martinet CM, Caron G, et al. Liposome/water lipophilicity: methods, information content and pharmaceutical applications. Med Res Rev. 2004;24(3):299–324. doi:10.1002/med.10063
37. Lalatsa A, Statts L, Adriana de Jesus J, et al. Topical buparvaquone nano-enabled hydrogels for cutaneous leishmaniasis. Int J Pharm. 2020;588:119734. doi:10.1016/j.ijpharm.2020.119734
38. Pineros I, Slowing K, Serrano DR, de Pablo E, Ballesteros MP. Analgesic and anti-inflammatory controlled-released injectable microemulsion: pseudo-ternary phase diagrams, in vitro, ex vivo and in vivo evaluation. Eur J Pharm Sci. 2017;101:220–227. doi:10.1016/j.ejps.2016.12.030
39. Serrano DR, Gordo MJ, Matji A, González S, Lalatsa A, Torrado JJ. Tuning the transdermal delivery of hydroquinone upon formulation with novel permeation enhancers. Pharmaceutics. 2019;11(4):167. doi:10.3390/pharmaceutics11040167
40. Zhang Q, Jiang X, Jiang W, Lu W, Su L, Shi Z. Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int J Pharm. 2004;275:85–96. doi:10.1016/j.ijpharm.2004.01.039
41. Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. AAPS Pharm Sci Tech. 2006;7(1):E8. doi:10.1208/pt070108
42. Elshafeey AH, Bendas ER, Mohamed OH. Intranasal microemulsion of sildenafil citrate: in vitro evaluation and in vivo pharmacokinetic study in rabbits. AAPS Pharm Sci Tech. 2009;10(2):361–367. doi:10.1208/s12249-009-9213-6
43. El Tahir KE, Abdel-Kader M. Chemical and pharmacological study of Cymbopogon proximus volatile oil. Res J Med Plants. 2008;2(2):52–60.
44. Grassi M, Coceani N, Magarotto L. Mathematical modeling of drug release from microemulsions: theory in comparison with experiments. J Colloid Interface Sci. 2000;228:141–150. doi:10.1006/jcis.2000.6945
45. lu Y, Zhang Y, Yang Z, Tang X. Formulation of an intravenous emulsion loaded with a clarithromycin phospholipid complex and its pharmacokinetics in rats. Int J Pharm. 2009;366(1–2):160–169. doi:10.1016/j.ijpharm.2008.09.008
46. Fahr A, van Hoogevest P, May S, Bergstrand N, Leigh MLS. Transfer of lipophilic drugs between liposomal membranes and biological interfaces: consequences for drug delivery. Eur J Pharm Sci. 2005;26:251–265. doi:10.1016/j.ejps.2005.05.012
47. Mohammed HA, Al-Omar MS, El-Readi MZ, Alhowail AH, Aldubayan MA, Abdellatif AAH. Formulation of ethyl cellulose microparticles incorporated pheophytin a isolated from suaeda vermiculata for antioxidant and cytotoxic activities. Molecules. 2019;24:1501. doi:10.3390/molecules24081501
48. Taha M, Alshamrani FJ, Rahim F, et al. Synthesis, characterization, biological evaluation, and kinetic study of indole base sulfonamide derivatives as acetylcholinesterase inhibitors in search of potent anti-Alzheimer agent. J King Saud Univer. 2021;33(3):101401. doi:10.1016/j.jksus.2021.101401
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.