Back to Journals » International Journal of Nanomedicine » Volume 14
Nano-biotechnology: a new approach to treat and prevent malaria
Authors Rahman K, Khan SU , Fahad S, Chang MX, Abbas A , Khan WU, Rahman L, Haq ZU, Nabi G, Khan D
Received 12 October 2018
Accepted for publication 22 January 2019
Published 21 February 2019 Volume 2019:14 Pages 1401—1410
DOI https://doi.org/10.2147/IJN.S190692
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Khaista Rahman,1,* Shahid Ullah Khan,2,* Shah Fahad,2,3 Ming Xian Chang,4,5 Aqleem Abbas,6 Wasim Ullah Khan,7 Lutfur Rahman,8 Zaheer Ul Haq,9 Ghulam Nabi,4,5 Dilfaraz Khan10
1College of Animal Sciences/State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan 430070, China; 2College of Plant Sciences and Technology/National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University, Wuhan 430070, China; 3Department of Agriculture, The University of Swabi, Khyber Pakhtunkhwa, Anbar, Pakistan; 4State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, the Chinese Academy of Sciences, Wuhan 430072, Hubei, China; 5University of the Chinese Academy of Sciences, Shijingshan District, Beijing, China; 6Provincial Key Lab of Plant Pathology of Hubei Province, Huazhong Agricultural University, Hongshan District, Wuhan 430070, China; 7School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou, China; 8Molecular Systematics & Applied Ethno Botany Lab (MoSEL), Department of Biotechnology, Quaid I Azam University, Islamabad, Pakistan; 9School of Chemistry and Chemical Engineering, Shanghai Jiao tong University, Shanghai, China; 10Institute of Chemical Sciences, Gomal University, Dera Ismail Khan, Pakistan
*These authors contributed equally to this work
Abstract: Malaria, the exterminator of ~1.5 to 2.7 million human lives yearly, is a notorious disease known throughout the world. The eradication of this disease is difficult and a challenge to scientists. Vector elimination and effective chemotherapy for the patients are key tactics to be used in the fight against malaria. However, drug resistance and environmental and social concerns are the main hurdles in this fight against malaria. Overcoming these limitations is the major challenge for the 21st-century malarial researchers. Adapting the principles of nano-biotechnology to both vector control and patient therapy is the only solution to the problem. Several compounds such as lipids, proteins, nucleic acid and metallic nanoparticles (NPs) have been successfully used for the control of this lethal malaria disease. Other useful natural reagents such as microbes and their products, carbohydrates, vitamins, plant extracts and biodegradable polymers, are also used to control this disease. Among these particles, the plant-based particles such as leaf, root, stem, latex, and seed give the best antagonistic response against malaria. In the present review, we describe certain efforts related to the control, prevention and treatment of malaria. We hope that this review will open new doors for malarial research.
Keywords: malaria, vectors, chemotherapy, drug resistance, nano-biotechnology
Introduction
Malaria is the most significant and malicious of all the parasitic human diseases. The causal agent of malaria is the single cell protozoa called Plasmodium. Protozoal vector-borne diseases are the most common infections in developing regions and result in more than a million deaths from malaria every year worldwide.1,2 According to the WHO, malaria is one of the world’s most lethal diseases which caused 214 million new infections and nearly 438,000 malaria-associated deaths in 2015 worldwide.1,3,4 More than 216 million people are still infected by the malarial parasite each year.5 Although the disease is widespread, it is most severe in tropical and subtropical regions. In 2006, ~400,000 cases of malaria were registered in hospitals across the Amazon basin region of Brazil. Due to the overuse of antimalarial drugs, in particular chloroquine, the parasite has developed a variety of resistance mechanisms against antimalarial drugs. Among these Plasmodium spp., Plasmodium falciparum has now become drug resistant and there has been a global resurgence in malaria in recent years. Nowadays, its derivatives are considered to be a first-line antimalarial drug;6–8 however, the parasite is rapidly showing resistance to it.9–11
Four strategies help in controlling malaria. The first one is to eradicate the mosquitoes’ breeding grounds. The second, indoor residual spraying (IRS), has been proven useful in malaria control. Third, there is the use of chemically treated bed nets. The fourth option is effective chemotherapy for infected individuals.12 The first three options were the most effective control tactics against Anopheles mosquitoes to prevent the spread of malaria. Over several decades, the use of chemical compounds, ie, phenols, Paris Green, mercuric chloride, cresols, naphthalene, Bordeaux mixture, rosin fish oil soap and many others as conventional pesticides, was considered reliable sources to control malaria.13 The first synthetic organic insecticide, dichlorodiphenyltrichloroethane (DDT), was synthesized in the 19th century, and this invention was the primary method for vector control.14 The application of IRS, such as DDT and other insecticides, initially eradicated the female mosquitoes responsible for malaria with great success.15 The application of these insecticides, however, has decreased the annual parasite index (API) drastically throughout the world. The reduction in API has stimulated the WHO to develop and implement various control strategies.16 Many researchers are involved in controlling and targeting the adult female at its larval stages. The chemicals that effectively target the adult female mosquitoes are Paris Green (copper acetoarsenite)17 and petroleum oils.18 Many other larvicides, ie, synthetic pyrethroids,19 and many organophosphates20 were rarely exploited against the adult female during this time. The synthetic pyrethroids, although effective, are at the same time extremely lethal to aquatic nontarget organisms, mostly fish.21 The persistent and toxic effects of the applied insecticides were serious impediments to apply these chemicals against malaria. The rise of insecticide-resistant mosquito strains is another major challenge.22
The World Health Assembly (WHA) resolution called for approving and implementing alternate measures in managing malaria through ecologically friendly insecticides rather than through ecologically unfriendly insecticides. The integrated vector management (IVM) approach was adopted which seeks to control the female mosquitoes, either at the immature larval stages or at the mature adult stages by exploiting biological agents, by using biological tools such as viruses, bacteria, fungi, oomycetes, azolla (aquatic fern), and through natural predators.23,24 Although this was seen as the best strategy, very soon various ecological, environmental, social and economic concerns were raised.25 Using the products of these organisms against mosquitoes was an alternative biological control strategy; hence, its low availability,26 high cost27 and the incidence of resistance to larvicides of mosquito larvae are the main concerns to be noted.28 The application of a versatile type of biologically synthesized nanoparticles (NPs) introduced a novel scope of research to study for their utility against mosquitocidal activities in the hope that these NPs make the mosquito body more susceptible due to their biogenic nature as well as being eco-friendly with a minimal dosage and host specificity.29
Nano-biotechnology
New multifunctional gadgets and schemes for higher biochemical evaluation with outstanding qualities, such as better sensitivity, specificity and a higher rate of recognition, have been produced through the utilization of molecular biology with engineering. The word “nano” is a Greek word which means small or dwarf. Similarly, NPs can be defined as the particles, which range in size from 1 to 100 nm in either direction, and can be considered to be up to several 100 nm.2,30 Actually, NPs are aggregates of atoms, ions and molecules. In other words, the term “nano” is used to represent one billionth of a meter as 10−9 m. Similarly, the concept of nanotechnology was first introduced by professor Norio Taniguchi in 1974, and after that the field of nanotechnology has received remarkable attention, especially since the 1980s.1,2,31,32
Nano-biotechnology is, therefore, a combination of both engineering and molecular biology. Nanotechnology has revolutionized the world with nano-objects which include nanotubes, nano-channels (Figure 1), NPs, nano-pores and nano-capacitors.33 These nano-objects have significant analytical applications in the world. Nano-biotechnology can also be defined as the junction of nanotechnology and biotechnology, which intends to create, improve, and utilize nanoscale structures for advanced biotechnology.34 The significant study of nanotechnology is the amalgamation of various types of NPs in different sizes, shapes and chemical compositions with precise discrepancies. In recent years, the production of noble metal NPs, ie, gold, silver, palladium, platinum and zinc from natural sources has procured significance and consideration due to the required need to develop eco-friendly sociable technologies in the material.35,36 The potential advantages of nano-biotechnology emerged when NPs were biosynthesized using naturally found organic compounds such as vitamins, proteins, lipids, carbohydrates, botanical extracts, biodegradable polymers and microorganisms. These developments cause the production of a few inorganic NPs, mostly metal NPs (Figure 2), several metal oxides and salts. Many of the aforementioned raw materials, including plant-based materials, can be exploited to biosynthesize large-scale NPs which would certainly be the best eco-friendly candidates.37 Various plant parts, ie, leaf, root, latex, seed, and stem have been currently utilized to biosynthesize metal NPs. The polyphenols present in the plant parts play a key biochemical role in the biosynthesis of these metal NPs. These polyphenols are widely distributed in the kingdom plantae. The advantages of the biosynthesis of NPs over other chemicals are that it is robust, ecological, recyclable, reproducible, cost-effective and eco-friendly.38 Microbes, such as fungus, bacteria and others can also be utilized to biosynthesize NPs, but the speed of biosynthesis is relatively slower. Moreover, NPs which are limited in shape and size are produced from microbes as compared with plant-based materials. Recently, fungi have drawn more attention as the best candidates for the biosynthesis of AgNPs.39 Anyway, biological materials produce eco-friendly NPs rather than artificially synthesized NPs. More importantly, the routes related to biological-based materials are safe, and there are not any toxic chemicals involved which can affect the producer’s health.40 Furthermore, the mechanisms of these bio-reductive variations in the catalytic actions of substances received through these routes are genuinely precious.
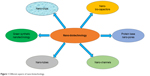  | Figure 1 Different aspects of nano-biotechnology. |
Here, we report on recent developments in this area relevant to NPs and nano-drops for the treatment and prevention of malaria. We also discuss further developmental pathways and the emergence of order in this somewhat chaotic, yet promising, new field.
Nano-biotechnology applied to the treatment of malaria
Nano-biotechnology is the ultimate solution to fight several parasitic diseases such as schistosomiasis, lymphatic filariasis, soil transmitted helminthiasis, parasitic zoonoses, onchocerciasis, African trypanosomiasis, leishmaniasis ectoparasitic skin infections, chagas diseases and others such as malaria, tuberculosis dengue, buruli ulcer and leprosy. Nano-biotechnology works to eradicate malaria by providing satisfactory therapeutic approaches to vector elimination, as well as directly targeting the parasite. The following are some nano-biotechnological efforts that help in the easy and safe treatment of malaria.
Lipid-based NPs
The use of liposomes for the treatment of malaria and leishmaniasis was reviewed over 20 years ago.41 In general, liposomal formulation seems superior when compared with treatments with the free drug. There are several examples which illustrate that, upon targeting a high amount of drugs to the infected tissues, consequently the toxicity of the drug is dramatically reduced. Moreover, the effectiveness of the remedy is improved with the aid of enhancing the dose rather than increasing the dosage supplied to patients because of the safety profile of liposomal formulations. Considering malaria as a case study where the parasites are commonly positioned in red blood cells (RBCs).
Consequently using nanotechnology can lessen the toxicity of the drug molecules. Furthermore, some studies have to remain in the or have to engage with infected RBCs.34 The administration of the antimalarial drug with liposomes targeted to infected RBCs with a tagged antibody against infected erythrocytes surface antigens on the chloroquine liposomes against drug-resistant Plasmodium berghei, presented a cure of 75%–90% in infected mice.42 Artesunate liposomes were used to reduce the dosing frequency by using a low release for 24 hours43 and with encapsulated beta-artemether for a malaria-resistant treatment.37 Artemether and lumefantrine co-loaded into small lipid nano-drops has a higher efficacy and can easily access the target site.44 Recently, it has been reported that altimeter alone loaded into lipid NPs is more efficient for the treatment of meningeal malaria in animal models.45
Different kinds of liposomes, either encapsulated or coupled with recombinant human tumor necrosis factor against experimental cerebral malaria (ECM) induced in P. berghei K 173-infected mice were also proven to be efficient.38 This review showed that liposome coupled with a human necrosis factor was more efficient than its free form in preventing ECM-associated mortality by suppressing mice parasitemia.
Liposomes with a Plasmodium amino acid sequence were effective against P. berghei-infected mice. The peptide which contained a conserved region I as well as a consensus heparin sulfate proteoglycan-binding sequence attached to the distal end of a lipid Y polyethylene glycol bio-conjugated, was incorporated into phosphatidylcholine liposomes.39 Moreover, mice immunization with RTS, S malaria antigen encapsulated in liposomes containing lipid A-induced high levels of antibody and cytotoxic T cell immune response in comparison with non-fusion RTS, S.40 Recently, a vaccine based on an RTS, S antigen has been successfully used in preventing malaria in African children.46
Poly ethylene glycol (PEG)-coated halofantrine loaded poly-D,L-lactic acid (PLA) nano-capsules were studied against malaria, and the reduction in its cardiotoxicity was evaluated in mice infected with P. berghei. In the experiment, NPs of primaquine on poly (diethylmethylidene malonate) were assessed in mice infected with P. berghei which demonstrates a higher increased life span (ILS) index for primaquine-loaded NPs.47 The same molecule was encapsulated in albumin and gelatin NPs of different sizes47 for liver targeting. Very recently, primaquine was formulated in lipid nano-emulsion (10–200 nm) and was very effective as an antimalarial agent against P. berghei infection in Swiss albino mice.48
Chloroquine phosphate, an antimalarial drug, was encapsulated in gelatin NPs and swelling-controlled delivery was demonstrated in a physiological medium (pH 7.4), while a lower release was observed in the acidic pH range.49 Transferrin-conjugated solid lipid NPs (SLNs) were studied with the target of reaching the brain for cerebral malaria treatment. This study demonstrated significantly enhanced brain uptake of quinine compared with the unconjugated SLNs or drug solution.50 Currently, the anti-plasmodial (antimalarial) biological properties of violacein were turned into validated in vitro and in vivo.51
In a study, the NP violacein was investigated against Plasmodium chabaudi taking the mouse as a model animal and an increment of the anti-plasmodial activity was found on a daily basis. However, noninfected mice procuring the same doses of violacein did not exhibit any significant change in anti-plasmodial activity. Similarly, in vitro assays against the P. falciparum revealed that violacein is 300 times more effective than the commonly found antimalarial drug quinine.52
Currently, the effects of antimalarial drugs loaded into immunoliposomes targeted with the packed red blood cells (pRBC)-specific monoclonal antibody have been studied.53 More recently, Urbán et al54 encapsulated chloroquine and fosmidomycin in the liposomes and observed that immunoliposome encapsulation of chloroquine and fosmidomycin improves by tenfold the efficacy of antimalarial drugs.
Nucleic acid-based nano-therapy of malaria
MicroRNAs are small regulatory noncoding RNAs that are involved in targeting and silencing genes. These microRNAs, although they are physiological regulators, can be used as therapeutic agents for many diseases.55 These microRNAs have played a satisfactory role in preventing malaria. An effort has been made which showed that P. falciparum has a susceptibility to antisense oligonucleotide NPs (ODN-NS).56
This fascinating method generally utilizes antisense oligodeoxy (OD) N-chitosan particles which are 50 mm in size. These particles increase the antisense ODN internalization by P. falciparum infected erythrocytes through erythrocyte permeation pathways that target the Plasmodium topoisomerase II gene.57 ODN chitosan NPs, both negatively and positively charged surfaces as well as free antisense ODNs in a concentration of 0.5 μM, exhibited a sequence-specific inhibition compared with sense sequence controls. The major difference between the ODN–chitosan NPs and free ODNs is specificity. ODN–chitosan NPs were found to be more sequence specific in their antisense effect than free ODNs. ODN–chitosan NPs were found to be more sequence-specific inhibitors in their antisense effect as compared with free ODNs. Similarly, the negatively charged surface of ODN–chitosan NPs showed the pronounced effect of about 87% on the P. berghei growth while the positive charged surface showed 74% and free ODNs were 68%. It was the first report which demonstrated the susceptibility of P. falciparum to these microRNAs.58
Protein-based nano-therapy for malaria
Presently, work is going on to make protein-based nanocarriers for antimalarial drug delivery. The only option until now is the use of a collagen denatured biodegradable and biocompatible protein called gelatin.59 Gelatin is a compound where amide groups rarely make the gelatin positively charged having an isoelectric point.60 Furthermore, gelatin is a pharmaceutical adjuvant and approved plasma expander due to its safety record.61 Due to its gelling quality, it can be utilized in cosmetic products and also as a food additive. Among the natural polymers which are used in pharmaceutical nanotechnology research, gelatin has a key role in the nano-delivery system for bioactive compounds. The loading of drugs then proceeds via polyion complexation between drug molecules and surface-charged groups on the gelatin.60 The gelatin NPs were obtained by a double dissolution process which may then be stabilized by a suitable cross-linking agent and can be used for the optimal delivery of chloroquine at a physiological pH.49 However, satisfactory antimalarial activity and safety have not been seen.
Recently, nano-protein adjuvants have been used to carry malaria-specific antigens to the target receptors successfully. These adjuvants in conjugation with specific antigens ranging in size from 16 to 73 nm diameter upon injection into the mice showed a better immune response against malaria as compared with antigens alone.62 However, the extrinsic protein adjuvants have a limited use due to their low compatibility with target vaccine/antigens.63
More recently, Kaba et al64 has developed and designed a self-assembling protein nanoparticles (SAPNs) containing epitopes from the Plasmodium falciparum circumsporozoite protein (PfCSP) and portions of the tool-like receptor 5 (TLR5) agonist flagellin as an intrinsic adjuvant, which was more immunogenic and protective in the mouse model.
Green nano-biotechnology is a future promising field for the treatment of malaria
The biogenic synthesis of metallic NPs such as silver, gold copper and zinc using various biological materials has potential antimalarial activities against different Plasmodium species (Table 1). Until now, the green NPs such as silver, palladium, and platinum have proven more effective in controlling mainly the malarial parasites. The biologically synthesized silver has a significant role in overwhelming malarial production.65 The biologically synthesized NPs have tremendous advantages over other methods. These NPs are eco-friendly. In green synthesis, health perilous stabilizing and reducing agents can be replaced by important biomolecules such as carbohydrates, lipids, proteins, which are produced by organisms ie, fungi, algae, bacteria, yeast and plants. In this section we précis some biological systems which are eco-friendly for the synthesis of AgNPs. The synthesis of AgNPs via plants is the most valuable, eco-friendly and cost-effective method.66 Krishnaraj et al67 and Veerasamy et al68 used the leaf extracts of the medicinal plants Acalypha indica and Garcinia mangostana and synthesized AgNPs in the range of 20–30 and 35 nm, respectively. Chandran et al69 and Li et al70 also used the leaf extracts of the medicinal plants Aloe vera and Capsicum annuum for the synthesis of AgNPs, respectively.
  | Table 1 Effects of some photosynthetic metallic NPs against malaria parasites |
Additionally, it was found that the biogenic synthesized AgNPs from the leaf extract of Euphorbia hirta (40–50 nm) showed strong activity.71
Like plants, both Gram-positive and Gram-negative bacteria, have been used for the green synthesis of AgNPs.72 A few bacteria have the capability to produce extracellular AgNPs, at the same time as others that can synthesize intracellular AgNPs. Fascinatingly, some bacteria including Calothrix pulvinata, Anabaena flos-aquae,73 Vibrio alginolyticus,74 Aeromonas spp. SH10,75 Plectonema boryanum UTEX 485,75,76 and Lactobacillus spp.77 have the potential to produce extra- and intracellular AgNPs. Recently, it was demonstrated that the Bacillus licheniformis was used for the synthesis of AgNPs. Forty- and fifty-nanometer AgNPs were synthesized, respectively.78,79 Fungus is also used in an eco-friendly way for the synthesis of AgNPs. Recently, two reported fungal strains, ie, Penicillium expansum HA2N and Aspergillus terreus HA1N, produced 14–25 nm and 10–18 nm AgNPs, respectively. Both species showed excellent antifungal potential.80 Further studies demonstrated that the biogenic synthesized AgNPs from the Aspergillus fumigatus and Fusarium oxysporum, size 5–25 and 5–50 nm, respectively, had strong potential against fungal strains.81,82 Recently, studies showed that algae have also been used for the green production of AgNPs. Red aquatic algae were used for the production of AgNPs. The Venkatpurwar and Pokharkar83 reported that the synthesized AgNPs demonstrated strong activity against bacteria. Like other biological system, polysaccharides have also been extensively used in the production of AgNPs.66 The starch solution acts as a reducing/capping agent and when treated with AgNO3 stable AgNPs in the range of 10–34 nm were produced.84 Similarly, gum ghatti and gum kondagogu were used as a reducing and a stabilizing agent for the green synthesis of AgNPs.85,86
Using gum ghatti, narrow-sized (4.8–6.4 nm) AgNPs were produced, whereas gum kondagogu produced (2–9 nm) AgNPs.86 It was also found that cellulose also plays an excellent role in the production of AgNPs. While in Ag ion formation, aldehyde and alcohol functional groups in cellulose play a major role in reduction and stabilization.87
Similarly, other biological systems/biomolecules, proteins and DNA are also used for the green synthesis of AgNPs. Recently, it was demonstrated that the fabrication of AgNPs decorated graphene oxide (GO), as an effective antibacterial agent.88 Furthermore, it was found that the synthesis of AgNPs decorated on magnetic GO nano carbons (NCs) was shown to have a highly effective inhibitory property and reusability, even at a very low concentration (12.5 ppm).89
Nano-biotechnology for the control of malaria vectors
The investigation into a single control tool for use against malaria has been, and could probably remain, the using dynamic potency for maximum scientists convoluted in malaria research. Although anti-vector measures in malaria control need to be the goal for an economic of the transmission potential ideally to under the crucial stage for sustained transmission.90 Nano-biotechnology has the potential to control the population of malarial vectors by targeting different biochemical,91 physiological and molecular92 activities; currently, we are discussing the role of green nanotechnology in mosquito control.
Green nanotechnology as a future promising field for controlling malaria vectors
The green synthesis of NPs has received tremendous attention due to its cost-effective and eco-friendly properties.93 Presently, fungi are being utilized in nanotechnology for the production of NPs; synthesis using fungi has shown that this environmentally benign and renewable source can be used as an effective reducing agent for the synthesis of metallic NPs by using a filamentous fungus Cochliobolus lunatic which have strong activities against Anopheles stephensi.94 The extracellular synthesized gold NPs formed by using Aspergillus niger are highly toxic for malarial vectors. However, no side effects were seen in the environment.95 Similarly, the same author described that Chrysosporium tropicum, a pathogenic fungus-mediated silver and gold NPs, can kill A. stephensi.95 Many biologically synthesized NPs of gold and silver by using soil fungi to kill A. stephensi.96–98 Many entomopathogenic fungi such as Trichoderma harzianum that are used conventionally for the biological control of pests can be used to synthesize metallic NPs that can kill the malaria vector at any stage of development.99
Despite fungal mediated NPs, bacteria and plants also have the ability to stabilize metallic NPs with different biological activities. Bacteria synthesized NPs of silver, copper, gold zinc and cobalt by using Bacillus thuringiensis that can be used for the control of malaria vectors in many parts of the world.100,101 Over the next 2 decades, the photosynthesis of NPs of different metals have gained attention for use as a tool for controlling mosquitoes. In the future biosynthesized silver NPs (AgNPs), which cause lower ecological damage, will be a potential replacement for synthetic chemical insecticides. Hence, the need to use green synthesized NPs for the control of mosquitoes causing malaria (Table 2);102 however, the potential of plants for the biosynthesis of NPs against malaria vectors are yet to be fully explored.
  | Table 2 Effects of some photosynthetic metallic NPs against malaria vectors |
Nano-biotechnology in laboratory and the market: limitations
Although nano-biotechnology have a promising field to treat malaria and control the parasites but, no proper mechanism of action of these particles have been illustrated.103 Chemically, physically synthesized NPs have their particular dimension and structure and can be toxic as a particular matter.104 Thus, despite their therapeutic potential these NPs may also affect other tissues.105 Most of the nanotechnological approaches associated with the delivery of drugs cannot define the correct concentration of a particular drug and have very short term effects. However, currently Bakshi et al106 designed a long-acting injectable formulation of atovaquone solid drug NPs that have defined concentration and long-lasting effects.
From an entomological point of view, research focused on the toxicity of NPs against larval and pupal stages of mosquitoes, but there is limited information available about the effects of these particles on the adult mosquitoes and ovicidal property of these particles.107 Thus, despite extensive research in this field, there are still many puzzles that needed to be resolve.
Conclusion
Malaria is still a persistent challenge for modern research. In the early 20th century, scientists were trying to describe an effective method to eradicate malaria. However, no satisfactory and future promising method was explained due to drug resistance, and social and environmental concerns. However, for the past 2 decades, the development of advanced techniques in nano-biotechnology, such as the introduction of green nanotechnology, designing liposomes, the development of tissue-specific nano-pores and nano–bio-circuits have opened the door for a safe and eco-friendly therapeutic method for malaria. However, more study is needed in the field to explore the correct mechanism of action and the side effects.
Acknowledgment
This work was supported by grants (31672687 and 31873046) from the National Natural Science Foundation of China.
Disclosure
The authors report no conflicts of interest in this work.
References
Khan SU, Anjum SI, Ansari MJ, et al. Antimicrobial potentials of medicinal plant’s extract and their derived silver nanoparticles: a focus on honey bee pathogen. Saudi J Biol Sci. 2018. | ||
Ullah Khan S, Saleh TA, Wahab A, et al. Nanosilver: new ageless and versatile biomedical therapeutic scaffold. Inter J Nanomedicine. 2018;13:733–762. | ||
Kolluri N, Klapperich CM, Cabodi M. Towards Lab-on-a-Chip diagnostics for malaria elimination. Lab Chip. 2018;18(1):75–94. | ||
Ismail M, Ling L, Du Y, Yao C, Li X. Liposomes of dimeric artesunate phospholipid: a combination of dimerization and self-assembly to combat malaria. Biomaterials. 2018;163:76–87. | ||
Alonso P, Noor AM. The global fight against malaria is at crossroads. Lancet. 2017;390(10112):2532–2534. | ||
Talisuna AO, Bloland P, D’Alessandro U. History, dynamics, and public health importance of malaria parasite resistance. Clin Microbiol Rev. 2004;17(1):235–254. | ||
White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113(8):1084–1092. | ||
World Health Organization. Guidelines for the Treatment of Malaria: World Health Organization; 2015. | ||
Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32(13):1655–1660. | ||
Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol. 2006;36(14):1427–1441. | ||
Amato R, Pearson RD, Almagro-Garcia J, et al. Origins of the current outbreak of multidrug-resistant malaria in Southeast Asia: a retrospective genetic study. Lancet Infect Dis. 2018;18(3):337–345. | ||
World Health Organization. World Malaria Report. Geneva: World Health Organization; 2008. | ||
Kamareddine L. The biological control of the malaria vector. Toxins. 2012;4(9):748–767. | ||
Hassall KA. The Chemistry of Pesticides: Their Metabolism, Mode of Action and Uses in Crop Protection. Macmillan; 1982. | ||
Wakabi W. Africa counts greater successes against malaria. Lancet. 2007;370(9603):1895–1896. | ||
Pant CP, Rishikesh N, Bang YH, Smith A. Progress in malaria vector control. Bull World Health Organ. 1981;59(3):325–333. | ||
Rozendaal JA. Vector control: methods for use by individuals and communities. World Health Org. 1997. | ||
Pal R, Gratz NG. Larviciding for mosquito control. Int J Pest Manag. 1968;14(4):447–455. | ||
Parvez S, Al Wahaibi S. Comparison of three larviciding options for malaria vector control. East Mediterr Health J. 2003;9(4):627–636. | ||
Pattanayak S, Sharma V, Kalra N, et al. Malaria paradigms in India and control strategies. Indian J Malariol. 1994;31(4):141–199. | ||
Saha S, Kaviraj A. Acute toxicity of synthetic pyrethroid cypermethrin to some freshwater organisms. Bull Environ Contam Toxicol. 2008;80(1):49–52. | ||
Brown AW. Laboratory studies on the behaviouristic resistance of Anopheles albimanus in Panama. Bull World Health Organ. 1958;19(6):1053. | ||
Chapman HC. Biological control of mosquito larvae. Annu Rev Entomol. 1974;19(1):33–59. | ||
Floore TG. Biorational control of mosquitoes. Am Mosq Control Assoc. 2007;23(2):1–2. | ||
Beier JC. Malaria control in the highlands of Burundi: an important success story. Am J Trop Med Hyg. 2008;79(1):1–2. | ||
Aiub CA, Coelho EC, Sodré E, Pinto LF, Felzenszwalb I. Genotoxic evaluation of the organophosphorous pesticide temephos. Genet Mol Res. 2002;1(2):159–166. | ||
Pinheiro VCS, Tadei WP. Evaluation of the residual effect of temephos on Aedes aegypti (Diptera, Culicidae) larvae in artificial containers in Manaus, Amazonas state, Brazil. Cad Saúde Pública. 2002;18(6):1529–1535. | ||
Braga IA, Lima JB, Soares Sda S, Valle D. Aedes aegypti resistance to temephos during 2001 in several municipalities in the states of Rio de Janeiro, Sergipe, and Alagoas, Brazil. Mem Inst Oswaldo Cruz. 2004;99(2):199–203. | ||
Haldar KM, Haldar B, Chandra G. Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (wall.). Parasitol Res. 2013;112(4):1451–1459. | ||
Ping G, Huimin L, Xiaoxiao H, et al. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology. 2007;18(28):285604. | ||
Fox CL, Modak SM. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob Agents Chemother. 1974;5(6):582–588. | ||
Taniguchi N, Arakawa C, Kobayashi T. (1974). On the basic concept of ‘nano-technology’. In Proceedings of the International Conference on Production Engineering, 1974;8(2):18–23. | ||
Fortina P, Kricka LJ, Surrey S, Grodzinski P. Nanobiotechnology: the promise and reality of new approaches to molecular recognition. Trends Biotechnol. 2005;23(4):168–173. | ||
Goodsell DS. Bionanotechnology: Lessons from Nature. John Wiley & Sons; 2004. | ||
Jia L, Zhang Q, Li Q, Song H. The biosynthesis of palladium nanoparticles by antioxidants in Gardenia jasminoides Ellis: long lifetime nanocatalysts for p-nitrotoluene hydrogenation. Nanotechnology. 2009;20(38):385601. | ||
Song JY, Kwon EY, Kim BS. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst Eng. 2010;33(1):159–164. | ||
Chimanuka B, Gabriëls M, Detaevernier MR, Plaizier-Vercammen JA. Preparation of beta-artemether liposomes, their HPLC-UV evaluation and relevance for clearing recrudescent parasitaemia in Plasmodium chabaudi malaria-infected mice. J Pharm Biomed Anal. 2002;28(1):13–22. | ||
Postma NS, Crommelin DJ, Eling WM, Zuidema J. Treatment with liposome-bound recombinant human tumor necrosis factor-alpha suppresses parasitemia and protects against Plasmodium berghei k173-induced experimental cerebral malaria in mice. J Pharmacol Exp Ther. 1999;288(1):114–120. | ||
Longmuir KJ, Robertson RT, Haynes SM, Baratta JL, Waring AJ. Effective targeting of liposomes to liver and hepatocytes in vivo by incorporation of a Plasmodium amino acid sequence. Pharm Res. 2006;23(4):759–769. | ||
Richards RL, Rao M, Wassef NM, et al. Liposomes containing lipid A serve as an adjuvant for induction of antibody and cytotoxic T-cell responses against RTS,S malaria antigen. Infect Immun. 1998;66(6):2859–2865. | ||
Alving CR. Liposomes as drug carriers in leishmaniasis and malaria. Parasitol Today. 1986;2(4):101–107. | ||
Owais M, Varshney GC, Choudhury A, Chandra S, Gupta CM. Chloroquine encapsulated in malaria-infected erythrocyte-specific antibody-bearing liposomes effectively controls chloroquine-resistant Plasmodium berghei infections in mice. Antimicrob Agents Chemother. 1995;39(1):180–184. | ||
Gabriëls M, Plaizier-Vercammen J. Physical and chemical evaluation of liposomes, containing artesunate. J Pharm Biomed Anal. 2003;31(4):655–667. | ||
Parashar D, Aditya NP, Murthy RS. Development of artemether and lumefantrine co-loaded nanostructured lipid carriers: physicochemical characterization and in vivo antimalarial activity. Drug Deliv. 2016;23(1):123–129. | ||
Vanka R, Kuppusamy G, Praveen Kumar S, et al. Ameliorating the in vivo antimalarial efficacy of artemether using nanostructured lipid carriers. J Microencapsul. 2018;35(2):121–136. | ||
Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS, S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364(9443):1411–1420. | ||
Mbela TKM, Poupaert JH, Dumont P. Poly(diethylmethylidene malonate) nanoparticles as primaquine delivery system to liver. Int J Pharm. 1992;79(1–3):29–38. | ||
Singh KK, Vingkar SK. Formulation, antimalarial activity and biodistribution of oral lipid nanoemulsion of primaquine. Int J Pharm. 2008;347(1–2):136–143. | ||
Bajpai AK, Choubey J. Design of gelatin nanoparticles as swelling controlled delivery system for chloroquine phosphate. J Mater Sci Mater Med. 2006;17(4):345–358. | ||
Gupta Y, Jain A, Jain SK. Transferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrochloride to the brain. J Pharm Pharmacol. 2007;59(7):935–940. | ||
Costa FT, Avril M, Nogueira PA, Gysin J. Cytoadhesion of Plasmodium falciparum-infected erythrocytes and the infected placenta: a two-way pathway. Braz J Med Biol Res. 2006;39(12):1525–1536. | ||
Durán N, Justo GZ, Ferreira CV, et al. Violacein: properties and biological activities. Biotechnol Appl Biochem. 2007;48(Pt 3):127–133. | ||
Urbán P, Estelrich J, Cortés A, Fernàndez-Busquets X. A nanovector with complete discrimination for targeted delivery to Plasmodium falciparum-infected versus non-infected red blood cells in vitro. J Control Release. 2011;151(2):202–211. | ||
Urbán P, Estelrich J, Adeva A, Cortés A, Fernàndez-Busquets X. Study of the efficacy of antimalarial drugs delivered inside targeted immunoliposomal nanovectors. Nanoscale Res Lett. 2011;6(1):620. | ||
Rahman K, Shah AA, Khan MH, et al. In silico profiling of regulatory MicroRNA targets in GJB3 gene. Global J Biotechnol Biochem. 2014;9(2):41–49. | ||
Gujjar R, Marwaha A, El Mazouni F, et al. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J Med Chem. 2009;52(7):1864–1872. | ||
Phillips MA, Gujjar R, Malmquist NA, et al. Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite Plasmodium falciparum. J Med Chem. 2008;51(12):3649–3653. | ||
James TY, Pelin A, Bonen L, et al. Shared signatures of parasitism and phylogenomics unite Cryptomycota and microsporidia. Curr Biol. 2013;23(16):1548–1553. | ||
Yang D, Li Y, Nie J. Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs. Carbohydrate Polymers. 2007;69(3):538–543. | ||
Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109(1–3):256–274. | ||
Kuntworbe N, Martini N, Shaw J, Al-Kassas R. Malaria intervention policies and pharmaceutical nanotechnology as a potential tool for malaria management. Drug Dev Res. 2012;73(4):167–184. | ||
Scaria PV, Chen B, Rowe CG, et al. Protein-protein conjugate nanoparticles for malaria antigen delivery and enhanced immunogenicity. PLoS One. 2017;12(12):e0190312. | ||
Karch CP, Li J, Kulangara C, et al. Vaccination with self-adjuvanted protein nanoparticles provides protection against lethal influenza challenge. Nanomedicine. 2017;13(1):241–251. | ||
Kaba SA, Karch CP, Seth L, et al. Self-assembling protein nanoparticles with built-in flagellin domains increases protective efficacy of a Plasmodium falciparum based vaccine. Vaccine. 2018;36(6):906–914. | ||
Kuppusamy P, Yusoff MM, Maniam GP, Govindan N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications – An updated report. Saudi Pharm J. 2016;24(4):473–484. | ||
Ullah Khan S, Saleh TA, Wahab A, et al. Nanosilver: new ageless and versatile biomedical therapeutic scaffold. Int J Nanomedicine. 2018;13:733–762. | ||
Krishnaraj C, Jagan EG, Rajasekar S, et al. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids and Surfaces B Biointerfaces. 2010;76(1):50–56. | ||
Veerasamy R, Xin TZ, Gunasagaran S, et al. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc. 2011;15(2):113–120. | ||
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22(2):577–583. | ||
Li S, Shen Y, Xie A, et al. Green synthesis of silver nanoparticles using Capsicum annuum L. Extract. Green Chem. 2007;9(8):852–858. | ||
Elumalai E, Prasad T, Hemachandran J, et al. Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J Pharm Sci Res. 2010;2(9):549–554. | ||
Parikh RY, Ramanathan R, Coloe PJ, et al. Genus-wide physicochemical evidence of extracellular crystalline silver nanoparticles biosynthesis by Morganella spp. PLoS One. 2011;6(6):e21401. | ||
Brayner R, Barberousse H, Hemadi M, et al. Cyanobacteria as bioreactors for the synthesis of Au, Ag, PD, and Pt nanoparticles via an enzyme-mediated route. J Nanosci Nanotechnol. 2007;7(8):2696–2708. | ||
Rajeshkumar S, Malarkodi C, Paulkumar K, et al. Intracellular and extracellular biosynthesis of silver nanoparticles by using marine bacteria Vibrio alginolyticus. Nanosci Nanotechnol. 2013;3(1):21–25. | ||
Mouxing F, Qingbiao L, Daohua S, et al. Rapid preparation process of silver nanoparticles by bioreduction and their Characterizations1. Chin J Chem Eng. 2006;14(1):114–117. | ||
Lengke MF, Fleet ME, Southam G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(I) nitrate complex. Langmuir. 2007;23(5):2694–2699. | ||
Nair B, Pradeep T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des. 2002;2(4):293–298. | ||
Kalishwaralal K, Deepak V, Ramkumarpandian S, et al. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater Lett. 2008;62(29):4411–4413. | ||
Kalimuthu K, Babu RS, Venkataraman D, et al. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 2008;65(1):150–153. | ||
Ammar HA, El-Desouky TA. Green synthesis of nanosilver particles by Aspergillus terreus HA1N and Penicillium expansum HA2N and its antifungal activity against mycotoxigenic fungi. J Appl Microbiol. 2016;121(1):89–100. | ||
Bhainsa KC, D’Souza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids and Surfaces B: Biointerfaces. 2006;47(2):160–164. | ||
Ahmad A, Mukherjee P, Senapati S, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B Biointerfaces. 2003;28(4):313–318. | ||
Venkatpurwar V, Pokharkar V. Green synthesis of silver nanoparticles using marine polysaccharide: study of in-vitro antibacterial activity. Mater Lett. 2011;65(6):999–1002. | ||
Vigneshwaran N, Nachane RP, Balasubramanya RH, Varadarajan PV. A novel one-pot “green” synthesis of stable silver nanoparticles using soluble starch. Carbohydr Res. 2006;341(12):2012–2018. | ||
Kora A, Beedu S, Jayaraman A. Size-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org Med Chem Lett. 2012;2(1):17. | ||
Rastogi L, Kora AJ, Sashidhar RB. Antibacterial effects of gum kondagogu reduced/stabilized silver nanoparticles in combination with various antibiotics: a mechanistic approach. Appl Nanosci. 2015;5(5):535–543. | ||
Tummalapalli M, Deopura BL, Alam MS, Gupta B. Facile and green synthesis of silver nanoparticles using oxidized pectin. Mater Sci Eng C Mater Biol Appl. 2015;50:31–36. | ||
Bao Q, Zhang D, Qi P. Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J Colloid Interface Sci. 2011;360(2):463–470. | ||
Ocsoy I, Temiz M, Celik C, et al. A green approach for formation of silver nanoparticles on magnetic graphene oxide and highly effective antimicrobial activity and reusability. J Mol Liq. 2017;227:147–152. | ||
Coluzzi M. Malaria vector analysis and control. Parasitology Today. 1992;8(4):113–118. | ||
Blandin S, Moita LF, Köcher T, et al. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the defensin gene. EMBO Rep. 2002;3(9):852–856. | ||
Rayner JC. The merozoite has landed: reticulocyte-binding-like ligands and the specificity of erythrocyte recognition. Trend Parasitol. 2009;25(3):104–106. | ||
Suman TY, Elumalai D, Kaleena PK, Rajasree SRR. GC–MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia baccifera aerial extract and its larvicidal activity against malaria and filariasis vectors. Ind Crop Prod. 2013;47:239–245. | ||
Salunkhe RB, Patil SV, Patil CD, Salunke BK. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol Res. 2011;109(3):823–831. | ||
Soni N, Prakash S. Synthesis of gold nanoparticles by the fungus Aspergillus niger and its efficacy against mosquito larvae. Rep Parasitol. 2012;2:1–7. | ||
Soni N, Prakash S. Research article fungus generated novel nanoparticles: a new prospective for mosquito control. 2013;4(10):1481–1487. | ||
Soni N, Prakash S. Possible mosquito control by silver nanoparticles synthesized by soil fungus (Aspergillus niger 2587). 2013. | ||
Soni N, Prakash S. Microbial synthesis of spherical nanosilver and Nanogold for mosquito control. Ann Microbiol. 2014;64(3):1099–1111. | ||
Banu AN, Balasubramanian C. Myco-synthesis of silver nanoparticles using Beauveria bassiana against dengue vector, Aedes aegypti (Diptera: Culicidae). Parasitol Res. 2014;113(8):2869–2877. | ||
Wirth MC, Walton WE, Federici BA. Evolution of resistance to the Bacillus sphaericus bin toxin is phenotypically masked by combination with the mosquitocidal proteins of Bacillus thuringiensis subspecies israelensis. Environ Microbiol. 2010;12(5):1154–1160. | ||
Marimuthu S, Rahuman AA, Kirthi AV, et al. Eco-friendly microbial route to synthesize cobalt nanoparticles using Bacillus thuringiensis against malaria and dengue vectors. Parasitol Res. 2013;112(12):4105–4112. | ||
Santhoshkumar T, Rahuman AA, Rajakumar G, et al. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res. 2011;108(3):693–702. | ||
Foldbjerg R, Jiang X, Miclăuş T, et al. Silver nanoparticles – wolves in sheep’s clothing? Toxicol Res. 2015;4(3):563–575. | ||
Li Z, Hulderman T, Salmen R, et al. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environ Health Perspect. 2007;115(3):377–382. | ||
Nijhara R, Balakrishnan K. Bringing nanomedicines to market: regulatory challenges, opportunities, and uncertainties. Nanomedicine. 2006;2(2):127–136. | ||
Bakshi RP, Tatham LM, Savage AC, et al. Long-acting injectable atovaquone nanomedicines for malaria prophylaxis. Nat Commun. 2018;9(1):315. | ||
Benelli G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res. 2016;115(1):23–34. | ||
Panneerselvam C, Ponarulselvam S, Murugan K. Potential anti-plasmodial activity of synthesized silver nanoparticle using Andrographis paniculata Nees (Acanthaceae). Arch Appl Sci Res. 2011;3(6):208–217. | ||
Mishra A, Kaushik NK, Sardar M, Sahal D. Evaluation of antiplasmodial activity of green synthesized silver nanoparticles. Colloids Surf B Biointerfaces. 2013;111:713–718. | ||
Marimuthu S, Rahuman AA, Rajakumar G, et al. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol Res. 2011;108(6):1541–1549. | ||
Zahir AA, Rahuman AA. Evaluation of different extracts and synthesised silver nanoparticles from leaves of Euphorbia prostrata against Haemaphysalis bispinosa and Hippobosca maculata. Vet Parasitol. 2012;187(3–4):511–520. | ||
Rajakumar G, Abdul Rahuman A, Rahuman AA. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop. 2011;118(3):196–203. | ||
Arjunan NK, Murugan K, Rejeeth C, Madhiyazhagan P, Barnard DR. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector Borne Zoonotic Dis. 2012;12(3):262–268. | ||
Priyadarshini KA, Murugan K, Panneerselvam C, et al. Biolarvicidal and pupicidal potential of silver nanoparticles synthesized using Euphorbia hirta against Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res. 2012;111(3):997–1006. | ||
Suman TY, Ravindranath RRS, Elumalai D, et al. Larvicidal activity of titanium dioxide nanoparticles synthesized using Morinda citrifolia root extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus and its other effect on non-target fish. Asian Pac J Trop Dis. 2015;5(3):224–230. | ||
Sivapriyajothi S, Mahesh Kumar P, Kovendan K, Subramaniam J, Murugan K. Larvicidal and pupicidal activity of synthesized silver nanoparticles using leucas aspera leaf extract against mosquito vectors, Aedes aegypti and Anopheles stephensi. J Entomol Acarol Res. 2014;46(2):77–84. | ||
Murugan K, Benelli G, Panneerselvam C, et al. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp Parasitol. 2015;153:129–138. | ||
Vijayakumar S, Vinoj G, Malaikozhundan B, Shanthi S, Vaseeharan B. Plectranthus amboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochim Acta A Mol Biomol Spectrosc. 2015;137:886–891. | ||
Ramanibai R, Velayutham K. Bioactive compound synthesis of Ag nanoparticles from leaves of Melia azedarach and its control for mosquito larvae. Res Vet Sci. 2015;98:82–88. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.