Back to Journals » Cancer Management and Research » Volume 9
nab-Paclitaxel for the treatment of pancreatic cancer
Authors Kim G
Received 16 November 2016
Accepted for publication 30 December 2016
Published 16 March 2017 Volume 2017:9 Pages 85—96
DOI https://doi.org/10.2147/CMAR.S127840
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
George Kim
21st Century Oncology, University of Florida Health Oncology, Jacksonville, FL, USA
Background: Nanoparticle albumin-bound paclitaxel (nab-P) plus gemcitabine (Gem) became a standard treatment option for metastatic pancreatic cancer (MPC) following positive results from a global phase III trial (MPACT). A large number of studies have now published results on the use of nab-P/Gem to treat advanced and early-stage disease, warranting a comprehensive review. The main goal of this systematic review is to summarize the efficacy and safety data of nab-P/Gem for the treatment of pancreatic cancer (PC).
Methods: This systematic review includes results from studies that either published results in a peer-reviewed journal or presented the results at a major oncology conference.
Results: Sixty-two studies were included (50 in the advanced/metastatic setting and 12 in the locally advanced setting). Most studies on the treatment of MPC were exclusively first line (33/50). Nevertheless, the studies in this review comprised a broad spectrum of patients, including those <65 and ≥65 years of age and those with a Karnofsky performance status of 70–100. Median overall survival (OS) in studies of nab-P/Gem in the advanced/metastatic setting ranged from 8.7 to 13.5 months. In addition, 15 studies of patients with advanced/metastatic PC examined nab-P/Gem as a backbone on which to add a variety of agents, including cancer stem cell inhibitors, stromal disrupting agents, and immune-modulating agents (median OS, 6.9–17 months). Ongoing trials are investigating nab-P/Gem with or without other agents across disease settings.
Discussion: Studies conducted after MPACT have demonstrated that nab-P/Gem is an effective regimen for the first-line treatment of MPC for a wide range of patients. Regimens using nab-P/Gem as a backbone on which to combine additional agents are being studied actively, particularly in the advanced disease setting. Ongoing studies will yield valuable insights on the utility of nab-P–containing regimens to improve patient outcomes in PC in both earlier-stage and advanced disease.
Keywords: pancreatic cancer, nab-paclitaxel, metastatic, neoadjuvant, systematic review
Introduction
More than 50,000 new pancreatic cancer (PC) cases and >40,000 cancer-related mortalities due to PC are expected in the USA in 2016.1,2 The 5-year survival rate for all stages of PC combined is 8%. Although those with resectable disease have a more favorable prognosis (5-year survival ≈29%), ≈52% of patients are diagnosed with metastatic disease, which confers a less favorable outlook (5-year survival ≈3%).2 Since the approval of gemcitabine (Gem) in 1997, no phase III trial in advanced/metastatic disease had demonstrated a clinically and statistically significant improvement in overall survival (OS) over Gem alone3 until recently. The treatment landscape for metastatic disease has evolved to include 2 key regimens: folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) and nanoparticle albumin-bound paclitaxel (nab-P) plus Gem (nab-P/Gem). The FOLFIRINOX regimen was approved based on a French multicenter phase II/III trial that reported significant improvements in OS with FOLFIRINOX versus Gem (median, 11.1 vs 6.8 months; hazard ratio [HR], 0.57; P<0.001), but significant adverse events were also observed.4 The nab-P/Gem regimen was approved in many countries after the phase III MPACT trial demonstrated that the addition of nab®-P (Abraxane®; Celgene Corporation, Summit, NJ, USA) to Gem improved OS versus Gem (median, 8.7 vs 6.6 months; HR, 0.72; P<0.001).5 Currently, the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) recommend treatment with FOLFIRINOX or nab-P/Gem as standards of care for patients with metastatic pancreatic cancer (MPC).6,7 Age, performance status (PS), and other clinical factors are considered when deciding which regimen to use; Gem monotherapy is currently reserved for patients ineligible to receive combination chemotherapy.6
nab-P/Gem and FOLFIRINOX have not been approved for earlier-stage disease; however, numerous trials are exploring their utility. The NCCN recommends chemotherapy for unresectable locally advanced PC (LAPC) and chemoradiation for selected patients, preferably after induction chemotherapy for tumor control.6 Currently, no clear evidence exists to support the use of nab-P/Gem over FOLFIRINOX or vice versa, and several trials are investigating their efficacy and safety.8
A population-based study of >3,000 patients showed that nab-P/Gem is the most commonly used chemotherapy regimen for the first-line treatment of MPC in the USA,9 possibly due to the toxicity profile of FOLFIRINOX, which limits its use to younger/fitter patients. The extensive use of nab-P/Gem in both academic and community settings coupled with >100 current and active clinical trials in PC warrants a comprehensive review of clinical data to gain a better understanding of how this regimen is being used for the treatment of PC and associated outcomes. The overall goal of this review is to summarize recent data regarding the safety and efficacy of regimens that include nab-P/Gem for patients with PC.
Methods
The search terms “nab-paclitaxel and (pancreatic or pancreas)” were entered in PubMed to retrieve publications from January 1, 2011 to June 30, 2016. Abstracts from the annual meetings of the American Society of Clinical Oncology (ASCO) 2011–2016, the Gastrointestinal Cancers Symposium (ASCO GI) 2011–2016, the European Cancer Organisation/ESMO 2011–2015, the ESMO World Congress on Gastrointestinal Cancer 2015 and 2016, and the Italian Association of Medical Oncology (2014) were searched using the term “nab-paclitaxel.” Clinical trials and institutional analyses of nab-P in all stages of PC were included. Duplicates, electronic abstracts, case studies, cost studies, meta-analyses, and studies of the effects of eligibility criteria were excluded. The website www.clinicaltrials.gov was searched using the terms “nab-paclitaxel” OR “Abraxane” AND “pancreatic” AND “adenocarcinoma” to identify ongoing trials without results; only open, active, phase II–III trials with a sample size ≥100 were included.
Results
Studies of nab-P in advanced/metastatic PC
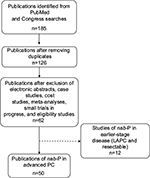
Fifty studies evaluating nab-P in MPC were retrieved (Figure 1; Table 1). Approximately one-half were retrospective analyses. MPACT was the only phase III study, and all other prospective trials were phase I or II. Two-thirds of studies evaluated nab-P in the first-line setting, and approximately one-third of those studies assessed nab-P/Gem with an additional agent. nab-P was most often evaluated at a dose of 125 mg/m2, which was given the first 3 of 4 weeks (qw 3/4). The tables in this systematic review cover MPC (Tables 1–3), neoadjuvant treatment or locally advanced disease (Table 4), and ongoing trials in all settings (Table 5).
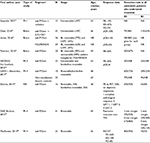
  | Table 1 Characteristics of advanced/MPC studies (n=50) Abbreviations: Gem, gemcitabine; MPC, metastatic pancreatic cancer; nab-P, nanoparticle albumin-bound paclitaxel. |
nab-P/Gem in MPC
Ten studies reported the median OS for first-line nab-P/Gem in patients with advanced PC;5,10–18 Table 2 lists 8 of these studies with a population >45 patients. The most commonly used dose and schedule were those used in the MPACT trial: nab-P 125 mg/m2 plus Gem 1,000 mg/m2 administered on a qw 3/4 schedule.5,14,19,20 Patients treated with this dose and schedule experienced a median OS ranging from 8.7 to 13.5 months5,18 and 1-year survival ranging from 35% to 62%.5,14,19,20 Most prospective studies evaluating this dose and schedule were single-arm trials.
nab-P/Gem in MPC – age
It may be expected that younger patients would experience longer survival and improved tolerability compared with older patients. However, most studies, including MPACT, suggest that older patients benefit from nab-P/Gem in terms of efficacy without increased risk of toxicity. Approximately 40% of patients enrolled in MPACT were >65 years.5 Median OS was 9.6 and 7.7 months for patients <65 and ≥65 years, respectively, and the toxicity profiles were similar between age groups.5 The combination in MPACT demonstrated significant OS benefit over Gem alone in both age groups: <65 years (HR, 0.65; P<0.001) and ≥65 years (HR, 0.80; P=0.048).
A study (N=37) including patients treated with first-line or ≥ second-line nab-P/Gem for MPC showed that OS was not significantly different between patients ≥66 years and those <66 years of age (median, 10.5 vs 9 months; P=0.49).21 Similarly, a large Italian database review of patients (N=208) with advanced PC treated with nab-P/Gem demonstrated that age (≥75 vs <75 years) was not significantly associated with efficacy or toxicity with respect to median OS (11.4 vs 11 months; P=0.86), disease control rate (69% vs 61%; P=0.64), grade 3/4 neutropenia (25% vs 28%), and neurotoxicity (9% vs 12%).22 Additionally, an exploratory analysis from MPACT showed that the percentages of patients requiring nab-P dose reductions were similar between age groups (42% for patients ≥65 years vs 40% for patients <65 years).23
nab-P/Gem in MPC – PS
Data on whether patients with a better PS might receive greater benefit from nab-P/Gem than patients with a poorer PS are inconclusive; however, similar to the literature in older patients, several studies suggest that less fit patients receive meaningful benefit from the regimen. Stratification of the MPACT population by Karnofsky PS (KPS) demonstrated significantly better OS in the fitter (KPS 90–100) versus less fit (KPS 70–80) group in the combination arm (median, 9.7 vs 7.6 months; HR, 0.76; P=0.009) and the Gem arm (median, 7.9 vs 4.3 months; HR, 0.57; P<0.001).5 In the KPS 70–80 subpopulation, nab-P/Gem extended median OS by >3 months compared with Gem alone (7.6 vs 4.3 months; HR, 0.59; P<0.001).
A small phase I/II trial examined the effect of nab-P/Gem in patients with an Eastern Cooperative Oncology Group (ECOG) PS of 2.24 The results of the phase I portion suggest that these patients were able to receive the standard dose of nab-P/Gem; the relative dose intensity was 100% in 6 patients who received nab-P 125 mg/m2 plus Gem 1,000 mg/m2 qw 3/4.
In a retrospective analysis of 39 patients with unresectable LAPC or MPC treated with nab-P/Gem,25 patients with an ECOG PS of 1 survived longer than patients with an ECOG PS of 2 (median OS, 15 vs 7 months; P=0.032).25 Similarly, the previously mentioned Italian retrospective analysis of patients with advanced PC (N=208) treated with nab-P/Gem showed a numerically shorter OS in the ECOG PS 2 versus ECOG PS 0–1 group (median, 8.7 vs 11.2 months; P=0.07), but the difference was not significant.22 In addition, toxicities did not appear to be influenced by PS, because similar percentages of patients with PS 0–1 and PS 2 developed neutropenia (31% and 34%, respectively) and neurotoxicity (17% in each group). Collectively, these studies suggest that, although PS may affect OS, nab-P/Gem seems to be effective regardless of PS.
nab-P/Gem in MPC – real-world comparative effectiveness studies
Although clinical trials comparing nab-P/Gem with FOLFIRINOX for the treatment of PC have not yet reported results, retrospective analyses have explored these standard-of-care regimens with one another and/or Gem for the treatment of MPC.11,12,26–29 One study reported a median OS of 10.2 months with nab-P/Gem (n=189) versus 11.2 months with FOLFIRINOX (n=666) and 7 months for Gem combined with other chemotherapies (n=1,567).11 Similar results were reported from another retrospective analysis: median OS of 11.6 months with nab-P/Gem (n=41) versus 13 months with FOLFIRINOX (n=101) and 7.5–9.1 months for Gem plus other chemotherapies (n=277).12 A real-world analysis based on electronic medical records of patients (N=202) receiving first-line treatment for advanced PC demonstrated similar comparative effectiveness for nab-P/Gem versus FOLFIRINOX (database persistence [proxy for OS], median, 8.6 months in both groups), despite patients in the FOLFIRINOX group being significantly younger.27 In addition, a retrospective analysis (N=150) of patients treated at 5 cancer centers in British Columbia, Canada, found that both nab-P/Gem and FOLFIRINOX produced similar outcomes and demonstrated longer OS versus Gem alone as treatment for unresectable PC (median, 11.6 and 11.2 vs 4.1 months, respectively; P<0.001 and P=0.039).29 Patients who received FOLFIRINOX were younger (median age, 61 vs 70 years) and fitter (ECOG PS≤1, 91% vs 54%) than those who received nab-P/Gem.29 Collectively, the OS with nab-P/Gem observed in MPACT was consistent with the OS observed in real-world observational data sets, and nab-P/Gem was comparable in effectiveness to FOLFIRINOX.
Subsequent therapies after first-line nab-P/Gem in MPC
Many recent analyses have examined the use of second-line therapies after nab-P/Gem.27,30–33 Patients in MPACT who received second-line therapy (n=170) after nab-P/Gem experienced a numerically longer median OS than those who did not (n=250; median total OS, 12.8 and 6.3 months, respectively).30 The longest total OS values were observed in patients who received first-line nab-P/Gem followed by fluoropyrimidine-containing second-line regimens (n=132; median, 13.5 months); a small number (n=18) received second-line FOLFIRINOX and experienced a median total OS of 15.7 months.30 Another retrospective analysis from the previously described Italian registry (N=250) demonstrated similar findings, that is, a median OS of 13.5 months in patients who received second-line treatment after first-line nab-P/Gem (n=122).31 More specifically, patients who received second-line FOLFOX/XELOX (n=56), FOLFIRI (n=24), and FOLFIRINOX (n=22) had median total OS values of 12.8, 13.2, and 13.8 months, respectively.31 Consistent findings have been observed in many other analyses, and the totality of data suggests that first-line nab-P/Gem followed by second-line therapy, particularly with regimens that contain a fluoropyrimidine, is feasible and beneficial to patients with advanced PC.27,30–33
Future directions
Future directions for nab-P/Gem include studies in which the regimen has been used as a backbone therapy (ie, with another agent) in MPC (Table 3) and as a doublet in locally advanced pancreatic cancer (Table 4). Table 5 displays a list of selected ongoing trials of nab-P/Gem with or without other agents as treatment for metastatic, locally advanced, and resectable disease.
nab-P/Gem as a backbone regimen in MPC (studies with results)
Because nab-P/Gem has demonstrated survival comparable to that with FOLFIRINOX and a more favorable toxicity profile, this regimen is commonly used as a chemotherapy backbone for other agents (Table 3). Agents combined with nab-P/Gem are diverse and include cancer stem cell inhibitors (demcizumab, vismodegib, tarextumab, and BBI-608), those with potential immune-modulating activities (indoximod), those directed against tumor stroma (PEGPH20 and 2-0, 3-0 desulfated heparin), chemotherapies (capecitabine ± cisplatin), hormone therapy (enzalutamide), and others (erlotinib and apatorsen). In 15 studies of patients with MPC treated with nab-P/Gem combined with other agents (including 10 phase I trials), the median OS ranged from 6.9 to 17 months.
nab-P/Gem as a backbone regimen in MPC (studies without results)
Thirty ongoing phase II and III trials of nab-P in PC with a sample size of ≥100 were identified, including 16 MPC trials (all first line); most included an additional agent (Table 5). For example, the phase II/III RESOLVE trial (N=326) is evaluating nab-P/Gem, with or without the Bruton tyrosine kinase inhibitor ibrutinib, as first-line treatment of MPC.34 Based on promising results from phase I/II trials (Table 3), a phase III trial (N=420) is investigating PEGPH20 in combination with nab-P/Gem in patients with high levels of hyaluronan, and demcizumab with nab-P/Gem is being evaluated in the phase II YOSEMITE trial (N=201).35 Another noteworthy ongoing trial is a phase II study (N=260) of nab-P/Gem plus istiratumab (MM-141; a bispecific antibody against ErbB3 and insulin-like growth factor-1 [IGF-1] receptor) for the first-line treatment of patients with MPC and high serum levels of free IGF-1.36 Finally, whether the combination of nab-P/Gem with checkpoint inhibitors will be an effective strategy for PC is an important question, because checkpoint inhibitors have recently provided breakthrough treatment options for several tumor types and are currently being explored in a number of PC trials. Data on such combinations (eg, nab-P/Gem and nivolumab)37 are preliminary at this point.
Neoadjuvant trials for patients with resectable, borderline resectable, or LAPC (studies with results)
Several recent studies (n=12) examined neoadjuvant nab-P/Gem as a strategy for improving R0 resection rates in resectable tumors or converting borderline resectable tumors to resectable tumors. One of the main pathologic predictors of survival after surgery is resection margin status; a negative resection margin (R0) is associated with better prognosis compared with a positive margin. Eight of the 12 studies had a total enrollment of ≥15 patients (Table 4). Noteworthy among these is a pilot phase II study in which patients with resectable PC (N=25) were treated with neoadjuvant nab-P/Gem for 3 cycles.38 Surgical resection was possible in 84% of patients and resulted in R0 resection in 95% of resected cases, or 80% of the intention-to-treat population.38 The phase II GAP study also evaluated neoadjuvant nab-P/Gem for 2 cycles in patients with resectable PC (N=41).39 After neoadjuvant treatment, 73% of the patients underwent pancreatic resection.39 Similar results were reported from another trial of neoadjuvant nab-P/Gem (administered for 2 cycles) in patients with resectable or borderline resectable tumors (N=16).40 Seventy-five percent of patients underwent surgery, and R0 resection was achieved in 69% of the intention-to-treat population – 92% of those who underwent surgery.
Neoadjuvant trials for patients with resectable, borderline resectable, or LAPC (studies without results)
The phase II NEOLAP trial (N=168) will examine the ability of neoadjuvant nab-P/Gem versus FOLFIRINOX to convert unresectable LAPC or borderline resectable tumors to resectable tumors (Table 5).41 Another phase II study (N=112) is comparing neoadjuvant nab-P/Gem versus FOLFIRINOX followed by resection in patients with potentially resectable tumors.42 The randomized phase II LAPACT study (N=110) is investigating time to treatment failure in patients with unresectable LAPC treated with nab-P/Gem.43,44
Ongoing adjuvant trials for patients with resectable PC
The ongoing phase III APACT study is evaluating nab-P/Gem versus Gem monotherapy as adjuvant treatment in patients who have undergone macroscopic complete resection for non-MPC (Table 5).45,46 Two other studies are also examining nab-P/Gem as adjuvant therapy: the phase II NEONAX study (N=166; nab-P/Gem as adjuvant only vs as neoadjuvant plus adjuvant)47 and a second-line adjuvant phase III trial in patients who experienced disease relapse during Gem-based adjuvant therapy (N=300).48
Discussion
Multiple studies have demonstrated that first-line treatment with nab-P/Gem improves survival in patients with MPC, with OS similar to or better than that observed in MPACT. These studies have helped to confirm the dose and schedule of nab-P 125 mg/m2 plus Gem 1,000 mg/m2 qw 3/4 as an effective and tolerable option for patients with MPC. Retrospective analyses of comparisons between nab-P/Gem and FOLFIRINOX suggested similar efficacy outcomes between the regimens, despite differences in patient populations; nab-P/Gem was used in a broader spectrum of patients.
Most studies demonstrated an OS benefit with nab-P/Gem regardless of age group; similarly, patients seem to derive substantial clinical benefit from nab-P/Gem regardless of PS. The demonstrated efficacy of first-line nab-P/Gem has led to a number of studies examining regimens afterward as second-line therapy.27,30–33 These studies showed that second-line treatment after nab-P/Gem is feasible and that fluoropyrimidine-containing regimens, and not exclusively FOLFIRINOX, are appropriate options in this setting.
There are currently >100 ongoing trials (combined target enrollment >9,500 patients) assessing different nab-P regimens for the treatment of PC, and these studies will provide critical information regarding optimal combinations for specific patient populations.49
Conclusion
In summary, nab-P/Gem is an effective and well-tolerated regimen for patients with PC. Ongoing trials will evaluate nab-P in all stages of PC. The combination of nab-P/Gem has become a standard of care for MPC and a backbone onto which novel therapies are added in ongoing trials. Future directions in this field will revolve around improving our understanding of PC, including its molecular biology, and identifying subsets of patients that may benefit from specific treatments.
Acknowledgments
Medical writing assistance was provided by John McGuire, PhD, MediTech Media, Ltd, funded by Celgene Corporation. The author is fully responsible for content and editorial decisions for this manuscript. The author is on the speaker’s bureau and is a consultant for Celgene Corporation.
Disclosure
The author reports no other conflicts of interest in this work.
References
American Cancer Society, editor. Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society; 2016. | ||
Surveillance, Epidemiology, and End Results Program. SEER stat facts sheets: pancreas cancer; updated 2016. Available from: http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed September 15, 2016. | ||
Ciliberto D, Botta C, Correale P, et al. Role of gemcitabine-based combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomised trials. Eur J Cancer. 2013;49(3):593–603. | ||
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. | ||
Goldstein D, El-Maraghi RH, Hammel P, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107(2):1–10. | ||
NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. V1. 2016. Available form: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed August 1, 2016. | ||
Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–v68. | ||
Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(22):2654–2668. | ||
Abrams TA, Meyer G, Moloney J, et al. Patterns of chemotherapy (CT) use in a population-based US-wide cohort of patients (pts) with metastatic pancreatic cancer (MPC). Poster presented at: ASCO 2014 Annual Meeting [abstract 4131]. | ||
Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–4554. | ||
Cartwright TH, Ginsburg A, Wilfong LS, Harrell RK, Hoverman JR. Use of first-line chemotherapy for advanced pancreatic cancer: FOLFIRINOX versus gemcitabine-based therapy. Poster presented at: ASCO 2014 Annual Meeting [abstract 4132]. | ||
Santoni M, Bittoni A, Andrikou K, et al. Does first-line therapy affect the outcome of patients with pancreatic cancer? Poster presented at: ESMO 2014 Annual Meeting [abstract 695P]. | ||
Krishna K, Blazer M, Wei L, et al. Modified gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer (MPC): a single institution experience. Poster presented at: ASCO 2015 Gastrointestinal Cancers Symposium [abstract 366]. | ||
Giordano G, Febbraro A, Vaccaro V, et al. Nab-paclitaxel (nab-P) and gemcitabine (G) as first line chemotherapy (CT) in advanced pancreatic cancer (APDAC) patients (pts): an Italian “real life” study. Poster presented at: ESMO 2015 Annual Meeting [abstract 2334]. | ||
Hammel P, Bachet JB, Desrame J, et al. Nab-paclitaxel plus gemcitabine or plus simplified LV5FU2 as first-line therapy in patients with metastatic pancreatic adenocarcinoma: a GERCOR randomized phase II study. Poster presented at: ASCO 2016 Annual Meeting [abstract 4120]. | ||
Zhang DS, Wang DS, Wang ZQ, et al. Phase I/II study of albumin-bound nab-paclitaxel plus gemcitabine administered to Chinese patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2013;71(4):1065–1072. | ||
Shen L, Yu X, Hao J, et al. A phase II study of Chinese patients (pts) treated with nab-paclitaxel (nab-P) plus gemcitabine (gem) for metastatic pancreatic cancer (MPC). Poster presented at: ASCO 2016 Annual Meeting [abstract 327]. | ||
Ueno H, Ikeda M, Ueno M, et al. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77(3):595–603. | ||
Kasuga A, Ueno H, Ikeda M, et al. Efficacy, safety and pharmacokinetics of weekly nab-paclitaxel plus gemcitabine in Japanese patients with metastatic pancreatic cancer (MPC): phase I/II trial. Presentation at the 2014 APA/JPS Meeting [abstract 14200]. | ||
Bachet J, Hammel P, Desrame J, et al. Nab-paclitaxel plus gemcitabine or plus simplified LV5FU2 as first-line therapy in patients with metastatic pancreatic adenocarcinoma. A GERCOR randomized phase II study (AFUGEM). Poster presented at: ESMO 2015 Annual Meeting [abstract P317]. | ||
Lo Re G, Santeufemia DA, Foltran L, Bidoli E, Basso SM, Lumachi F. Prognostic factors of survival in patients treated with nab-paclitaxel plus gemcitabine regimen for advanced or metastatic pancreatic cancer: a single institutional experience. Oncotarget. 2015;6(10):8255–8260. | ||
Giordano G, De Vita F, Melisi D, et al. Analysis of activity, efficacy and safety of first line nab-paclitaxel (nab-P) and gemcitabine (G) in advanced pancreatic cancer (APDAC) frail and elderly patients (pts). Poster presented at: ESMO 2015 Annual Meeting [abstract P297]. | ||
Scheithauer W, Von Hoff DD, Ramanathan R, et al. Dose delivery in a phase III trial (MPACT) of weekly nab-paclitaxel (nab-P) plus gemcitabine (gem) vs gem alone for patients with metastatic adenocarcinoma of the pancreas. Poster presented at: ESMO 2013 Annual Meeting [abstract 2.586]. | ||
Guillen-Ponce C, Lopez R, Macarulla T, et al. A phase I/II trial to evaluate the efficacy and safety of nab-paclitaxel in combination with gemcitabine for the treatment of frail patients with advanced or metastatic pancreatic cancer: safety results of the phase I trial. Poster presented at: ESMO 2014 Annual Meeting [abstract 700]. | ||
Montes AF, Villarroel PG, Ayerbes MV, et al. Retrospective analysis of prognostic and predictive markers in patients with locally advanced unresectable or metastatic pancreatic adenocarcinoma treated with gemcitabine/nabpaclitaxel: influence of the presence of stent. Poster presented at: ASCO 2015 Annual Meeting [abstract 483]. | ||
Park PYS, McAferty KV, Ahmadi M, et al. Radiological markers of treatment responsiveness in patients (pts) with metastatic pancreatic ductal adenocarcinomas (mPDAC) receiving systemic chemotherapy. Poster presented at: ASCO 2016 Annual Meeting [abstract 403]. | ||
Braiteh FS, Patel M, Parisi M, Ni Q, Park SY, Faria C. Comparative effectiveness and resource utilization of nab-paclitaxel plus gemcitabine (nab-P+G) versus FOLFIRINOX (FFX) in first-line treatment of advanced pancreatic adenocarcinoma (PDAC) in a U.S. community oncology setting. Poster presented at: ASCO 2016 Annual Meeting [abstract 433]. | ||
Braiteh FS, Patel M, Parisi M, Ni Q, Park SY, Faria C. Comparative effectiveness and resource utilization of nab-paclitaxel plus gemcitabine (nab-P+G) versus gemcitabine monotherapy (G) in first-line treatment of advanced pancreatic adenocarcinoma (PDAC) in a U.S. community oncology setting. Poster presented at: ASCO 2016 Annual Meeting [abstract 429]. | ||
Wang Y, Chen L, Camateros P, Gill S, Renouf DJ, Cheung WY. Comparative effectiveness of FOLFIRINOX or nab-paclitaxel plus gemcitabine in locally advanced or metastatic pancreatic cancer: a population-based analysis. Poster presented at: ASCO 2016 Annual Meeting [abstract 6561]. | ||
Chiorean EG, Von Hoff DD, Tabernero J, et al. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br J Cancer. 2016;115(2):188–194. | ||
Giordano G, Febbraro A, Milella M, et al. Impact of second-line treatment (2L T) in advanced pancreatic cancer (APDAC) patients (pts) receiving first line nab-paclitaxel (nab-P) + gemcitabine (G): an Italian multicentre real life experience. Poster presented at: ASCO 2016 Annual Meeting [abstract 4124]. | ||
Schmidt SL, Durkal V, Jayavalsan SP, et al. Outcomes in metastatic pancreatic adenocarcinoma (MPAC) patients treated with FOLFIRINOX (FFX)/FOLFOX(FX) and gemcitabine + nab-paclitaxel (NabG). Poster presented at: ASCO 2016 Annual Meeting [abstract 397]. | ||
Idrees K, Parikh A, Postlewait LM, et al. Treatment of borderline resectable (BR) and locally advanced (LA) pancreatic cancer in the era of FOLFIRINOX and gemcitabine plus nab-paclitaxel: a multi-institutional study. Poster presented at: ASCO 2016 Annual Meeting [abstract 451]. | ||
ClinicalTrials.gov. Study of ibrutinib vs placebo, in combination with nab-paclitaxel and gemcitabine, in the first line treatment of patients with metastatic pancreatic adenocarcinoma (RESOLVE). Available from: https://clinicaltrials.gov/ct2/show/NCT02436668. Accessed August 1, 2015. | ||
ClinicalTrials.gov. Study of gemcitabine, ABRAXANE® plus placebo versus gemcitabine, ABRAXANE® plus 1 or 2 truncated courses of demcizumab in subjects with 1st-line metastatic pancreatic ductal adenocarcinoma (YOSEMITE). Available from: https://clinicaltrials.gov/ct2/show/NCT02289898. Accessed August 1, 2016. | ||
ClinicalTrials.gov. A phase 2 study of MM-141 plus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Available from: https://clinicaltrials.gov/ct2/show/NCT02399137. Accessed August 1, 2016. | ||
Hochster HS, Wainberg ZA, Gutierrez M, et al. Phase 1 study of nivolumab + nab-paclitaxel +/- gemcitabine in pancreatic cancer: safety evaluation of patients treated with nivolumab + nab-paclitaxel in arm A. Poster presented at: AACR: Special Conference on Pancreatic Cancer 2016 [abstract A72]. | ||
MacKenzie S, Zeh H, McCahill L, et al. A pilot phase II multi center study of nab-paclitaxel and gemcitabine as preoperative therapy for potentially resectable pancreatic cancer (PC). Poster presented at: ASCO 2013 Annual Meeting [abstract 4038]. | ||
Barbour A, O’Rourke N, Chan H, et al. Initial survival outcomes for the AGITP GAP study – a phase II study of perioperataive nab-paclitaxel and gemcitabine for resectable pancreatic ductal adenocarcinoma (PDAC). Poster presented at: ASCO 2016 Annual Meeting [abstract 4105]. | ||
Alvarez R, Musteanu M, Garcia-Garcia E, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109(4):926–933. | ||
ClinicalTrials.gov. Trial to investigate intensified neoadjuvant chemotherapy in locally advanced pancreatic cancer (NEOLAP). Available from: https://clinicaltrials.gov/ct2/show/NCT02125136?term=nab-paclitaxel+AND+pancreatic+AND+adenocarcinoma&rank=55. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Phase II study of preoperative FOLFIRINOX versus gemcitabine/nab-paclitaxel in patients with resectable pancreatic cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT02243007?term=nab-paclitaxel+AND+pancreatic+AND+adenocarcinoma&rank=59. Accessed August 1, 2016. | ||
ClinicalTrials.gov. First line treatment of patients with metastatic pancreatic adenocarcinoma (GABRINOX). Available from: https://clinicaltrials.gov/ct2/show/NCT01964287. Accessed August 1, 2016. | ||
Philip PA, Lacy J, Dowden SD, et al. LAPACT: an open-label, multicenter phase II trial of nab-paclitaxel (nab-P) plus gemcitabine (gem) in patients (pts) with locally advanced pancreatic cancer (LAPC). Poster presented at: ASCO 2016 Annual Meeting [abstract TPS477]. | ||
ClinicalTrials.gov. Nab-paclitaxel and gemcitabine vs gemcitabine alone as adjuvant therapy for patients with resected pancreatic cancer (the “apact” study) (apact). Available from: https://clinicaltrials.gov/ct2/show/NCT01964430?term=nab-paclitaxel+AND+pancreatic+AND+adenocarcinoma&rank=24. Accessed August 1, 2016. | ||
Tempero MA, Coussens LM, Fong L, Manges R, Singh P, Li Y. A randomized, multicenter, double-blind, placebo-controlled study of the bruton tyrosine kinase inhibitor, ibrutinib, versus placebo in combination with nab-paclitaxel and gemcitabine in the first-line treatment of patients with metastatic pancreatic adenocarcinoma (RESOLVE). Poster presented at: ASCO 2016 Annual Meeting [abstract TPS483]. | ||
ClinicalTrials.gov. Neoadjuvant plus adjuvant or only adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer (NEONAX). Available from: https://clinicaltrials.gov/ct2/show/NCT02047513?term=nab-paclitaxel+AND+pancreatic+AND+adenocarcinoma&rank=34. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Second-line adjuvant therapy with nab-paclitaxel plus gemcitabine versus oxaliplatin plus folinic acid and fluorouracil for gemcitabine-refractory pancreatic cancer after curative resection. Available from: https://clinicaltrials.gov/ct2/show/NCT02506842?term=nab-paclitaxel+AND+pancreatic+AND+adenocarcinoma&rank=42. Accessed August 1, 2016. | ||
ClinicalTrials.gov. 2016. Available from: https://clinicaltrials.gov. Accessed August 1, 2016. | ||
Giordano G, Febbraro A, Vaccaro V, et al. Nab-paclitaxel (nab-P) and gemcitabine (G) as first line chemotherapy (CT) in advanced pancreatic cancer (APDAC) elderly patients (pts): an Italian “real life” study. Poster presented at: ESMO 2015 Annual Meeting [abstract P296]. | ||
Sueyoshi H, Ioka T, Tamura T, et al. Phase I study of chemoradiation therapy (nab-paclitaxel/gemcitabine) in 15 patients with unresectable locally advanced pancreatic cancer (UR-LAPC). Poster presented at: ASCO 2015 Annual Meeting [abstract 475]. | ||
Dean AP, Spry N, McGrath A. Nab-paclitaxel plus gemcitabine followed by radiotherapy with concurrent 5-fu in locally advanced unresectable pancreatic cancer: a Western Australian experience. Poster presented at: ASCO 2016 Annual Meeting [abstract 430]. | ||
Peterson S, Loaiza-Bonilla A, Ben-Josef E, et al. Neoadjuvant nab-paclitaxel and gemcitabine (AG) in borderline resectable (BR) or unresectable (UR) locally advanced pancreatic adenocarcinoma (LAPC) in patients ineligible for FOLFIRINOX. Poster presented at: ASCO 2016 Annual Meeting [abstract 328]. | ||
Van Laethem J, Bali MA, Borbath I, et al. Preoperative gemcitabine-nab-paclitaxel (G-NP) for (borderline) resectable (BLR) or locally advanced (LA) pancreatic ductal adenocarcinoma (PDAC): feasibility results and early response monitoring by diffusion-weighted (DW) MR. Poster presented at: ASCO 2016 Annual Meeting [abstract 4116]. | ||
Sliesoraitis S, Desai NV, Trevino JG, et al. Final results for gemcitabine with nab-paclitaxel in neoadjuvant treatment of resectable pancreatic adenocarcinoma: GAIN-1 study. Poster presented at: ASCO 2014 Annual Meeting [abstract e15201]. | ||
Cohen SJ, O’Neil BH, Berlin J, et al. A phase 1b study of erlotinib in combination with gemcitabine and nab-paclitaxel in patients with previously untreated advanced pancreatic cancer: an Academic Oncology GI Cancer Consortium study. Cancer Chemother Pharmacol. 2016;77(4):693–701. | ||
Ko AH, Truong TG, Kantoff E, et al. A phase I trial of nab-paclitaxel, gemcitabine, and capecitabine for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2012;70(6):875–881. | ||
De Jesus-Acosta A, O’Dwyer PJ, Ramanathan RK, et al. A phase II study of vismodegib, a hedgehog (hh) pathway inhibitor, combined with gemcitabine and nab-paclitaxel (nab-P) in patients (pts) with untreated metastatic pancreatic ductal adenocarcinoma (PDA). Poster presented at: ASCO 2014 Annual Meeting [abstract 257]. | ||
O’Reilly EM, Smith LS, Bendell JC, et al. Final results of phase Ib of anticancer stem cell antibody tarextumab (OMP-59R5, TRXT, anti-notch 2/3) in combination with nab-paclitaxel and gemcitabine (nab-P+Gem) in patients (pts) with untreated metastatic pancreatic cancer (mPC). Poster presented at: ASCO 2015 Annual Meeting [abstract 278]. | ||
Hidalgo M, Cooray P, Carrato A, et al. A phase 1b study of the anti-cancer stem cell agent demcizumab (DEM) and gemcitabine (GEM) +/- nab-paclitaxel in patients with pancreatic cancer. Poster presented at: ASCO 2016 Annual Meeting [abstract 341]. | ||
Hingorani S, Bullock A, Harris W, et al. Final analysis of stage 1 data from a randomized phase 2 study of PEGPH20 plus nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients, utilizing VENTANA companion diagnostic assay. Poster presented at: ESMO World Congress on Gastrointestinal Cancers 2016 [abstract PD-006]. | ||
Hingorani SR, Harris WP, Seery TE, et al. Interim results of a randomized phase II study of PEGPH20 added to nab-paclitaxel/gemcitabine in patients with stage IV previously untreated pancreatic cancer. Poster presented at: ASCO 2016 Annual Meeting [abstract 439]. | ||
Bullock AJ, Hingorani SR, Wu XW, et al. Final analysis of stage 1 data from a randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer (pts), utilizing VENTANA companion diagnostic assay. Poster presented at: ASCO 2016 Annual Meeting [abstract 4104]. | ||
O’Reilly EM, Mahalingam D, Roach JM, et al. Safety, pharmacokinetics, pharmacodynamics, and antitumor activity of necuparanib combined with nab-paclitaxel and gemcitabine in patients with metastatic pancreatic cancer: updated phase 1 results. Poster presented at: ASCO 2016 Annual Meeting [abstract 4117]. | ||
Bhattacharyya GS, Babu KG, Bondarde SA, et al. Effect of coadministered beta blocker and cox-2 inhibitors to patients with pancreatic cancer prior to receiving albumin-bound paclitaxel. Poster presented at: ASCO 2015 Annual Meeting [abstract 302]. | ||
Mahipal A, Springett GM, Burke N, et al. Phase I trial of enzalutamide in combination with gemcitabine and nab-paclitaxel for the treatment of advanced pancreatic cancer. Poster presented at: ASCO 2015 Annual Meeting [abstract 467]. | ||
Reni M, Belli C, Balzano G, et al. Phase IB trial of nab-paclitaxel plus gemcitabine, capecitabine, and cisplatin (PAXG regimen) in patients with stage III pancreatic adenocarcinoma. Poster presented at: ESMO World Congress on Gastrointestinal Cancer 2014 [abstract 711P]. | ||
Sigal D, Marcus SG, Rosen PJ, et al. Association of 2-O, 3-O desulfated heparin (ODSH) plus combination gemcitabine (G)/nab-paclitaxel (A) with preliminary benefit in untreated metastatic pancreatic cancer plus combination gemcitabine (G)/nab-paclitaxel (A) with preliminary benefit in untreated metastatic pancreatic cancer. Poster presented at: ASCO 2013 Annual Meeting [abstract 284]. | ||
Ko AH, Murphy PB, Peyton JD, et al. A randomized, double-blinded, placebo-controlled phase II trial of gemcitabine (gem) plus nab-paclitaxel (nab-P) plus apatorsen (A) or placebo (pl) in patients (pts) with metastatic pancreatic cancer (mPC): the RAINIER trial. Poster presented at: ASCO 2016 Annual Meeting [abstract 4119]. | ||
El-Rayes BF, Shahda S, Starodub A, O’Neil BH, Hanna WT, Oh C. A phase Ib extension study of cancer stemness inhibitor BB608 (napabucasin) in combination with gemcitabine and nab-paclitaxel (nab-PTX) in patients (pts) with metastatic pancreatic cancer. Poster presented at: ASCO 2016 Annual Meeting [abstract 4128]. | ||
Bahary N, Garrido-Laguna I, Wang-Gillam A, et al. Results of the phase Ib portion of a phase I/II trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of metastatic pancreatic cancer. Poster presented at: ASCO 2016 Annual Meeting [abstract 452]. | ||
Borad MJ, Kwak EL, Wang-Gillam A, Ibrahim A, Aldridge J, Olszanski AJ. Evofosfamide combined with gemcitabine/nab-paclitaxel in patients with previously untreated locally advanced or metastatic pancreatic adenocarcinoma (PAC): results of a phase I trial. Poster presented at: ASCO 2016 Annual Meeting [abstract 4114]. | ||
ClinicalTrials.gov. Quality of life study in patients with locally advanced or metastatic pancreatic cancer treated with gemcitabine in combination with nab-paclitaxel (QOLINPAC). Available from: https://clinicaltrials.gov/ct2/show/NCT02106884?term=nab-paclitaxel+AND+pancreatic+AND+adenocarcinoma&rank=54. Accessed August 1, 2016. | ||
ClinicalTrials.gov. First-line treatment of metastatic pancreatic cancer with nab-paclitaxel and gemcitabine (ALPACA). Available from: https://clinicaltrials.gov/ct2/show/NCT02564146?term=NCT02564146&rank=1. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Gemcitabine and nab-paclitaxel combined with momelotinib in participants with previously untreated metastatic pancreatic ductal adenocarcinoma. Available from: https://clinicaltrials.gov/ct2/show/NCT02101021. Accessed August 1, 2016. | ||
ClinicalTrials.gov. A study of PEGylated recombinant human hyaluronidase in combination with nab-paclitaxel plus gemcitabine compared with placebo plus nab-paclitaxel and gemcitabine in participants with hyaluronan-high stage IV previously untreated pancreatic ductal adenocarcinoma. Available from: https://clinicaltrials.gov/ct2/show/NCT02715804. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Study of nanoliposomal irinotecan (nal-IRI)-containing regimens in patients with previously untreated, metastatic pancreatic adenocarcinoma. Available from: https://clinicaltrials.gov/ct2/show/NCT02551991. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Sequential treatment of nab-paclitaxel + gemcitabine / FOLFIRI.3 vs nab-paclitaxel + gemcitabine in 1st line treatment of metastatic pancreatic cancer (FIRGEMAX). Available from: https://clinicaltrials.gov/ct2/show/NCT02827201. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Phase I/II study of nab-paclitaxel and gemcitabine followed by AG-mFOLFOX in patients with metastatic pancreatic adenocarcinoma (SEQUENCE). Available from: https://www.clinicaltrials.gov/ct2/show/NCT02504333. Accessed August 1, 2016. | ||
ClinicalTrials.gov. A phase I-II study of PAXG in stage III-IV pancreatic adenocarcinoma (PACT-19). Available from: https://clinicaltrials.gov/ct2/show/NCT01730222?term=nab-paclitaxel+AND+pancreatic+AND+adenocarcinoma&rank=12. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Phase I/II study to evaluate nab-paclitaxel in substitution of CPT11 or oxaliplatin in FOLFIRINOX schedule as first line treatment in metastatic pancreatic cancer (NabucCO). Available from: https://clinicaltrials.gov/ct2/show/NCT02109341. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Paclitaxel albumin-stabilized nanoparticle formulation and gemcitabine hydrochoride with or without WEE1 inhibitor MK-1775 in treating patients with previously untreated pancreatic cancer that is metastatic or cannot be removed by surgery. Available from: https://clinicaltrials.gov/ct2/show/NCT02194829. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Phase 2 nab™-paclitaxel (Aabraxane®) plus gemcitabine in subjects with locally advanced pancreatic lancer (LAPC) (LAPACT). Available from: http://www.clinicaltrials.gov/ct2/show/NCT02301143. Accessed August 1, 2016. | ||
ClinicalTrials.gov. S1505: combination chemotherapy or gemcitabine hydrochloride and paclitaxel albumin-stabilized nanoparticle formulation before surgery in treating patients with pancreatic cancer that can be removed by surgery. 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02562716. Accessed September 15, 2016. | ||
ClinicalTrials.gov. Pancreas cancer: molecular profiling as a guide to therapy before and after surgery (“personalized medicine”). Avalable from: https://clinicaltrials.gov/ct2/show/NCT02562716. Accessed August 1, 2016. | ||
ClinicalTrials.gov. Systemic therapy and chemoradiation in advanced localised pancreatic cancer-2 (SCALOP-2). Available from: https://clinicaltrials.gov/ct2/show/NCT02024009. Accessed August 1, 2016. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.