Back to Journals » Vascular Health and Risk Management » Volume 10
N-terminal fragment of probrain natriuretic peptide is associated with diabetes microvascular complications in type 2 diabetes
Authors Hamano K, Nakadaira I, Suzuki J, Gonai M
Received 14 May 2014
Accepted for publication 4 August 2014
Published 3 October 2014 Volume 2014:10 Pages 585—589
DOI https://doi.org/10.2147/VHRM.S67753
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
Kumiko Hamano, Ikue Nakadaira, Jun Suzuki, Megumi Gonai
Department of Diabetes and Endocrinology, Kanto Rosai Hospital, Kawasaki, Japan
Aim/introduction: Circulating levels of N-terminal fragment of probrain natriuretic peptide (NT-proBNP) are established as a risk factor for cardiovascular disease and mortality in patients with diabetes, as well as in the general population. We sought to examine the possibility of NT-proBNP as a biomarker of microvascular complications in patients with type 2 diabetes.
Materials and methods: In total, 277 outpatients with type 2 diabetes were consecutively enrolled as a hospital cohort. Two hundred and seventeen of these patients (132 males; mean age, 63.4 years) were designated as cases with any of the diabetic complications (retinopathy, neuropathy, nephropathy, ischemic heart disease, strokes, peripheral artery disease), and 60 (42 males; mean age, 54.1 years) were set as controls without clinical evidence of diabetic complications. Diabetic complications were evaluated by medical record and routine laboratory examinations. NT-proBNP was measured and investigated with regard to the associations with diabetic complications.
Results: Mean NT-proBNP levels were significantly higher in patients with any of the diabetic complications (59 versus 33 pg/mL; P<0.0001). In logistic regression analysis, NT-proBNP levels >79 pg/mL, which was the highest tertile, were independently associated with a 5.04 fold increased risk of all complications (P<0.0051) compared to the lowest tertile (NT-proBNP levels <31 pg/mL). Odd ratios of cardiovascular disease and nephropathy, neuropathy, and retinopathy were 9.33, 6.23, 6.6 and 13.78 respectively, in patients with NT-proBNP values in the highest tertile (>79 pg/mL), independently of age, sex, duration of diabetes or other risk factors, such as body mass index or hemoglobin A1c. In addition, NT-proBNP levels were associated with surrogate markers of atherosclerosis, such as brachial-ankle pulse wave velocity (r=0.449, P<0.0001) and left ventricular hypertrophy (r=0.212, P<0.001).
Conclusion: In this hospital-based cohort of type 2 diabetes, the NT-proBNP levels were associated with systemic atherosclerosis and comorbid diabetic microvascular as well as macrovascular complications. It is useful to stratify high-risk diabetic patients by measuring NT-proBNP and to start comprehensive care for preventing the progression of diabetic complications. It is necessary to elucidate the underlying mechanism for the progression of diabetic complications represented by an elevation of NT-proBNP and to demonstrate the ability of NT-proBNP as a predictive global biomarker for diabetic complications in Japanese type 2 diabetic patients.
Keywords: NT-proBNP, diabetic complication, biomarker
A Corrigendum for this paper has been published
Introduction
The N-terminal fragment of probrain natriuretic peptide (NT-proBNP) is postulated as a diagnostic and prognostic biomarker of heart failure and cardiovascular mortality in type 2 diabetes.1,2 It has been reported that patients with type 2 diabetes had increased levels of NT-proBNP even though they had no overt cardiovascular disease (CVD).3 The mechanism for the elevation of BNP was not fully elucidated. We recently reported that NT-proBNP could be a marker of silent myocardial ischemia in type 2 diabetes.4
Diabetes mellitus retinopathy (DMR) is a common chronic microvascular complication. DMR has been associated with increased all-cause and CVD mortality risk in both type 2 and type 1 diabetes.5 Considering these data, identification of DMR could possibly add to the diabetic patient’s CVD risk stratification.6 Also, there are several reports – including ours7 – that indicate the interrelationships of diabetic microangiopathy with macroangiopathy.8 Microalbuminuria reflects a generalized disturbance of microvascular function related to the endothelium-dependent mechanism. Thus, microalbuminuria is established as not only a marker for the risk of retinopathy, nephropathy, and neuropathy but also as a predictor for CVD.9 Recently, NT-proBNP was reported to be associated with diabetic complications in a large cohort of type 1 diabetes.10 It was proposed that NT-proBNP is a novel vascular risk factor reflecting systemic inflammation.
The aim of the present study was to test whether NT-proBNP levels were associated with diabetic microvascular complications in Japanese type 2 diabetes.
Materials and methods
In total, 277 type 2 diabetes patients in outpatient settings were consecutively enrolled as a hospital-based cohort and a cross-sectional case-controlled study was designed. Two hundred and seventeen of these patients (132 males) were designated as cases with diabetic complications and 60 (42 males) were controls without apparent diabetic complications. Diagnosis of type 2 diabetes was performed according to Japan Diabetes Society criteria. Patients with heart failure (New York Heart Association Functional Classification > II) and on hemodialysis were excluded, since levels of NT-proBNP are strongly influenced. Also, patients with acute illness, malignancy, or pregnancy were excluded.
The examination included full medical histories, physical examinations, and blood samples. All patients underwent ophthalmologic examinations and were graded as background or proliferative retinopathy. Albumin excretion rate (AER), measured on two serial 24-hour urine collections, was categorized as normoalbuminuria (<30 μg/min), microalbuminuria (30–300 μg/min), and macroalbuminuria (>300 μg/min). Estimated glomerular filtration rate (eGFR) was calculated by the equation from the Modification of Diet in Renal Disease study for Japanese using the four-component abbreviated equation:
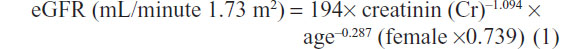
Distal symmetrical polyneuropathy was diagnosed by the absence of ankle reflexes or abnormal vibration perception threshold by a 128 Hz tuning fork on the big toe and the loss of touch sensation by a 10 g Monotouch filament on the sole of the foot. CVD was defined as a composite of a history of myocardial infarction, angina, coronary artery bypass graft, or procedures of angioplasty, ischemic strokes, or transient ischemic attacks. Peripheral artery disease (PAD) was defined by a history of intermittent claudication, vascular intervention, or amputation concomitant with a decreased ankle brachial index <0.9.
In the present study, diabetic complications were defined as DMR, nephropathy, neuropathy, CVD, and PAD. Left ventricular hypertrophy (LVH) was defined by the electrocardiogram criteria (S V1 + R V5 or V6 >35 mm). Brachioankle pulse wave velocity (baPWV) was measured by form ABI/PWV (Omron Colin, Komaki, Japan). Serum NT-proBNP levels were measured by a two-site sandwich electrochemiluminescence immunoassay (ECLusys proBNP; Hoffman-La Roche Ltd, Basel, Switzerland).
The Institutional Review Board of Kanto Rosai Hospital approved the study protocol, and all the participants gave written informed consent. Variables distributed normally are presented as means (standard deviation), while variables with skewed distribution were analyzed after logarithmic transformation (NT-proBNP; AER). Variables were compared by a Student’s t-test or one-way analysis of variance. Pearson correlation analyses were performed to analyze the associations between NT-proBNP and other numerical variables. To assess the pattern of odds ratios (ORs) across increasing NT-proBNP values, the NT-proBNP values were categorized by the tertile distribution.
Logistic regression analysis was used separately to estimate the ORs of NT-proBNP for any complication, and 95% confidence interval (CI) was given. Adjustment confounding variables were age, sex, duration of diabetes, body mass index (BMI), and hemoglobin A1c (HbA1c). A P-value of <0.05 was considered to be statistically significant. All reported P-values are two-sided. All analysis was performed using JMP version 9.0.0 (SAS Institute Inc., Cary, NC, USA).
Results
The characteristics of subjects are shown in Table 1. The NT-proBNP levels were negatively associated with eGFR and positively with age (data not shown) in accordance with previous reports. Levels of NT-proBNP were also correlated with electrocardiogram LVH (r=0.212; P<0.001) and baPWV (r=0.449; P<0.0001).
The NT-proBNP values were significantly higher in cases with any of the complications (DMR, DSN [distal sensory neuropathy], nephropathy, CVD, and PAD) than controls (59 versus 33 pg/mL; P<0.0001). The effect size for NT-proBNP was 0.26. Of the 217 cases, DMR was present in 74 (background, 83.7%; proliferative, 16.3%). Nephropathy was present in 97 (microalbuminuria, 78.4%; macroalbuminuria, 21.6%) and DSN in 97 cases. CVD was present in 88 (ischemic heart disease, 63.6%; strokes, 36.4%) and 28 cases had PAD.
In logistic regression analysis adjusted for age, sex, duration of diabetes, BMI, and HbA1c, those in the higher tertiles (>79 pg/mL) had significantly higher ORs for all complications as well as for each complication examined separately compared to subjects in the lower tertile (<31 pg/mL) (Table 2). It is noteworthy that OR for DMR is prominent as 13.78 (95% CI 3.34–70.75) in the highest tertile of NT-proBNP, compared to that in the lowest tertile. Many of the subjects had more than one complication, and, as a consequence, the number of complications increased progressively through the tertiles of NT-proBNP. This tendency was observed even after adjustment for age, sex and duration of diabetes.
Discussion
In the present study, we showed that NT-proBNP elevation was associated with diabetic microvascular complications. We also demonstrated that NT-proBNP was correlated with surrogate markers of cardiac and vascular structural change, namely LVH or vascular stiffness (baPWV). It may be argued that increased NT-proBNP in patients with diabetes might be explained by minimally reduced glomerular filtration. It was shown that increased BNP has been found in patients with impaired renal function than healthy controls, and yet urinary NT-proBNP was positively correlated with increased plasma NT-proBNP.11,12
These inverse correlations between renal function and urinary NT-proBNP indicate that renal retention is not the only reason for increased NT-proBNP, suggesting an increased release of BNP and NT-proBNP from cardiac myocytes. In fact, in a previous paper, we have shown a good correlation of NT-proBNP and cardiac parameters.4 Recently, subclinical abnormalities in cardiac structure have been associated with longitudinal kidney function decline indicating a close relationship of two organs, ie, cardiorenal continuum.13
The novel point of the present results is that subjects with higher NT-proBNP had diabetic complications, such as DMR, DSN, and PAD, the target organs not directly related to kidney or heart. Similar results have just been shown in a large cohort of type 1 diabetes.7 It is conceivable that the longer the duration of diabetes, the worse the kidney function, and advanced age – all together – contribute to the elevation of NT-proBNP. However, in the present study, elevation of NT-proBNP was associated with DSN or DMR independent of disease duration in addition to kidney function or age. Data regarding the association between DSN and NT-proBNP levels were scarce and repetitive microhypoxic insults to nerve fibers’ arterial supply was suggested by Jurado et al in a group of type 2 diabetic patients.14
Moreover, subjects with strokes (data not shown) or PAD had higher NT-proBNP even with normal kidney and cardiac status. A similar finding has been recently reported in a Japanese large scale general population cohort study.15
A variety of factors other than myocardial stretch have been shown to stimulate secretion of BNP, such as myocardial ischemia, endocrine and paracrine factors such as endothelin, angiotensin II, and tumor necrosis factor α (TNFα). In the most recent report in type 1 diabetes, TNFα was postulated as a key molecule for the elevation of BNP and was shown to play an important role in the development of diabetic complications.10
Unfortunately, in the present study, the TNFα or other cytokines were not measured. Another possibility is that cardiac biomarkers may represent systemic vascular inflammation or oxidative stress that may have impact on disease progression in systemic vasculature.
There are several limitations in the present study. First, the conclusions may be limited, due to the small number of the subjects. Second, the diagnostic criteria are based on clinical practice, and we might have underestimated the prevalence of complications, such as silent myocardial ischemia. Third, in the present study, nonclassical diabetic complications, such as periodontal disease, cognitive function, or depression, were not evaluated.
In the context of clinical practice under limited resources, aggressive and expensive imaging procedures are not necessary for all diabetic patients; instead, a reliable and simple biomarker focusing on classical complications is helpful to identify individuals at high risk.
The major point of our findings is that NT-proBNP might be a universal biomarker for detecting diabetic vascular complications. It is suggested that the complex interplay of heart, kidney, and other vasculature may happen very early in diabetes.8 It is necessary to explore the causal relationships between BNP and the systemic vasculatures in diabetes.
In conclusion, the stable and reproducible assay of NT-proBNP might be widely used in clinical settings, and it is possible to stratify high risk subjects among diabetics and implement intensive interventions.
Acknowledgment
Parts of this study were presented at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, USA, June 21–25, 2013.
Disclosure
The authors report no conflicts of interest in this work.
References
Gaede P, Hildebrandt P, Hess G, Parving HH, Pedersen O. Plasma N-terminal pro-brain natriuretic peptide as a major risk marker for cardiovascular disease in patients with type 2 diabetes and microalbuminuria. Diabetologia. 2005;48(1):156–163. | |
Tarnow L, Gall MA, Hansen BV, Hovind P, Parving HH. Plasma N-terminal pro-B-type natriuretic peptide and mortality in type 2 diabetes. Diabetologia. 2006;49(10):2256–2262. | |
Magnusson M, Melander O, Israelsson B, Grubb A, Groop L, Jovinge S. Elevated plasma levels of Nt-proBNP in patients with type 2 diabetes without overt cardiovascular disease. Diabetes Care. 2004;27(8):1929–1935. | |
Hamano K, Abe M, Komi R, Kobayashi S. N-terminal fragment of pro-brain natriuretic peptide (NT-proBNP) for predicting silent myocardial ischaemia in type 2 diabetes mellitus independent of microalbuminuria. Diabetes Metab Res Rev. 2010;26(7):534–539. | |
van Hecke MV, Dekker JM, Stehouwer CD, et al; EURODIAB prospective complications study. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care. 2005;28(6):1383–1389. | |
Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care. 2007;30(2):292–299. | |
Hamano K, Nitta A, Ohtake T, Kobayashi S. Associations of renal vascular resistance with albuminuria and other macroangiopathy in type 2 diabetic patients. Diabetes Care. 2008;31(9):1853–1857. | |
Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53(Suppl 5):S35–S42. | |
Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. | |
Gruden G, Barutta F, Chaturvedi N, et al. NH2-terminal probrain natriuretic peptide is associated with diabetes complications in the EURODIAB Prospective Complications Study: the role of tumor necrosis factor-α. Diabetes Care. 2012;35(9):1931–1936. | |
Ng LL, Geeranavar S, Jennings SC, Loke I, O’Brien RJ. Diagnosis of heart failure using urinary natriuretic peptides. Clin Sci (Lond). 2004;106(2):129–133. | |
Totsune K, Takahashi K, Satoh F, et al. Urinary immunoreactive brain natriuretic peptide in patients with renal disease. Regul Pept. 1996;63(2–3):141–147. | |
Park M, Shlipak MG, Katz R, et al. Subclinical cardiac abnormalities and kidney function decline: the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol. 2012;7(7):1137–1144. | |
Jurado J, Ybarra J, Ferrandiz M, Comerma L, Pou JM. Amino-terminal brain natriuretic peptide is related to the presence of diabetic polyneuropathy independently of cardiovascular disease. Diabetes Care. 2007;30(8):e86. | |
Doi Y, Ninomiya T, Hata J, et al. N-terminal pro-brain natriuretic peptide and risk of cardiovascular events in a Japanese community: the Hisayama study. Arterioscler Thromb Vasc Biol. 2011;31(12):2997–3003. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



