Back to Journals » Psoriasis: Targets and Therapy » Volume 7
Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management
Authors Haneke E
Received 10 July 2017
Accepted for publication 7 September 2017
Published 16 October 2017 Volume 2017:7 Pages 51—63
DOI https://doi.org/10.2147/PTT.S126281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Uwe Wollina
Eckart Haneke1–4
1Department of Dermatology, Inselspital, University of Bern, Bern, Switzerland; 2Dermatology Practice Dermaticum, Freiburg, Germany; 3Centro de Dermatología Epidermis, Instituto CUF, Porto, Portugal; 4Department of Dermatology, University Hospital, Gent, Belgium
Abstract: Psoriasis is the skin disease that most frequently affects the nails. Depending on the very nail structure involved, different clinical nail alterations can be observed. Irritation of the apical matrix results in psoriatic pits, mid-matrix involvement may cause leukonychia, whole matrix affection may lead to red lunulae or severe nail dystrophy, nail bed involvement may cause salmon spots, subungual hyperkeratosis, and splinter hemorrhages, and psoriasis of the distal nail bed and hyponychium causes onycholysis whereas that of the proximal nail fold causes psoriatic paronychia. The more extensive the involvement, the more severe is the nail destruction. Pustular psoriasis may be seen as yellow spots under the nail or, in case of acrodermatitis continua suppurativa, as an insidious progressive loss of the nail organ. Nail psoriasis has a severe impact on quality of life and may interfere with professional and other activities. Management includes patient counseling, avoidance of stress and strain to the nail apparatus, and different types of treatment. Topical therapy may be tried but is rarely sufficiently efficient. Perilesional injections with corticosteroids and methotrexate are often beneficial but may be painful and cannot be applied to many nails. All systemic treatments clearing widespread skin lesions usually also clear the nail lesions. Recently, biologicals were introduced into nail psoriasis treatment and found to be very effective. However, their use is restricted to severe cases due to high cost and potential systemic adverse effects.
Keywords: nail psoriasis, etiology, pathology, quality of life, impact, treatment
Introduction
Psoriasis is a chronic inflammatory disease with a strong genetic background but highly influenced by environmental factors. Its prevalence is ~1–2% of the world population with considerable differences among regions and individuals with different skin types. It is the skin disease that most frequently affects the nail. At the time of consultation, roughly one half of the patients suffer from nail changes. Over lifetime, up to 90% of all psoriatics will have had nail alterations. The prevalence of nail psoriasis is even higher in psoriatic arthritis.1 Nail lesions often appear around 10 years later than skin lesions, which may in part be the reason for nail psoriasis being observed less frequently in children. In general, cutaneous psoriasis is more severe in individuals with nail involvement.
Etiology and pathogenesis
Neither gender nor race predilection appears to exist. There is no association of HLA-C0602 with nail and joint involvement, but nail psoriasis is often associated with an inflammation at the insertion points of tendons and ligaments giving rise to enthesitis. Thus, the nail lesions were believed to represent an abnormal response to tissue stressing of the integrated nail-joint apparatus, rather than being due to autoimmunity. The nail and joint disease may be linked to tissue-specific factors, including tissue biomechanical stressing and microtrauma, that lead to activation of aberrant innate immune responses.2 However, a case of skin and nail psoriasis definitely disappearing after allogeneic bone marrow transplantation is more in favor of predominant immunogenetic factors.3
Clinical characteristics of nail psoriasis
Psoriasis causes a variety of both specific as well as ambiguous nail lesions. Fingernails are more frequently affected than toe nails, probably because they grow faster. Particularly, for scientific purposes, nail psoriasis is often divided into matrix and nail bed involvement or both, which is also reflected in many trials differentiating the response into general, more on matrix or nail bed psoriasis. Pits are the most characteristic and most frequent signs and are seen as small, sharply delimited depressions in the nail surface. They are of remarkably even size and depth. Their distribution may be haphazard or they may sometimes be arranged in parallel transverse or short longitudinal lines. They are the result of tiny psoriatic foci in the apical matrix producing parakeratosis, which breaks off when it grows out from under the proximal nail fold then leaving these depressions. Sometimes, the parakeratosis remains and is seen as an ivory-colored spot in the proximal half of the nail plate. Pits may be single, which is not yet psoriasis specific, or multiple. Ten pits in one nail or >50 pits on all nails are regarded as proof of psoriasis. Red spots in the lunula usually represent a very active psoriasis lesion with dilatation of the capillaries and thinning of the suprapapillary plate. Red or mottled lunulae are due to the dilatation of matrix blood vessels.4
Total matrix affection results in complete nail destruction with crumbling of the plate, whereas leukonychia is seen when the mid- to distal matrix is affected and parakeratotic cells are incorporated into the nail plate making it optically opaque. In most cases, psoriatic leukonychia is an ill-defined white transverse band. Splinter hemorrhages are very narrow, some millimeters long reddish-darkbrown to black streaks. They are analogous to Auspitz’s phenomenon of the skin and either due to hemorrhage from the dilated capillaries in the nail bed or due to blood clots in these longitudinally arranged small vessels. Salmon or oil spots are very frequent and represent psoriatic plaques in the most distal matrix and the nail bed. This area looks like paper on which a drop of oil has fallen: a yellowish-brownish spot with a red margin shines through the plate because the psoriatic squames compressed under the nail are imbibed with serum. When a salmon spot reaches the hyponychium, the parakeratosis breaks out and psoriatic onycholysis develops. This typically has a reddish proximal margin differentiating it from most other causes of onycholysis, such as onychomycosis. Subungual hyperparakeratosis may be thick and then no oil drop phenomenon is seen. The hyperkeratosis may be very marked and at times so extreme as to resemble pachyonychia congenita. Psoriasis affecting the dorsal as well as the ventral surface of the proximal nail fold results in swelling and rounding of its free edge. This leads to a spontaneous loss of the cuticle characterizing the pattern of chronic paronychia.
As mentioned earlier, psoriatic arthritis is very frequently associated with severe nail involvement and psoriatic paronychia, complete nail destruction, and swelling of the distal interphalangeal joint. This has a serious negative influence on the quality of life.
In contrast, psoriatic pachydermoperiostosis is closely related to psoriatic arthritis but usually without obvious nail changes. Mainly the big toe is considerably swollen and often painful.
Three different forms of pustular psoriasis are differentiated. Nail changes are seen in all of them. The nail changes in the palmar plantar pustular psoriasis of Barber–Königsbeck are similar to the common type of psoriasis, but the surface defects may be larger and are called elkonyxis. Yellow subungual spots represent large Munro’s abscesses. Subungual abscesses are frequent in the generalized pustular psoriasis of von Zumbusch. Acrodermatitis continua suppurativa of Hallopeau is the most notorious form of pustular psoriasis of the nails. It often begins with one single digit where the skin of the distal phalanx turns red and develops some pustules migrating under the nail and causing nail dystrophy. With time, the entire nail unit may disappear leaving a red smooth digit tip until the disease slowly wanes off. Less frequently, acrodermatitis continua suppurativa may initially involve several fingers and toes and run a rapid and severe course. Recently, a mutation in the interleukin 36 receptor antagonist gene leading to a defect in interleukin 36 antagonist was identified in generalized pustular psoriasis and acrodermatitis continua suppurativa supporting the view that it belongs to the autoinflammatory diseases group.5,6 Pustulosis palmoplantaris is histopathologically and genetically different and rarely affects the periungual skin.7
Reiter’s disease is also known as reactive arthritis. It is a systemic condition with characteristic joint, mucosal, eye, genito-urinary, skin, and nail changes. The latter are very similar to pustular psoriasis. However, they often have a more brownish tint due to a higher content of erythrocytes in the pustules. Histopathology with extensive spongiform pustules is virtually identical to pustular psoriasis.8
Nail psoriasis in children
Psoriasis may occur at any age. Although rare, it was also observed in the newborn. Erythrodermic psoriasis in children usually shows ungual involvement with nail dystrophy and marked subungual hyperkeratosis similar to pityriasis rubra pilaris. Even pustular psoriasis and psoriatic arthritis were observed in children.6
Diagnosis of nail psoriasis
In most cases, nail psoriasis follows cutaneous psoriasis and is therefore easy to diagnose. However, ~5% of nail psoriasis occurs isolated and may pose diagnostic challenges. This is particularly the case when even the nail alterations are atypical such as a single nail in a child or of a toe, isolated nail bed psoriasis without pits and salmon spots. Histopathology is usually diagnostic, provided the biopsy is sufficient, which is, unfortunately, often not the case. It has to be remembered that matrix lesions cause changes of the nail plate and those of the nail bed are seen under the nail plate. The biopsy has to be taken slightly more proximal than anticipated and must include enough subungual soft tissue. In contrast, nail clippings are diagnostic for the most important differential diagnosis, the various onychomycosis forms, and sometimes give a strong hint at nail psoriasis. Furthermore, nail psoriasis exhibits some features not commonly seen in cutaneous lesions.1 Dermatoscopy makes the clinical signs more obvious and helps in the diagnosis. Videodermatoscopy allows higher magnifications than the usual hand-held dermatoscopes. Capillaroscopy shows the dilated tortuous capillaries of the proximal nail fold. This is even better visible in laser confocal microscopy. The features of high-frequency ultrasound are less reliable but may be of help for the very experienced. Optical coherence microscopy uses a similar principle but has a much higher resolution.
Differential diagnosis
In most cases, nail psoriasis is diagnosed on clinical grounds.8 Skin lesions elsewhere with one or several psoriatic nail features suggest the correct diagnosis. With a good biopsy, histopathology is usually pathognomonic and helps to delineate nail psoriasis from other conditions, particularly onychomycosis. The clinical diagnosis of pustular psoriasis is made on the basis of red skin areas with a rim of small pustules. Reiter’s disease requires additional laboratory examinations.1
Onychomycosis is said to be the most frequent nail disease. It has many features in common with nail psoriasis, both clinically and histopathologically (Table 1).1
  | Table 1 Differential diagnosis of nail psoriasis and onychomycosis Note: Copyright © 2009. Haneke E. Adapted from Haneke E. Non infectious inflammatory disorders of the nail apparatus. J Dtsch Dermatol Ges. 2009;7:787–797.10 Data from Haneke E.1 |
Another important differential diagnosis is the asymmetric gait nail unit syndrome seen mainly in the big toenail as an onycholysis without further criteria of nail psoriasis or onychomycosis.9 Furthermore, nonspecific nail dystrophy, particularly of toenails, is very common in the elderly, in subjects with peripheral arterial disease, chronic venous stasis, after trauma to the leg, in peripheral neuropathy, and in some dermatoses such as eczema, nail lichen planus, Darier’s disease, Hailey-Hailey disease, alopecia areata, and many drugs.1,8
Grading and assessment of nail psoriasis
Reliable repeatable specific validated severity and outcome measures are necessary to evaluate a disease and its response to a specific treatment.11 This was missing in nail psoriasis until the nail psoriasis severity index (NAPSI), target NAPSI, and its many variants were established.12 NAPSI is calculated by dividing each nail into four quadrants. Each quadrant is evaluated for the presence of psoriasis manifestations of the nail matrix, such as pitting, leukonychia, red spots in the lunula, and nail plate crumbling, as well as of the nail bed, such as oil-drop phenomenon, onycholysis, subungual hyperkeratosis, and splinter hemorrhages. If any of these signs is present in all four quadrants, a score of 4 is given. A score of 0 represents no signs in any quadrant. Each nail is evaluated for a matrix and a nail bed score of 0–4. They are combined to yield a maximal score of 0–8 for each nail. All nails may be evaluated, with the total NAPSI score being the sum of the scores, up to 80 if only fingers are considered, or up to 160 if fingers plus toes are included.12 If only the most seriously affected nail is evaluated, it is called target NAPSI; this is often done to assess the effects of a therapeutic regimen.11 Many therapeutic studies use (target) NAPSI-50, NAPSI-75, and NAPSI-90 to indicate the percentage of patients that reach a (target) NAPSI improvement of 50, 75, or 90%, respectively. The NAPSI has some disadvantages, such as being too time-consuming to be used in clinical practice, and that the NAPSI scores often do not correspond with the clinical severity of nail psoriasis.13 A new scoring system, the N-NAIL, overcomes many of these limitations, but it has yet to prove its clinical practicability.13 The use of many different scoring systems, major differences in study design, inclusion criteria, and follow-up make it difficult if not impossible to compare the results of most nail psoriasis trials.11 In addition, subjective and objective patient factors such as quality of life, satisfaction with treatment ease and outcome, adverse effects and not the least practicability, and cost of treatment are important factors.11 Such an evaluation and assessment tool for nail psoriasis has recently been published under the term of nail assessment in psoriasis and psoriatic arthritis.14
Associations
Psoriasis is a frequent skin disease. In the last decades, a metabolic syndrome associated with psoriasis has been described; however, this is not of particular importance for ungual psoriasis except in psoriatic arthritis. Associations and co-occurrence with other skin disorders involving the nail are rather common. The most important differential diagnosis is onychomycosis. Both conditions may look very similar. A psoriatic nail may be colonized with pathogenic fungi, and a true infection of the psoriatic nail is not infrequent (Table 1).1,15
Impact on quality of life
Nail psoriasis has a profound negative influence on all aspects of quality of life as well as on daily, sports, and professional activities.16–20 Women try to hide their nails and cover them with nail lacquer; although common nail varnishes are not harmful, artificial nails, particularly when long, increase the mechanical stress and strain to the nail plate – nail bed attachment acting as a Köbner phenomenon and worsening nail psoriasis. Similarly, professional activities with particular use of the fingers may have a deteriorating effect on the disease. Matrix involvement scores higher than pure nail bed affection as it results in more obvious nail plate damage.19
Course
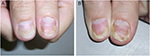
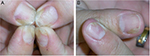
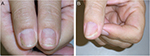
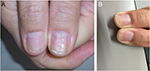
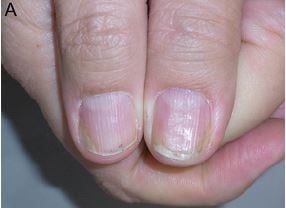
Nail psoriasis is chronic but often improves and worsens without known reasons (Figures 1–4). Trauma may play an important role in the exacerbation of nail psoriasis. There may be periods without any nail alterations.1,8,10
Management of nail psoriasis
Management of the disease includes patient education, avoidance of trauma to the nails, and different therapeutic approaches with physical and pharmaceutical procedures and agents.
Patient counseling includes education on the nature of psoriasis, how life may be influenced by nail involvement, about the specific problems of treatment, that nail psoriasis is not due to an allergy or an “unhealthy” diet and thus is not treatable with particular foods. However, smoking increases the risk of psoriasis and obesity and alcohol use are associated with a higher risk for psoriasis. It is important to avoid trauma to the nail unit that will inevitably exacerbate the condition or induce recurrences. Manicure and nail cleaning have to be performed cautiously without further traumatizing the hyponychium and attachment of the nail to the nail bed. It is helpful to explain that genes are the most important etiological factors and that the skin and nail lesions are amenable to treatment but that the genes cannot be corrected. Many genes contribute to the psoriatic personality, which may explain the enormous variability of the clinical features including the response to treatment. Particularly, chronic repeated trauma is thought to be an aggravating factor. The development of pits may be the result of microtraumata to the enthesis of the extensor tendon and the dorsal aponeurosis of the distal interphalangeal joint.2 They may be masked using nail varnish.
All nail psoriasis treatments require a long time, as the nail is a slow-growing cutaneous appendage. The effect of any treatment can usually not be evaluated before 3–6 months, and it may take a year or longer to reach the maximum improvement achievable with a given therapy. Pretreatment photographs are highly recommended and should be repeated at all follow-up visits to show the therapeutic result to the patients. Concomitant onychomycosis may prevent clinical cure, and it is a must to exclude fungal infection when starting nail psoriasis therapy; a single remaining altered nail during an otherwise efficacious therapy may be a hint at a concomitant mycotic infection.1,21
Many different therapeutic measures are available. Their choice depends on various factors such as severity of nail involvement and its impact on quality of life, associated skin lesions, psoriatic arthritis, comorbidities, profession, age, patient preferences, potential risks, and not the least costs and their reimbursement.
Nail psoriasis is very recalcitrant to almost all topical treatments, whereas systemic therapies clearing the skin are usually effective also in nail psoriasis.22 The problem of all topical drugs is their limited penetration to the diseased tissue: through all layers of the proximal nail fold with the underlying nail in matrix lesions, through the nail plate, and subungual hyperkeratosis in nail bed psoriasis. Hence, pits, though often being rather inconspicuous, are the most resistant to treatment. Nevertheless, a 3-month trial of a potent antipsoriatic topical preparation is warranted (Figure 1). The less nail is left the easier the penetration of the drug to the very psoriatic lesion is. Clipping the onycholytic nail over the nail bed is essential to reach nail bed psoriasis. Thinning the nail by filing or grinding, or drilling holes into the nail plate with mechanical burrs23 or with ablative lasers, is often used to enhance nail penetration.24
Corticosteroids are often used in nail psoriasis. They have to be class IV (high potency) and applied once or twice a day on the proximal nail fold in case of matrix and on the nail plate in nail bed affection. Although probably still the most commonly used drugs, corticosteroids were rarely tested in controlled studies. Both clobetasol and betamethasone dipropionate were tested in clinical trials and showed comparable results11 concerning pitting, salmon patches, subungual hyperkeratosis, and onycholysis.25,26 All high-potency steroids carry the risk of skin atrophy when used on the proximal nail fold, often associated with hypopigmentation. Whether or not using them for 4–5 days a week as a “pulse” treatment is equally effective and reduces this risk remains to be proven. Clobetasol 8% was tested as a nail lacquer with good results depending of the duration of the treatment.27
Perilesional injections are another type of local treatment. This increases the concentration of the drug at the site of disease while minimizing the dose for the whole organism. Perilesional injection of corticosteroids is by far the most often performed (Figure 2), either by injection with a needle or by high air-pressure devices.28 Injection with a 30 G needle and using some distraction techniques such as vibration and pressure around the area to be injected make the procedure tolerable although some patients prefer the needle-less technique. Apparently, the efficacy of different high-pressure injectors varies considerably (O Correia, Inst CUF, personal communication, 2014). We inject 0.1 mL of a triamcinolone acetonide suspension (10 mg/mL) into both sides of the proximal nail fold; injection into or under the nail bed is extremely painful and requires prior anesthesia.29,30 The injections are repeated on a monthly basis for 6 months and, then, followed by every 6 weeks and later every other months. However, not all patients tolerate the injections. Matrix and nail bed signs of psoriasis respond slightly differently with salmon spots and subungual hyperkeratosis usually showing the best effect. Adverse effects are not uncommon with subungual hematoma and temporary nail deformation being relatively frequent29,30 and the disappearing digit, atrophy of the terminal phalanx bone, and rupture of the extensor tendon being the most serious ones.31–34 Epidermoid inclusion cysts were observed after jet injections necessitating amputation.35
The combination of the vitamin D3 derivate calcipotriol and a potent corticosteroid, such as betamethasone dipropionate, has shown good results;26,27 personal experience has shown that twice or even thrice daily application may be worth trying. Another vitamin D derivative, such as tacalcitol, was used alternatively with 8% clobetasol nail lacquer.33
Vitamin D3 (calcitriol) and its analogs calcipotriol and tacalcitol are well established in the therapy of psoriasis vulgaris due to their effects on epidermal differentiation and proliferation and regulation of production and release of proinflammatory cytokines.11 Most studies were done with calcipotriol and tacalcitol.36,37 Apparently, their effect on nail bed lesions is more marked than on matrix signs.
Calcineurin inhibitors have a profound inhibitory effect on T-cell functions that are implicated in the pathogenesis of psoriasis. Used systemically, cyclosporin A (CyA) is highly active against psoriasis, but topical application of CyA showed ambiguous results.11 The new calcineurin inhibitor tacrolimus shows much better skin and nail penetration. It demonstrated good activity on nail bed and matrix psoriasis in a controlled study.38 No studies with pimecrolimus were published until now.11
Tazarotene is a synthetic retinoid with antiinflammatory and antiproliferative actions on keratinocytes. Tazarotene 0.1% was used for the treatment of nail psoriasis. The results were variable, but one study compared it with clobetasol showing equal results.25 It was also used in childhood nail psoriasis.39 Side effects are mainly skin irritation with redness and desquamation.11
5-Fluorouracil (5-FU) is an antimitotic and antiproliferative agent, which is active against disorders with a high proliferative activity, such as psoriasis. Only one study of topical 5-FU with 20% urea as a penetration enhancer showed good effects on nail psoriasis, but inflammation, infection, onycholysis, and discoloration were observed as adverse effects.40 Other investigators did not see a beneficial response. This does not make 5-FU a favorite nail psoriasis agent.11
Dithranol was once the most commonly used antipsoriatic topical, but because of its unpleasant cosmesis, it is rarely used nowadays. One study reported some improvement of nail bed lesions, but the staining of the nails made it unacceptable.41
Indigo naturalis extract regulates proliferation and differentiation of epidermal keratinocytes, restores the epidermal barrier function, and inhibits inflammatory reactions. Twice daily application of an oily extract reduced nail bed lesions such as hyperkeratosis and onycholysis by about one half; it was thus more effective than calcipotriol solution.42,43 Studies are in progress to make it colorless and cosmetically more acceptable (CH Yang, Chang Gung Memorial Hospital, personal communication, May 8, 2017).
Methotrexate was also used for perimatrical and nail bed injections with acceptable results. The dose was 0.1 mL of a 25 mg/mL solution. Good results were seen after 6 months and 15 weeks, respectively.44,45
Systemic treatments are indicated in widespread psoriasis. Those therapies proven successful for skin lesions usually also improve nail psoriasis. However, many physicians and patients are reluctant to treat isolated ungual psoriasis systemically. A European Consensus Paper on the treatment of psoriasis defines the involvement of particularly sensitive areas such as the head and neck, genito-anal area, and nails as moderate to severe. The selection of the mode of treatment then depends on the severity of the nail disease, its impact on quality of life, on professional, sports, and social activities, and in particular on potential associated psoriatic arthritis.
Systemic corticosteroids are not a good option for psoriasis vulgaris and in particular for nail psoriasis. High doses are necessary with a considerable risk of serious side effects, break-through phenomena, and development of pustulation of hitherto not pustular psoriasis.
Methotrexate has been introduced into the treatment of cutaneous psoriasis and psoriatic arthritis in the 1960s. It is an inexpensive drug with good efficacy in skin lesions; however, as an antimetabolite, it slows down the nail growth rate and improvement in nail lesions is therefore often slow and seen very late (Figure 3). Furthermore, there is a risk of severe side effects such as hepatotoxicity, lymphopenia, lekopenia, nausea, and erosive stomatitis. Long-term toxicity includes liver, lung, and heart fibrosis. The dose is usually slowly increased to reach ~10–20 mg/week. Both oral and injection therapies are possible with virtually equal doses as the bioavailability of methotrexate is very good.46–48 NAPSI improvement is between 25 and maximally 50%. Methotrexate has also been injected intralesionally with a good result.44,45
Ciclosporin A is another established systemic antipsoriatic drug. It is a calcineurin inhibitor with strong immunosuppressive action. Its positive effect on cutaneous and ungual psoriasis is well established, both in single-drug studies as well as in comparative ones.49,50 The dose is usually 3–5 mg/kg daily, but half the dose is often given in Japan after initial improvement.51 Although ciclosporin is probably the most active “classical” systemic antipsoriatic drug, it is limited to a treatment period of 6–12 months because of potentially serious adverse effects such as disturbance of renal function, arterial hypertension, diabetes mellitus, nausea, hypertrichosis, gingival hyperplasia, paresthesia, fatigue, and headache.
Synthetic retinoids have been used to treat extensive skin psoriasis. The first of these drugs was etretinate. Although it had a good effect on nail changes in some cases,52,53 etretinate is no longer used and substituted by its derivative acitretin.
Acitretin is the follower product of etretinate with a shorter half-life in the body. It is usually given in a dose of 0.5–1 mg/kg/day.54,55 Its action is slow and, in most cases, does not reach >50% improvement of nail psoriasis.56 All retinoids have a number of side effects, particularly when given in a dose >0.5 mg/kg/day, such as dry and cracking lips, dry mouth, hair loss, and in children ossification disturbances. High-dose retinoids can have an onychodestructive effect and are no longer recommended as the first-line nail psoriasis treatment;57 however, good results were seen in generalized pustular psoriasis and acrodermatitis continua suppurativa.57 Acitretin is occasionally used in combination with photochemotherapy with ultraviolet (UV) A and narrow band UV B.
Fumaric acid esters are used for psoriasis treatment in some countries, mainly in Europe, but their use was somewhat controversial. A case report described a good effect on nail psoriasis.58 Side effects are mainly gastrointestinal, flushing, lymphopenia, and rarely renal dysfunction.
Leflunomide is a disease-modifying antirheumatic agent with an effect on psoriatic arthritis and also a modest action on nail psoriasis.59 Sulfasalazine was used in one patient with a beneficial effect.60 Silicic acid was given orally and topically on skin lesions. Ten of the 12 patients treated had nail lesions, and five of them cleared completely, although the nails were not treated with the silicic acid gel; thus, a systemic effect was postulated.61
Apremilast is a new small-molecule oral phosphodiesterase 4 inhibitor reducing the expression of several proinflammatory mediators; it is more an antiinflammatory than immunosuppressive agent distinguishing it from most other systemic antipsoriatic compounds. It has an excellent safety profile with no known organ toxicity, thus obviating the need for laboratory controls.62 It is approved for the treatment of cutaneous psoriasis and psoriatic arthritis and has shown a good effect in nail psoriasis, although only after 32 weeks. Its cost may, however, limit its widespread use.63,64 A nail lacquer containing apremilast is being developed;65 however, human studies on nail psoriasis have not yet been published.
Tofacitinib is a small-molecule oral Janus kinase inhibitor interfering in the JAK–STAT pathway. It is active against psoriasis and alopecia areata including their nail manifestations. In four Phase III randomized controlled studies and compared to etanercept, a twice daily dose of 5 or 10 mg was shown to be noninferior to etanercept injected subcutaneously twice weekly with sustained effects up to 52 weeks.66–69
Biologicals are a new development in the treatment of many, mainly immunologically mediated diseases, among them also psoriasis. There are several classes, both concerning the nature of the antibody as well as their target, such as humanized and fully human antibodies and antibodies to tumor necrosis factor-α, various interleukins, and T-cell inhibitors, respectively. They all have profound immunosuppressive actions and are thus not without risk, particularly concerning infections and re-activation of tuberculosis, to mention but a few.11 Biologicals are usually considered second- or third-line treatments when other established topical and systemic antipsoriatic drugs were not or not sufficiently active in suppressing nail lesions.70 In contrast, nail psoriasis was found to be an indicator of poor prognosis for the treatment of psoriasis with biologicals independent of the specific substance used.71 In most cases, nail psoriasis responses lag behind those of cutaneous psoriasis, which can in part be explained by the slow growth of nails as nail plate changes have to grow out, whereas nail bed changes may be seen earlier. In comparison with “classical” systemic drugs, eg, methotrexate and cyclosporin, biologicals often show a dramatic and more rapid improvement. However, only 20–57% of the patients reach a 90% improvement of their NAPSI score with biologicals and the effect is lost after 47 months in average.72,73 The most likely mechanism is the formation of antidrug antibodies, but compensatory production of other proinflammatory cytokines and a particular individual reaction may be the cause that many patients stop this treatment.74 In many countries, biologicals are not automatically reimbursed by the social health insurance and patients and physicians have to give evidence that previous, less expensive treatments were not sufficiently efficacious.
TNF-α inhibitors were the first biologicals developed for psoriasis treatment. TNF-α is a cytokine with proinflammatory action that induces keratinocyte proliferation and prevents apoptosis. Most experience was gained with infliximab, the first of this group, but in general, the efficacy of all TNF-α inhibitors currently available for psoriasis treatment is virtually comparable. Also their side effects and limitations are the same. Activation of opportunistic infections, congestive heart failure, demyelinating disorder, antibodies against TNF-α inhibitors, and rarely lupus erythematosus may occur.11
Infliximab is a chimeric human-mouse IgG1 antibody binding membrane-bound and soluble TNF-α. This reduces epidermal T-lymphocyte infiltration. It exhibits certain antigenicity and may thus induce autoantibodies that may reduce its effectiveness thus requiring higher doses with time. It has to be given intravenously, and ~16% of the patients develop infusion reactions such as fever, chills, flush, urticaria, myalgia, arthralgia, nausea, hypotension, and dyspnea.75 Infliximab was associated with a higher rate of onychomycosis compared to the other TNF-α inhibitors.76 Patients with a high psoriasis area severity index (PASI) response also show a good NAPSI response. Almost one half of the patients demonstrated complete nail clearance after 50 weeks. NAPSI reduction by 50% was achieved by almost all patients, 80% reached NAPSI-75, 30% reached NAPSI-90, and 10% cleared completely.77 Infliximab appears to be the fastest acting TNF-α inhibitor. Its dosage is usually 5 mg/kg given on weeks 0, 2, and 6 and if necessary 8.
Adalimumab is a human monoclonal IgG1 antibody against TNF-α. It binds to cell surface proteins of the TNF-α receptor preventing its action. Its mechanism of action is similar to that of infliximab. Roughly 50–60% of NAPSI improvement are achieved.11,78
The combination with cyclosporin was shown to be particularly effective reaching a reduction of the NAPSI score of 100%.79 Adalimumab did not increase the rate of onychomycoses.76 The dose is 80 mg at baseline, then 40 mg every 2 weeks, but some authors gave 40 mg from the beginning (Figure 4).11
Certolizumab pegol is a PEGylated TNF-α inhibitor that is Fc free. It is effective in the treatment of rheumatoid arthritis and psoriasis with efficacy in nail psoriasis, enthesitis, and dactylitis.80,81
Being a fusion of the TNF receptor with the Fc part of the IgG1 antibody etanercept blocks the action of TNF-α. Thus, its mechanism of action is similar to that of infliximab and adalimumab. Several reports on its use in nail psoriasis demonstrated good results.82 There was no statistically different outcome with 50 mg once or twice weekly after 12 weeks and target NAPSI improvement between 71 and 76%.83 It was also effective in refractory acrodermatitis continua suppurativa.84
Golimumab is another TNF-α inhibitor approved for psoriatic arthritis with an effect on nail psoriasis. Target NAPSI improvement was over 40% after 24 weeks and 52% after 52 weeks of treatment with 50 mg every 4 weeks.85,86
All TNF-α inhibitors have the potential to paradoxically worsen psoriasis or even induce it.87,88 In many cases, this regresses despite continuation of the therapy or when another biological is used. This is apparently independent from the condition for which TNF-α inhibitors were administered.88–91 The mechanism of action may be an unabated interferon-α production by plasmacytoid dendritic cells, which might result in psoriasis flares and induction of psoriasiform lesions.91,92
T-cell inhibitors such as alefacept and efalizumab are not widely used because of their considerable adverse effect profile.93 Efalizumab is a monoclonal CD11a antibody and was withdrawn from the market because of cases of leukencephalopathy observed under treatment with this molecule. Alefacept is a fusion protein binding at the CD2 portion of the leukocyte function antigen-3 linked to the Fc portion of human IgG1 and targets T lymphocytes. No studies to evaluate the efficacy of these drugs in nail psoriasis were published.
Rituximab causes B-cell depletion. Its role in the treatment of nail psoriasis is not yet examined.93,94
New biologicals focus on the inhibition of interleukins involved in the propagation of the psoriatic process. Their nonspecific immunosuppressive action is less pronounced compared with the TNF-α inhibitors. The targets are mainly IL-12/IL-23 and IL-17. However, it was shown that anti-IL-12 action might be proinflammatory under certain circumstances and thus counterproductive for the therapy of psoriasis.95
Anti-interleukin 17 therapy is based on the fact that IL-17 plays a central role in the development of psoriatic lesions, but IL-17 is also important for the defense against extracellular pathogens and recruits neutrophils.
IL-17 inhibitors are secukinumab, ixekizumab, and brodalimumab, but there are no ongoing studies with the last one.
Secukinumab is a human monoclonal IL-17A antibody approved for plaque psoriasis and psoriatic arthritis that has also shown good efficacy in nail psoriasis. It has an early onset action and a sustained effect. Its safety profile is acceptable. It is administered subcutaneously with a 300 mg dose at weeks 0, 1, 2, 3, 4, and then every 4 weeks. At 16 weeks, both 150 and 300 mg secukinumab were superior to placebo with further improvement with longer treatment periods.96,97
Ixekizumab is another humanized monoclonal antibody directed against IL-17A recently approved for psoriasis. It demonstrated significant improvement of the NAPSI score as early as 2 weeks after start of the treatment, which sustained to week 20 when given in a dose of 75 mg at weeks 0, 2, 4, 8, and 12 and then 120 mg every 4 weeks from week 20 onward.98,99
Brodalumab is a human monoclonal IL-17A antibody active against plaque psoriasis. It was more active than ustekinumab in a head-to-head comparison. Although approved in the US and Japan, the clinical development was terminated as suicidal ideation and behavior were observed.100
IL-23 is another important proinflammatory cytokine involved in the pathogenesis of psoriasis.
Antibodies targeting the p40 subunit of IL-23 also have an action against IL-12 as they both share this subunit. Antibodies directed against the p19 subunit are selective for IL-23.
Ustekinumab is a monoclonal antibody directed against the p40 subunit of IL-12/23. It is active against psoriasis and psoriatic arthritis and was also shown to have a good action on nail psoriasis. Nail improvement was observed from week 4 onward with significant improvement of 90% at week 40. NAPSI and PASI improvement ran parallel. The dose is 45 and 90 mg if the weight is over 100 kg, usually at weeks 0, 4, 16, and 28.101–103 Efficacy and side effects are comparable to the other IL inhibitors.
Briakinumab was another monoclonal antibody directed at p40. After showing good clinical results, its development was stopped because of severe infections and a higher incidence of other severe side effects.104
Guselkumab is a fully human IgG1k monoclonal IL-23 antagonist directed against the unique p19 subunit of IL-23; it has no anti-IL-12 component. It has a profound action on moderate-to-severe plaque psoriasis and was superior to adalimumab.105 The dose given was 100 mg at weeks 0, 4, 12, 16, and 20 and then every 8 weeks.
Tildrakizumab and BI-655066 are also targeting the p19 subunit of IL-23. They are currently being studied for various indications including psoriasis.106,107 No results are yet available concerning nail psoriasis.
In summary, most new biological drugs have a good and reliable action on nail psoriasis with an acceptable adverse effect profile. They are more active than most of the classical systemic antipsoriatic drugs.
Radiotherapy is an “old“ treatment modality, which has come out of time not only because of potential long-term adverse effects but also because most dermatologists no longer operate X-ray machines because of the difficulties to comply with the bureaucratic challenges associated with the use of therapeutic ionizing rays. However, some studies report favorable results with Grenz rays,108 superficial X-rays,109,110 and electron beam therapy.111
Light has been used for a long time, but as nails are virtually impermeable for UV B and allow <2% of UV A to penetrate, the effect is rather limited.112 Potentiation of UV by specific photosensitizers, called photochemotherapy, was beneficial in some studies48,113 but is often associated with multiple melanonychias.114
Intense pulsed light (IPL) is a broad-spectrum light source with a high-energy intensity. With a 550 nm filter, it has been used for the treatment of plaque psoriasis. A trial on 20 patients with finger and toenail psoriasis using IPL with a cutoff filter of 550 nm and a median of 8.6 sessions resulted in significant improvement in the NAPSI with nail bed lesions showing a reduction of 71% and matrix lesions of 32%. A relapse was seen in three patients after 6 months.115
Lasers have also been used to treat nail psoriasis. The pulsed dye laser (PDL) is the device of choice as it targets the dilated capillaries in the matrix and nail bed. In many studies, 1.5–6 ms pulses were used with a good effect on matrix and nail bed lesions.116,117 A comparative study using 6 ms pulse length and 9 J/cm2 and 0.45 ms and 6 J/cm2 gave almost the same improvement of matrix and nail bed lesions with significantly less pain with the shorter pulse.118 Side effects are mainly pain, hemorrhage, and pigmentation. These results were confirmed in another trial.119 PDL with tazarotene was significantly more effective than tazarotene alone.120 A comparison of the PDL with the excimer laser gave significantly better improvement with the PDL.121 In another comparative study, PDL was compared with the Nd:YAG laser. Both groups were treated with calcipotriol betamethasone in addition. Whereas the results were comparably good, the Nd–YAG was significantly more painful.122
Photodynamic therapy (PDT) uses light and a photoactive substance that both generate reactive oxygen species able to kill those cells that accumulated the photosensitizer. In a comparative study, no difference was found between PDL and PDT.117 However, there is considerable heat development during the illumination of the target and this is often not tolerated by the patients.
Conclusion
Nail involvement in psoriasis is common. It is an indicator of poor prognosis and of a higher risk to develop psoriatic arthritis. Surprisingly, nail psoriasis is only briefly mentioned in most national and European guidelines on the diagnosis and treatment of psoriasis; however, the European Nail Society is now working on recommendations for the treatment of nail psoriasis.
The many treatments available give evidence that hitherto none is the ideal therapy. Topicals have to fight with the difficulties to get through the nail and nail fold to the diseased structures. Injections that bring the remedy to the site of the disease process and greatly avoid systemic effects are painful and carry the risk of local side effects. Systemic drugs are often not used for isolated nail psoriasis, although this is accepted as a severe psoriasis considerably impairing quality of life. However, it is known that virtually all systemic treatments that improve the skin lesions are also beneficial for the nails, although often with a delayed and less pronounced response. The potential systemic adverse effects have to be kept in mind before and during such a therapy. The development of new biologicals has revolutionized psoriasis treatment and thus also that of ungual psoriasis. Finally, there are some physical modalities such as ionizing rays, various light qualities including photodynamic treatment, and lasers.
Many treatment possibilities may make it delicate to choose the right approach. It is certainly wise to begin with a topical antipsoriatic preparation (Table 2). This has to be used for a minimum of 4–6 months before its efficacy can be evaluated. If this does not help sufficiently, a classical antipsoriatic drug such as methotrexate, fumaric acid ester, and cyclosporine would be the second choice while keeping in mind all potential contraindications. If the results are not satisfying a biological may be chosen. Again, there are many contraindications that have to be carefully looked for before starting such a treatment. The choice is huge now, and the treating physician has to select among TNF-α blockers, agents interfering with T-lymphocyte functions, and IL-23 and IL-17 inhibitors.
  | Table 2 Treatment algorithm for nail psoriasis Abbreviations: CyA, cyclosporin A; FAE, fumaric acid ester; MTX, methotrexate; NP, nail psoriasis; NAPSI, nail psoriasis severity index. |
In summary, nail psoriasis is still an underestimated part of psoriasis, but the outlook is bright with many new treatments available.
Disclosure
The author reports no conflicts of interest in this work.
References
Haneke E. Nail disorders. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffel DJ, Wolff K, editors. Fitzpatrick’s Dermatology in General Medicine. 9th ed. New York: McGraw-Hill; In press 2017. | ||
McGonagle D, Tan AL, Benjamin M. The nail as a musculoskeletal appendage-implications for an improved understanding of the link between psoriasis and arthritis. Dermatology. 2009;218:97–102. | ||
Yokota A, Hukazawa M, Nakaseko C, et al. Resolution of psoriasis vulgaris following allogeneic bone marrow transplantation for aplastic anemia. Rinsho Ketsueki. 1996;37(1):35–39. | ||
Peña-Romero A, Toussaint-Caire S, Domínguez-Cherit J. Mottled lunulae in nail psoriasis: report of three cases. Skin Appendage Disord. 2016;2(1–2):70–71. | ||
Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365(7):620–628. | ||
Kelati A, Baybay H, Najdi A, Zinoune S, Mernissi FZ. Pediatric psoriasis: should we be concerned with comorbidities? A cross sectional study. Pediatr Int. 2017;59(8):923–928. | ||
Misiak-Galazka M, Wolska H, Rudnicka L. What do we know about palmoplantar pustulosis? J Eur Acad Dermatol Venereol. 2017;31(1):38–44. | ||
Jiaravuthisan MM, Sasseville D, Vender RB, Murphy F, Muhn CY. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol. 2007;57(1):1–27. | ||
Zaias N, Rebell G, Escovar S. Asymmetric gait nail unit syndrome: the most common worldwide toenail abnormality and onychomycosis. Skinmed. 2014;12(4):217–223. | ||
Haneke E. Non infectious inflammatory disorders of the nail apparatus. J Dtsch Dermatol Ges. 2009;7:787–797. | ||
Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76(6):675–705. | ||
Rich P, Scher RK. Nail psoriasis severity index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49(2):206–212. | ||
Klaassen KM, van de Kerkhof PC, Bastiaens MT, Plusje LG, Baran RL, Pasch MC. Scoring nail psoriasis. J Am Acad Dermatol. 2014;70(6):1061–1066. | ||
Augustin M, Blome C, Costanzo A, et al. Nail assessment in psoriasis and psoriatic arthritis (NAPPA): development and validation of a tool for assessment of nail psoriasis outcomes. Br J Dermatol. 2014;170(3):591–598. | ||
Klaassen KM, Dulak MG, van de Kerkhof PC, Pasch MC. The prevalence of onychomycosis in psoriatic patients: a systematic review. J Eur Acad Dermatol Venereol. 2014;28(5):533–541. | ||
Alpsoy E, Polat M, Fettahlıoğlu-Karaman B, et al. Internalized stigma in psoriasis: a multicenter study. J Dermatol. 2017;44(8):885–891. | ||
Malakouti M, Brown GE, Leon A, et al. The dermatologic intimacy scale: quantitatively measuring the impact of skin disease on intimacy. J Dermatolog Treat. 2017;28(4):347–352. | ||
Paek SY, Thompson JM, Qureshi AA, Merola JF, Husni ME. Comprehensive assessment of the psoriasis patient (CAPP): a report from the GRAPPA 2015 annual meeting. J Rheumatol. 2016;43(5):961–964. | ||
de Jong EM, Seegers BA, Gulinck MK, Boezeman JB, van de Kerkhof PC. Psoriasis of the nails associated with disability in a large number of patients: results of a recent interview with 1728 patients. Dermatology. 1996;193(4):300–303. | ||
van der Velden HM, Klaassen KM, van de Kerkhof PC, Pasch MC. The impact of fingernail psoriasis on patients’ health-related and disease-specific quality of life. Dermatology. 2014;229(2):76–82. | ||
de Vries AC, Bogaards NA, Hooft L, et al. Interventions for nail psoriasis. Cochrane Database Syst Rev. 2013;(1):CD007633. | ||
Haneke E. Histopathology of the Nail – Onychopathology. Boca Raton: CRC Press; 2017. | ||
Brem J. Effective topical method of therapy for onychomycosis. Cutis. 1981;27(1):69–76. | ||
Tsai MT, Tsai TY, Shen SC, et al. Evaluation of laser-assisted trans-nail drug delivery with optical coherence tomography. Sensors (Basel). 2016;16(12):iiE2111. | ||
Rigopoulos D, Gregoriou S, Katsambas A. Treatment of psoriatic nails with tazarotene cream 0.1% vs. clobetasol propionate 0.05% cream: a double-blind study. Acta Derm Venereol. 2007;87(2):167–168. | ||
Tosti A, Piraccini BM, Cameli N, et al. Calcipotriol ointment in nail psoriasis: a controlled double-blind comparison with betamethasone dipropionate and salicylic acid. Br J Dermatol. 1998;139(4):655–659. | ||
Baran R, Tosti A. Topical treatment of nail psoriasis with a new corticoid-containing nail lacquer formulation. J Dermatol Treat. 1999;10:201–204. | ||
Nantel-Battista M, Richer V, Marcil I, Benohanian A. Treatment of nail psoriasis with intralesional triamcinolone acetonide using a needle-free jet injector: a prospective trial. J Cutan Med Surg. 2014;18(1):38–42. | ||
de Berker DA, Lawrence CM. A simplified protocol of steroid injection for psoriatic nail dystrophy. Br J Dermatol. 1998;138(1):90–95. | ||
Saleem K, Azim W. Treatment of nail psoriasis with a modified regimen of steroid injections. J Coll Physicians Surg Pak. 2008;18(2):78–81. | ||
Wolf R, Tur E, Brenner S. Corticosteroid-induced ‘disappearing digit’. J Am Acad Dermatol. 1990;23(4 pt 1):755–756. | ||
Deffer TA, Goette DK. Distal phalangeal atrophy secondary to topical steroid therapy. Arch Dermatol. 1987;123(5):571–572. | ||
Bjorkman A, Jorgsholm P. Rupture of the extensor pollicis longus tendon: a study of aetiological factors. Scand J Plast Reconstr Surg Hand Surg. 2004;38(1):32–35. | ||
Jakubik J. Finger tendon rupture following local application of triamcinolone-acetonide (Kenalog A-40). Acta Chir Plast. 1981;23(3):180–188. | ||
Mascaró JM. Epidermoid cyst formation after jet injection of triamcinolone for nail psoriasis. In: 29th Conf Coll Ibero Latino Am Dermatol CILAD; Sevilla; September 16–19, 2012. | ||
Sanchez Regaña M, Martin Ezquerra G, Umbert Millet P. Nail psoriasis: a combined treatment with 8% clobetasol nail lacquer and tacalcitol ointment. J Eur Acad Dermatol Venereol. 2008;22(8):963–969. | ||
Tzung TY, Chen CY, Yang CY, Lo PY, Chen YH. Calcipotriol used as monotherapy or combination therapy with betamethasone dipropionate in the treatment of nail psoriasis. Acta Derm Venereol. 2008;88(3):279–280. | ||
De Simone C, Maiorino A, Tassone F, D’Agostino M, Caldarola G. Tacrolimus 0.1% ointment in nail psoriasis: a randomized controlled open-label study. J Eur Acad Dermatol Venereol. 2013; 27(8):1003–1006. | ||
Diluvio L, Campione E, Paternò EJ, Mordenti C, El Hachem M, Chimenti S. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24(3):332–333. | ||
Fritz K. Successful local treatment of nail psoriasis with 5-fluorouracil [in German]. Z Hautkr. 1989;64(12):1083–1088. | ||
Yamamoto T, Katayama I, Nishioka K. Topical anthralin therapy for refractory nail psoriasis. J Dermatol. 1998;25(4):231–233. | ||
Lin YK, Chang YC, Hui RC, et al. A Chinese herb, Indigo naturalis, extracted in oil (Lindioil) used topically to treat psoriatic nails: a randomized clinical trial. JAMA Dermatol. 2015;151(6):672–674. | ||
Lin YK, See LC, Huang YH, et al. Efficacy and safety of Indigo naturalis extract in oil (Lindioil) in treating nail psoriasis: a randomized, observer-blind, vehicle-controlled trial. Phytomedicine. 2014;21(7):1015–1020. | ||
Sarıcaoglu H, Oz A, Turan H. Nail psoriasis successfully treated with intralesional methotrexate: case report. Dermatology. 2011;222(1):5–7. | ||
Daulatabad D, Grover C, Singal A. Role of nail bed methotrexate injections in isolated nail psoriasis: conventional drug via an unconventional route. Clin Exp Dermatol. Epub 2017 Apr 10:doi: 10.1111/ced.13087. | ||
Bauzá A, Redondo P, Aquerreta D. Psoriatic onycho-pachydermo periostitis: treatment with methotrexate. Br J Dermatol. 2000;143(4):901–902. | ||
Gümüşel M, Özdemir M, Mevlitoğlu I, Bodur S. Evaluation of the efficacy of methotrexate and cyclosporine therapies on psoriatic nails: a one-blind, randomized study. J Eur Acad Dermatol Venereol. 2011;25(9):1080–1084. | ||
Sanchez-Regana M, Sola-Ortigosa J, Alsina-Gibert M, Vidal-Fernandez M, Umbert-Millet P. Nail psoriasis: a retrospective study on the effectiveness of systemic treatments (classical and biological therapy). J Eur Acad Dermatol Venereol. 2011;25(5):579–586. | ||
Arnold WP, Gerritsen MJ, van de Kerkhof PC. Response of nail psoriasis to cyclosporin. Br J Dermatol. 1993;129(6):750–751. | ||
Mahrle G, Schulze HJ, Farber L, Weidinger G, Steigleder GK. Low-dose short-term cyclosporine versus etretinate in psoriasis: improvement of skin, nail, and joint involvement. J Am Acad Dermatol. 1995;32(1):78–88. | ||
Syuto T, Abe M, Ishibuchi H, Ishikawa O. Successful treatment of psoriatic nails with low-dose cyclosporine administration. Eur J Dermatol. 2007;17(3):248–249. | ||
Rabinovitz HS, Scher RK, Shupack JL. Response of psoriatic nails to the aromatic retinoid etretinate. Arch Dermatol. 1983;119(8):627–628. | ||
Gajardo J. Experiencia clinica con etretinato (Tigason) en 26 pacientes portadores de psoriasis. [Clinical experience with etretinate (Tigason) in 26 patients with psoriasis] [Spanish]. Rev Med Chil. 1989;117(5):516–522. | ||
Murray HE, Anhalt AW, Lessard R, et al. A 12-month treatment of severe psoriasis with acitretin: results of a Canadian open multicenter study. J Am Acad Dermatol. 1991;24(4):598–602. | ||
Brazzelli V, Martinoli S, Prestinari F, Borroni G. An impressive therapeutic result of nail psoriasis to acitretin. J Eur Acad Dermatol Venereol. 2004;18(2):229–230. | ||
Tosti A, Ricotti C, Romanelli P, Cameli N, Piraccini BM. Evaluation of the efficacy of acitretin therapy for nail psoriasis. Arch Dermatol. 2009;145(3):269–271. | ||
Baran R. Therapeutic assessment and side-effects of the aromatic retinoid on the nail apparatus (French). Ann Dermatol Venereol. 1982;109(4):367–371. | ||
Vlachou C, Berth-Jones J. Nail psoriasis improvement in a patient treated with fumaric acid esters. J Dermatolog Treat. 2007;18(3):175–177. | ||
Behrens F, Finkenwirth C, Pavelka K, et al. Leflunomide in psoriatic arthritis: results from a large European prospective observational study. Arthritis Care Res (Hoboken). 2013;65(3):464–470. | ||
Gerster JC, Hohl D. Nail lesions in psoriatic arthritis: recovery with sulfasalazine treatment. Ann Rheum Dis. 2002;61(3):277. | ||
Lassus A. Colloidal silicic acid for the treatment of psoriatic skin lesions, arthropathy and onychopathy. A pilot study. J Int Med Res. 1997;25(4):206–209. | ||
Torres T, Puig L. Apremilast: a novel oral treatment for psoriasis and psoriatic arthritis. Am J Clin Dermatol. Epub 2017 Jun 8:doi: 10.1007/s40257-017-0302-0. | ||
Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (efficacy and safety trial evaluating the effects of apremilast in psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49. | ||
Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–1399. | ||
Kushwaha AS, Repka MA, Narasimha Murthy S. A novel apremilast nail lacquer formulation for the treatment of nail psoriasis. AAPS PharmSciTech. Epub 2017 Apr 28:doi: 10.1208/s12249-017-0776-3. | ||
Di Lernia V, Bardazzi F. Profile of tofacitinib citrate and its potential in the treatment of moderate-to-severe chronic plaque psoriasis. Drug Des Devel Ther. 2016;10:533–539. | ||
Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two, randomised, placebo-controlled, phase 3 trials. Br J Dermatol. 2015;173:949–961. | ||
Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–561. | ||
Merola JF, Elewski B, Tatulych S, Lan S, Tallman A, Kaur M. Efficacy of tofacitinib for the treatment of nail psoriasis: two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2017;77(1):79.e–87.e. | ||
Langley RG, Saurat JH, Reich K; on behalf of the Nail Psoriasis Delphi Expert Panel. Recommendations for the treatment of nail psoriasis in patients with moderate to severe psoriasis: a dermatology expert group consensus. J Eur Acad Dermatol Venereol. 2012;26(3):373–381. | ||
Bardazzi F, Lambertini M, Chessa MA, Magnano M, Patrizi A, Piraccini BM. Nail involvement as a negative prognostic facor in biological therapy for psoriasis: a retrospective study. J Eur Acad Dermatol Venereol. 2017;31:843–846. | ||
Gniadecki R, Kragballe K, Dam TN, Skov L. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol. 2011;164(5):1091–1096. | ||
Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol. 2015;172(1):244–252. | ||
Arnold T, Schaarschmidt ML, Herr R, Fischer JE, Goerdt S, Peitsch WK. Drug survival rates and reasons for drug discontinuation in psoriasis. J Dtsch Dermatol Ges. 2016;14(11):1089–1099. | ||
Callen JP. Complications and adverse reactions in the use of newer biologic agents. Semin Cutan Med Surg. 2007;26(1):6–14. | ||
Al-Mutairi N, Nour T, Al-Rqobah D. Onychomycosis in patients of nail psoriasis on biologic therapy: a randomized, prospective open label study comparing etanercept, infliximab and adalimumab. Expert Opin Biol Ther. 2013;13(5):625–629. | ||
Fabroni C, Gori A, Troiano M, Prignano F, Lotti T. Infliximab efficacy in nail psoriasis. A retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25(5):549–553. | ||
Irla N, Yawalkar N. Marked improvement in nail psoriasis during treatment with adalimumab. Dermatology. 2009;219(4):353–356. | ||
Karanikolas GN, Koukli EM, Katsalira A, et al. Adalimumab or cyclosporine as monotherapy and in combination in severe psoriatic arthritis: results from a prospective 12-month nonrandomized unblinded clinical trial. J Rheumatol. 2011;38(11):2466–2474. | ||
Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab0 certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180–190. | ||
Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73(1):48–55. | ||
Rallis E, Stavropoulou E, Rigopoulos D, Verros C. Rapid response of nail psoriasis to etanercept. J Rheumatol. 2008;35(3):544–545. | ||
Ortonne JP, Paul C, Berardesca E, et al. A 24-week randomized clinical trial investigating the efficacy and safety of two doses of etanercept in nail psoriasis. Br J Dermatol. 2013;168(5):1080–1087. | ||
Weisshaar E, Diepgen TL. Successful etanercept therapy in therapy-refractory acrodermatitis continua suppurativa Hallopeau. J Dtsch Dermatol Ges. 2007;5(6):489–492. | ||
Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a sub-cutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60(4):976–986. | ||
Kavanaugh A, van der Heijde D, McInnes IB, et al. Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum. 2012;64(8):2504–2517. | ||
Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by antitumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513–2518. | ||
Wollina U, Hansel G, Koch A, Schönlebe J, Köstler E, Haroske G. Tumor necrosis factor-α inhibitor-induced psoriasis or psoriasiform exanthemata. First 120 cases from the literature including a series of 6 new cases. Am J Clin Dermatol. 2008;9:1–14. | ||
Pink AE, Foni A, Smith CH, Barker JNWN. The development of sarcoidosis on antitumour necrosis factor therapy: a paradox. Br J Dermatol. 2010;163:641–666. | ||
Pirard D, Arco D, Debrouckere V, Heenem M. Anti-tumor necrosis factor alpha-induced psoriasiform eruptions: three further cases and current overview. Dermatology current overview. Dermatology. 2006;213:182–186. | ||
Conrad C, Lapointe AK, Gilliet M. Paradoxic psoriasis induced by anti-TNF treatment – a report of 8 cases and evidence for a new pathogenic mechanism. In: 93rd Ann Meet Swiss Soc Dermatol Venereol; Geneva; 2011; FC 11, Dermatol Helv 2011;6:30 | ||
Haneke E. Nail psoriasis. In: Soung J, Koo B, editors. Psoriasis. Rijeka: Intech; 2011:141–186. | ||
Menter A. The status of biologic therapies in the treatment of moderate to severe psoriasis. Cutis. 2009;84(suppl 4):14–24. | ||
Wcisło-Dziadecka D, Zbiciak M, Brzezińska-Wcisło M, Mazurek U. Anti-cytokine therapy for psoriasis – not only TNF-α blockers. Overview of reports on the effectiveness of therapy with IL-12/IL-23 and T and B lymphocyte inhibitors. Postepy Hig Med Dosw (online). 2016;70:1198–1205. | ||
Puig L. The role of IL 23 in the treatment of psoriasis. Expert Rev Clin Immunol. 2017;13(6):525–534. | ||
Reich K, Sullivan J, Arenberger P, et al. Secukinumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: 16 week results from the TRANSFIGURE study. Presented at: 23rd World Congress of Dermatology; Vancouver, Canada; June 8–15, 2015 | ||
Paul C, Reich K, Gottlieb AB, et al; CAIN457A2211 Study Group. Secukinumab improves hand, foot and nail lesions in moderate-to-severe plaque psoriasis: subanalysis of a randomized, double-blind, placebo-controlled, regimen-finding phase 2 trial. J Eur Acad Dermatol Venereol. 2014;28(12):1670–1675. | ||
Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–1199. | ||
Langley RG, Rich P, Menter A, et al. Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2015;29(9):1763–1770. | ||
Puig L. Brodalumab: the first anti-IL-17 receptor agent for psoriasis. Drugs Today (Barc). 2017;53(5):283–297. | ||
Rallis E, Kintzoglou S, Verros C. Ustekinumab for rapid treatment of nail psoriasis. Arch Dermatol. 2010;146(11):1315–1316. | ||
Vitiello M, Tosti A, Abuchar A, Zaiac M, Kerdel FA. Ustekinumab for the treatment of nail psoriasis in heavily treated psoriatic patients. Int J Dermatol. 2013;52(3):358–362. | ||
Rich P, Bourcier M, Sofen H, et al; PHOENIX 1 Investigators. Ustekinumab improves nail disease in patients with moderate-to-severe psoriasis: results from PHOENIX 1. Br J Dermatol. 2014;170(2):398–407. | ||
Gordon KB, Langley RG, Gottlieb AB, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol. 2012;132(2):304–314. | ||
Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. | ||
Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288. | ||
Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136(1):116.e–124.e. | ||
Lindelof B. Radiotherapy is rarely used in daily clinical care of patients suffering from nail psoriasis. Acta Derm Venereol. 1989;69(1):80–82. | ||
Finnerty EF. Successful treatment of psoriasis of the nails. Cutis. 1979;23(1):43–44. | ||
Yu RC, King CM. A double-blind study of superficial radiotherapy in psoriatic nail dystrophy. Acta Derm Venereol. 1992;72(2):134–136. | ||
Kwang TY, Nee TS, Seng KT. A therapeutic study of nail psoriasis using electron beams. Acta Derm Venereol. 1995;75(1):90. | ||
Stern DK, Creasey AA, Quijije J, Lebwohl MG. UV-A and UV- B penetration of normal human cadaveric fingernail plate. Arch Dermatol. 2011;147(4):439–441. | ||
Marx JL, Scher RK. Response of psoriatic nails to oral photochemotherapy. Arch Dermatol. 1980;116(9):1023–1024. | ||
MacDonald KJ, Hargreaves GK, Ead RD. Longitudinal melanonychia during photochemotherapy. Br J Dermatol. 1986;114(3):395–396. | ||
Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40(7):763–768. | ||
Oram Y, Karincaoglu Y, Koyuncu E, Kaharaman F. Pulsed dye laser in the treatment of nail psoriasis. Dermatol Surg. 2010;36(3):377–381. | ||
Fernández-Guarino M, Harto A, Sánchez-Ronco M, García-Morales I, Jaén P. Pulsed dye laser vs. photodynamic therapy in the treatment of refractory nail psoriasis: a comparative pilot study. J Eur Acad Dermatol Venereol. 2009;23(8):891–895. | ||
Treewittayapoom C, Singvahanont P, Chanprapaph K, Haneke E. The effect of different pulse durations in the treatment of nail psoriasis with 595-nm pulsed dye laser: a randomized, double- blind, intrapatient left-to-right study. J Am Acad Dermatol. 2012;66(5):807–812. | ||
Goldust M, Raghifar R. Clinical trial study in the treatment of nail psoriasis with pulsed dye laser. J Cosmet Laser Ther. Epub 2013 Oct 16. | ||
Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45(2):102–107. | ||
Al-Mutairi N, Noor T, Al-Haddad A. Single blinded left-to-right comparison study of excimer laser versus pulsed dye laser for the treatment of nail psoriasis. Dermatol Ther (Heidelb). 2014;4(2):197–205. | ||
Arango-Duque LC, Roncero-Riesco M, Usero Bárcena T, Palacios Álvarez I, Fernández López E. Treatment of nail psoriasis with pulse dye laser plus calcipotriol betametasona gel vs. Nd:YAG plus calcipotriol betamethasone gel: an intrapatient left-to-right controlled study. Actas Dermosifiliogr. 2017;108(2):140–144. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.