Back to Journals » Drug Design, Development and Therapy » Volume 9
n-Butanol extract from Folium isatidis inhibits lipopolysaccharide-induced inflammatory cytokine production in macrophages and protects mice against lipopolysaccharide-induced endotoxic shock
Authors Jiang L, Lu Y, Jin J, Dong L, Xu F, Chen S, Wang Z, Liang G, Shan X
Received 5 June 2015
Accepted for publication 3 August 2015
Published 12 October 2015 Volume 2015:9 Pages 5601—5609
DOI https://doi.org/10.2147/DDDT.S89924
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Wei Duan
Lili Jiang,1 Yili Lu,1 Jiahui Jin,1 Lili Dong,1 Fengli Xu,1 Shuangshuang Chen,1 Zhanyue Wang,2 Guang Liang,2 Xiaoou Shan1
1Department of Pediatrics, The Second Affiliated Hospital, 2Chemical Biology Research Center at The School of Pharmacy, Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
Abstract: Sepsis, which is caused by severe infection, is an important cause of mortality, but effective clinical treatment against sepsis is extremely limited. As the main component of the outer membrane of Gram-negative bacteria, lipopolysaccharide (LPS) plays a major role in inflammatory responses. Studies have shown beneficial pharmacological effects for Folium isatidis. The present study further illuminated the effects of n-butanol extract from Folium isatidis in LPS-induced septic shock and identified the main active chemical components. Our study showed that pretreatment with n-butanol extract from Folium isatidis not only significantly inhibited LPS-induced tumor necrosis factor-α and interleukin-6 production but also markedly and dose dependently enhanced the recruitment of MyD88, the phosphorylation of extracellular signal-regulated kinase, and the degradation of IκB-α. Additionally, the extract exhibited dramatic protective effects against lung injury and death in mice with septic shock. Eight main active compounds were identified, including organic acids, glycoside, indolinones, and flavonoids. These findings provide a perspective on the respiratory protection offered by n-butanol extract from Folium isatidis in LPS-induced sepsis and outline a novel therapeutic strategy for the treatment of sepsis.
Keywords: Folium isatidis, sepsis, inflammatory cytokine
Introduction
Sepsis is defined as a systemic inflammatory response syndrome occurring in response to an infectious process, according to the American College of Chest Physicians and the Society of Critical Care Medicine.1 Under normal conditions, the innate immune system protects the host from a wide variety of invading microbial pathogens, and an uncontrolled anti-inflammatory response is associated with poorer outcomes.2 As a result of excessive production of various pro-inflammatory cytokines and cellular injury, which lead to multiple organ dysfunction, septic shock is still the leading cause of mortality in critically ill patients, despite progress in modern medical science.3–6 For a variety of reasons, renewed interest has been focused on studying and evaluating naturally existing products to treat septic shock.7
Folium isatidis, consisting of the dry leaves of Isatis indigotica, known as “Da-qing-ye” in Chinese, has been linked to the concepts of heat clearing and detoxification since ancient times. In addition to curing febrile diseases, such as fever, dipsosis, hematemesis, and jaundice, among others, Folium isatidis is clinically used as an antiviral medicine to treat diseases, such as influenza, viral hepatitis, mumps, and epidemic encephalitis B.8–10 It was also shown to be effective during the 2003 SARS epidemic11 and the 2009 H1N1 flu outbreak.12 Recently, Folium isatidis received attention because it exhibits anti-endotoxic and antibacterial activity, with the potential to be developed into a natural antibiotic.13,14 Specifically, Folium isatidis has been shown to have beneficial immunomodulatory effects, preventing infections.14 However, although substantial evidence now exists to support beneficial pharmacological action for Folium isatidis, the pharmacological mechanism is still unknown due to the co-existence of multiple bioactive components and limited approaches for preparing extracts from Folium isatidis.
In the present study, we investigated the effect of n-butanol extract from Folium isatidis in sepsis and identified the main chemical components and structures of Folium isatidis using high-performance liquid chromatography. This analysis was coupled with the tandem mass spectrometry (MS/MS) technique, laying a solid basis for further research. We focused on lipopolysaccharide (LPS, also known as endotoxin), which is a key molecule present in the outer membrane of Gram-negative bacteria, as a model to identify a possible therapeutic role for n-butanol extract from Folium isatidis in the management of sepsis.
Materials and methods
Plant material and reagents
Folium isatidis approved by the National Institutes for Food and Drug Control was bought from Shanghai Jingke Chemical Technology Co. Ltd, and Professor Xiaoxia Ye (College of Pharmacy, Wenzhou Medical University) also authenticated the leaves. LPS was obtained from Sigma (St Louis, MO, USA). Antibodies against MyD88, p-p38, p38, p-c-jun N-terminal kinase (JNK), JNK, p-extracellular signal-regulated kinase (ERK), ERK, IκB-α, and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) enzyme-linked immunosorbent assay (ELISA) kits were obtained from eBioscience (San Diego, CA, USA).
Extraction and isolation
An extract was obtained using the maceration method (Figure 1). Dry leaves (10 kg) were powdered and immersed in 30 L of 95% ethanol at room temperature for 1 week and then filtered. The residue was macerated for another week. Extract residues were then combined and centrifuged to dryness under a rotary evaporator at 59°C, resulting in a brown residue (1,053 g). Part of the extract (350 g) was resuspended in 150 mL of water (7:3; v/v). Once completely dispersed, the homogenized sample was successively loaded into petroleum ether, ethyl acetate, and n-butanol according to the polarity of the solvent, and every solvent was extracted three times. The n-butanol fraction was collected and subsequently dried using a rotary evaporator at 45°C. Finally, the residue was dissolved to an appropriate concentration for pharmacodynamics study and liquid chromatography (LC)–MS/MS injection.
Animals
Male C57BL/6 mice (n=10 per group) weighing 18–22 g were obtained from the Animal Center of Wenzhou Medical University (Wenzhou, People’s Republic of China). All animals were housed at a constant room temperature with a 12:12-hour light–dark cycle and fed with a standard rodent diet and water. The animals were acclimatized to the laboratory for 7 days before being used. The animals were randomly assigned to the vehicle control, LPS-induced sepsis, or treatment group. Protocols involving the use of animals were approved by the Wenzhou Medical University Animal Policy and Welfare Committee (Approval Document No: wydw2014-0001).
Preparation of cells
Mouse peritoneal macrophages (MPMs) were isolated from the male C57BL/6 mice after 3 days using a 1 mL thioglycollate solution (Solabio, Beijing, People’s Republic of China) induction. Total MPMs were harvested by washing the peritoneal cavity with PBS containing 30 mM EDTA (8 mL per mouse), followed by centrifugation and resuspension in RPMI-1640 medium (Gibco/BRL Life Technologies, Eggenstein, Germany) containing 10% FBS (HyClone, Logan, UT, USA), 100 U/mL penicillin and 100 mg/mL streptomycin. Nonadherent cells were removed by washing with medium 4 hours after seeding. Before treatment, the MPMs were cultured in 35-mm plates and incubated overnight at 37°C in 5% CO2, humidified air.
Cytokine quantification
Cells were treated with LPS for 22 hours in the presence or absence of compounds and culture media, which were then collected. The TNF-α and IL-6 levels in the media as well as in serum were determined using ELISA kits (eBioscience) according to the manufacturer’s instructions.
Western blotting
Cells were lysed with lysis buffer, vibrated for 10 seconds and then centrifuged at 12,000 g for 10 minutes at 4°C. The supernatants were collected for 50 μg of the cell extract, subjected to SDS-PAGE and transferred onto nitrocellulose membranes. Each membrane was blocked with 5% nonfat milk. The blots were probed with the specific antibodies. After extensive washing, the blots were incubated with a horseradish peroxidase-conjugated secondary antibody and visualized using an enhanced chemiluminescence reagent (Bio-Rad, Hercules, CA, USA).
LPS-induced septic shock
Regarding the animal experiments, n-butanol extract from Folium isatidis was dissolved in 0.5% carboxymethyl cellulose sodium solution, which served as a vehicle for analysis. Male C57BL/6 mice weighing 18–22 g were orally pretreated with the n-butanol extract solution (5 g/kg/day) for 7 days before receiving an injection of LPS (20 or 10 mg/kg) into the caudal vein. Control animals received a similar volume (200 μL) of the vehicle. For septic survival analysis (LPS at 20 mg/kg), mortality was recorded every 12 hours for 7 days. For septic shock response evaluation (LPS at 10 mg/kg), mice were euthanized under ether anesthesia 4 hours after LPS injection, and then their lung tissues were embedded in paraffin for histopathological examination (n=10 in each group).
LC–MS/MS analysis
The Eksigent Nano Ultra 2D LC was used for compound separation and was coupled to an LTQ Orbitrap XL mass spectrometer for analyte detection and identification. Prepared samples were separated on a Waters BEH Shield RP18 Column (1.7 μm, 2.1 mm ×50 mm) maintained at 36°C. The mobile phase consisted of 0.1% formic acid containing 5 mM ammonium acetate (A) and methanol (B). The effluent was delivered at 0.25 mL/min, and the injection volume was 10 μL. Survey scans were acquired in the Orbitrap with simultaneous ESI (+) and ESI (-) ionization modes. Analytes were quantified using the multiple-reaction monitoring mode. The spray voltage was set to 4.5 kV, and the resolving power at 400 m/z was set to 30,000, with a scan range of 50–2,000.
Data analysis
All experiments were carried out in triplicate. The results are given as the mean ± SEM. Data analyses were performed using one-way analysis of variance with Tukey’s test. Comparisons were deemed significant at P<0.05.
Results
Inhibition of LPS-induced inflammatory response in macrophages
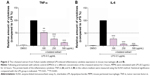
To examine whether the extract could affect the inflammatory response induced by LPS, the production of the pro-inflammatory cytokines TNF-α and IL-6 in the presence or absence of the extract was measured using ELISA. As shown in Figure 2, LPS-treated MPMs showed a significant increase in the secretion of TNF-α and IL-6 in the culture media after LPS stimulation, whereas the levels of TNF-α and IL-6 were significantly inhibited by the n-butanol extract from Folium isatidis compared with the levels in the LPS group (P<0.01). Furthermore, improved anti-inflammatory function could be achieved by increasing the drug concentration, indicating that the n-butanol extract from Folium isatidis possesses therapeutic effects against LPS-induced inflammatory responses.
Inhibition of LPS-induced IκB-α degradation and ERK phosphorylation
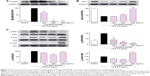
LPS is known to activate the toll-like receptor-4 (TLR-4)/MyD88 signaling pathway; thus, the effect of the n-butanol extract from Folium isatidis on LPS-induced MyD88 recruitment was evaluated. The results (Figure 3A) showed that pretreatment with the extract significantly reduced LPS-induced MyD88 recruitment. NF-κB has been shown to be a pivotal factor for the activation of pro-inflammatory molecules, and the degradation of IκB-α constitutes the first step in NF-κB activation. To investigate whether the effect of the n-butanol extract from Folium isatidis is attributable to suppression of this pathway, we evaluated the total IκB-α in total cell protein extracts. As illustrated in Figure 3B, LPS exposure for 1 hour resulted in a rapid loss of IκB-α in MPMs. Conversely, increased IκB-α was observed in cells pretreated with the n-butanol extract from Folium isatidis.
In addition to the NF-κB pathway, the mitogen-activated protein kinase (MAPK) pathway is another signal cascade that upregulates inflammatory cytokines. Therefore, we tested whether the extract could inhibit LPS-induced NF-κB and MAPK activation. The MAPK family consists of ERK, p38, and JNK, so we analyzed LPS-induced phosphorylation of all of these using western blotting. Figure 3C shows that all three proteins were activated by LPS stimulation. Pretreatment with the extract significantly decreased the LPS-induced phosphorylation of ERK in a dose-dependent manner, whereas it had no effect on the phosphorylation of p38 or JNK.
Protection against LPS-induced septic shock
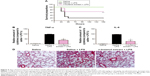
The n-butanol extract obtained from Folium isatidis exhibited significant effects in vitro, thus prompting us to investigate the effect of the extract on LPS-induced endotoxic shock in vivo. C57BL/6 mice were treated with the extract at a dosage of 5 g/kg/day for 7 consecutive days. All mice were then challenged with LPS (20 mg/kg, intravenous), and survival rates were monitored for 7 days. We compared the survival rates between the extract-treated and the LPS control mice. The extract significantly improved the survival rate of mice with LPS-induced sepsis (Figure 4A).
Sepsis can be thought of as falling within a continuum from infection to multiple organ failure syndrome.4 Acute lung injury is a significant change that occurs in animals treated with LPS and is characterized by edema, congestion, hemorrhage, and neutrophil and mononuclear cell accumulation.15 Whether pretreated with the n-butanol extract from Folium isatidis or not, mice were administered LPS (10 mg/kg, intravenous), and 4 hours later, serum was collected, and the lungs were dissociated and stained by H&E. The extract pretreatment at 5 g/kg/day significantly decreased the in vivo concentrations of TNF-α and IL-6 (Figure 4B and C) and abrogated the development of acute injury in the lungs (Figure 4D).
Identification of the main active ingredients and structures of the n-butanol extract
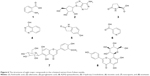
Extraction of the n-butanol fraction was successfully partitioned from air-dried and pulverized Folium isatidis by successively using petroleum ether, ethyl acetate, and n-butanol. By selecting the optimal experimental conditions for LC–MS/MS, the major active ingredients of the n-butanol extract obtained from Folium isatidis were well separated and effectively analyzed. Figure 5 shows the chromatograms of the n-butanol extract from Folium isatidis. Eight main active ingredients and structures were identified in the n-butanol extract by comparing the tandem mass spectral data and major fragment ions with those described in the related literature, as shown in Table 1 and Figure 6. Among the eight investigated constituents, compounds A, C, and F are organic acids; compound B is a glycoside; compounds D and E are indolinones; and compounds G and H are flavonoids. Additionally, the major active ingredients of the n-butanol extract obtained from Folium isatidis were well separated and effectively analyzed, but the compounds that dominate as anti-inflammatory agents and whether they have orchestrated effects remain to be further evaluated.
Discussion
Excessive production of TNF-α and IL-6 is a symbolic pathological change in acute inflammation that is also involved in systemic inflammation, and neutralization of TNF-α can improve survival in several types of preclinical models of sepsis.16,17 Several mediator-specific agents targeting pro-inflammatory cytokines have been proposed and studied in patients with sepsis. In the present study, we demonstrated that n-butanol extract from Folium isatidis significantly downregulated LPS-induced secretion of TNF-α and IL-6 in mouse macrophages.
It has long been recognized that the LPS-cell interaction initiated by the recognition of LPS-binding protein18,19 and CD14 facilitates the transfer of LPS–LPS-binding protein complexes to TLR-4.19 Once LPS binds to TLR-4, the TLR-4-mediated inflammatory cytokine cascade is activated. The signaling process involves MyD88-dependent and TRIF-dependent pathways. The MyD88-dependent pathway induces the early activation of NF-κB and AP1 for the induction of inflammatory cytokines via multiple signaling molecules, such as NF-κB and MAPKs.20 Our results indicate that the beneficial effect of n-butanol extract from Folium isatidis is consistent with marked inhibition of MyD88 recruitment, a decrease in NF-κB activation and blockade of ERK activation. Moreover, daily oral administration of n-butanol extract from Folium isatidis improved the survival rate of mice with LPS-induced septic shock.
A recent worldwide survey of relevant pathogens in intensive care units showed that among patients with sepsis who had positive blood cultures, 62% carried Gram-negative bacteria, 47% Gram-positive bacteria, and 19% fungi.21 LPS is the major component of the outer membrane of Gram-negative bacteria, greatly contributing to the production of inflammatory cytokines in many cell types. The crucial importance of LPS to Gram-negative bacteria makes this molecule a good candidate target for the development of new anti-inflammatory agents. It has been reported that humans are much more sensitive to LPS than other animals (eg, mice). For example, a dose of 1 μg/kg induces shock in humans, whereas mice will tolerate a dose up to a 1,000 times higher.22 The diversity of pathogens that cause infection and differences among species may explain why basic research has not supported clinical trials.
Folium isatidis is widely used in the clinic, with more than 70 types of preparations employed, and it has great clinical effects. In recent years, worldwide, researchers have successfully isolated nearly 30 types of compounds from Folium isatidis, such as alkaloids, organic acids, glycosides, and sterols.23–28 We have also extracted and identified some of these in the n-butanol extract studied here. Indigo and indirubin are the major compounds in Folium isatidis by qualitative and quantitative evaluation, according to Chinese pharmacopoeia. Nevertheless, differences in chemical compositions in categories or contents neither are conducive to standardized production of Chinese herbal medicine nor support research on therapeutic effects. Therefore, an index of quality control should be set up for each compound and both qualitatively and quantitatively characterized. As to the mechanism, in the current study, certain chemicals in the extract may have directly degraded or become inactivated, may have stimulated the immune system to promote an immunological response against endotoxin invasion, may have scavenged for free oxygen radicals, or perhaps exhibited the synergistic effect of several mechanisms. Due to the complexity of Chinese medicine itself and technical limitations, certain existing active practices in Chinese traditional medicine are likely to degrade or disappear during the process of separation. Additionally, the pharmacological actions of the monomers that we obtain do not represent those of raw materials. Therefore, there is an urgent need for appropriate technology to isolate active monomers, with the purpose of screening and launching studies on the pharmacodynamics of these compounds and further exploring these compounds’ mechanisms.
Conclusion
In summary, we demonstrated that n-butanol extract obtained from Folium isatidis has significant anti-endotoxin activity both in vitro and in vivo, and the main components of the extract were illustrated. The extract showed potential effects as an anti-inflammatory or natural antisepsis agent by inhibiting LPS-stimulated TNF-α and IL-6 production, recruitment of MyD88, phosphorylation of ERK, and degradation of IκB-α, thus protecting mice from LPS-induced septic shock-associated lung injury. The n-butanol extract also improved survival, indicating that the extract is a potential candidate for the treatment of septic shock.
Acknowledgment
This work was supported by the Provincial Natural Science Foundation of Zhejiang (Y2100530).
Disclosure
The authors report no conflicts of interest in this work.
References
Soong J, Soni N. Sepsis: recognition and treatment. Clin Med. 2012;12(3):276–280. | ||
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Eng J Med. 2003;348(2):138–150. | ||
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. | ||
Dellinger RP. Cardiovascular management of septic shock. Crit Care Med. 2003;31(3):946–955. | ||
Linde-Zwirble W, Angus D. Severe sepsis epidemiology: sampling, selection, and society. Critical Care. 2004;8(4):222. | ||
Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003*. Crit Care Med. 2007;35(5):1244–1250. | ||
Wang H, Xu T, Lewin MR. Future possibilities for the treatment of septic shock with herbal components. Am J Emerg Med. 2009;27(1):107–112. | ||
Deng YP, Liu YY, Liu Z, et al. Antiviral activity of Folium isatidis derived extracts in vitro and in vivo. Am J Chin Med. 2013;41(4):957–969. | ||
Boxing W. A review on experimental studies of counteracting hepatitis B virus by chinese herbal drugs [J]. New J Tradit Chin Med. 2001;2:3–5. | ||
Xu T, Zhang L, Sun X, Zhang H, Tang K. Production and analysis of organic acids in hairy-root cultures of Isatis indigotica Fort (indigo woad). Biotechnol Appl Biochem. 2004;39(1):123–128. | ||
Lau T, Leung P, Wong E, et al. Using herbal medicine as a means of prevention experience during the SARS crisis. Am J Chin Med. 2005;33(03):345–356. | ||
Liu Z, Yang ZQ, Xiao H. Antiviral activity of the effective monomers from Folium Isatidis against influenza virus in vivo. Virol Sin. 2010;25(6):445–451. | ||
Liu C-S, Cham T-M, Yang C-H, Chang H-W, Chen C-H, Chuang L-Y. Antibacterial properties of Chinese herbal medicines against nosocomial antibiotic resistant strains of Pseudomonas aeruginosa in Taiwan. Am J Chin Med. 2007;35(06):1047–1060. | ||
Zhang H, Zhang G, Qiao Z. Detection of indigo and indirubin in different part of Radix Isatidis and Folium Isatidis and antiendotoxic action comparison of some ingredients. J Pharm Pract. 2000;18:347. | ||
Spiller F, Orrico MI, Nascimento DC, et al. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am J Respir Crit Care Med. 2010;182(3):360–368. | ||
Lorente JA, Marshall JC. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock. 2005;24:107–119. | ||
Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18(3):335–343. | ||
Schumann RR, Flaggs G, Gray P, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249(4975):1429–1431. | ||
Pugin JM, Schürer-Maly C, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci. 1993;90(7):2744–2748. | ||
Park SH, Kim ND, Jung JK, Lee CK, Han SB, Kim Y. Myeloid differentiation 2 as a therapeutic target of inflammatory disorders. Pharmacology and therapeutics. 2012;133(3):291–298. | ||
Vincent J-L, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. J Am Med Assoc. 2009;302(21):2323–2329. | ||
Warren HS, Fitting C, Hoff E, et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis. 2010;201(2):223–232. | ||
Zhang Q, Hong B, Zheng L, Wang X, Cai D. Matrix solid-phase dispersion extraction followed by HPLC-diode array detection method for the determination of major constituents in a traditional Chinese medicine Folium isatidis (Da-qing-ye). J Sep Sci. 2012;35(18):2453–2459. | ||
Deng X, Gao G, Zheng S, Li F. Qualitative and quantitative analysis of flavonoids in the leaves of i Isatis indigatica Fort by ultra-performance liquid chromatography with PDA and electrospray ionization tandem mass spectrometry detection. J Pharm Biomedi Anal. 2008;48(3):562–567. | ||
LI W, CHEN F-k, Liu X. Chemical constituents of Folium isatidis. Journal of Shenyang Pharmaceutical University. 2005;1:004. | ||
Liu Y, Wu X-y, Fang J-g, Tang J. Studies on chemical constituents from Radix isatidis. Herald of Medicine. 2003;22(9):591–594. | ||
Deng X-Y, Gao G-h, Zheng S-n, LI F-m. Chemical constituents from Folium Isatidis [J]. J Shenyang Pharm Univ. 2009;4:007. | ||
Fang J-g, Peng J, Wang W-q, TANG J, MA Y-g. Study on the bioactive components in Folium Isatidis. by serum pharmacochemistry on rat. Chin J Hosp Pharm. 2008;28(6):434. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.