Back to Journals » Drug Design, Development and Therapy » Volume 12
N-acetylcysteine amide provides neuroprotection via Nrf2-ARE pathway in a mouse model of traumatic brain injury
Authors Zhou Y, Wang HD , Zhou XM, Fang J, Zhu L, Ding K
Received 5 July 2018
Accepted for publication 16 October 2018
Published 4 December 2018 Volume 2018:12 Pages 4117—4127
DOI https://doi.org/10.2147/DDDT.S179227
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Anastasios Lymperopoulos
Yuan Zhou,1 Han-dong Wang,1 Xiao-ming Zhou,2 Jiang Fang,2 Lin Zhu,2 Ke Ding2
1Department of Neurosurgery, Jinling Hospital, Jinling School of Clinical Medicine, Nanjing Medical University, Jiangsu, China; 2Department of Neurosurgery, Jinling Hospital, Jiangsu, China
Background: Increasing evidence demonstrate N-acetylcysteine amide (NACA) provides neuroprotection and attenuated oxidative stress in rats following traumatic brain injury (TBI). The nuclear factor erythroid 2-related factor 2 (Nrf2)–antioxidant response element (ARE) signal pathway is activated after TBI and provides a protective effect against TBI. However, the function and mechanism of NACA in mice after TBI remain unknown. This study was to evaluate the neuroprotection of NACA and the potential action of the Nrf2-ARE pathway in a weight-drop mouse model of TBI.
Materials and methods: Four groups of animals were randomly divided into sham, TBI, TBI+vehicle, and TBI+NACA (100 mg/kg, administered intraperitoneally). The protein levels of Nrf2, heme oxygenase-1 (HO-1), NAD(P)H: quinine oxidoreductase-1 (NQO1), cleaved caspase-3 and the mRNA levels of HO-1 and NQO1 were detected. The neurobehavior, neuronal degeneration, apoptosis and oxidative stress were also assessed.
Results: Treatment with NACA significantly improved neurologic status at days 1 and 3 following TBI. Moreover, NACA promoted Nrf2 activation a day after TBI. The protein and mRNA levels of HO-1 and NQO1 were upregulated by NACA. Meanwhile, NACA treatment significantly reduced the level of malondialdehyde (MDA) and enhanced the activity of superoxide dismutase (SOD) and glutathione peroxidase (GPx), which indicated NACA attenuated oxidative stress following TBI. NACA prominently reduced the protein level of cleaved caspase-3 and TUNEL-positive cells, indicating its antiapoptotic effect. Additionally, Fluoro-Jade C staining showed NACA alleviated neuronal degeneration a day after TBI.
Conclusions: Our study reveals that NACA potentially provides neuroprotection via the activation of the Nrf2-ARE signaling pathway after TBI in mice.
Keywords: N-acetylcysteine amide, traumatic brain injury, nuclear factor erythroid 2-related factor 2, heme oxygenase-1 (HO-1), NAD(P)H: quinine oxidoreductase-1, oxidative stress
Introduction
Traumatic brain injury (TBI) remains a major cause of disability and death in modern society.1,2 The pathological process in TBI includes both primary and secondary brain injury, and the latter is closely related to the prognosis of affected patients. It is generally believed that oxidative stress, activation of the inflammatory response, glutamate excitotoxicity, and loss of ionic homeostasis participate in secondary injury events.3 Within these complex mechanisms, oxidative stress is considered to be a critical factor.2 Oxidative stress after TBI can eventually result in neuronal dysfunction and death by producing excessive ROS and exhausting endogenous antioxidant factors.4 Therefore, most of the efforts to alleviate the secondary injury have focused on the mechanism underlying the oxidative stress.
As a known transcription factor, the nuclear factor erythroid 2-related factor 2 (Nrf2) is important in the reduction of oxidative stress.5 Ordinarily, Nrf2 is present in the plasma and binds to kelch ECH associating protein 1 (KEAP1). However, under oxidative stress, Nrf2 can decouple from KEAP1 and complete the nuclear import.6 Furthermore, cellular nuclear Nrf2 combines with the antioxidant response element (ARE) and activates a batch of endogenous substances, such as heme oxygenase-1 (HO-1), NAD(P)H: quinine oxidoreductase-1 (NQO1), superoxide dismutase (SOD), and glutathione peroxidase (GPx). The redox balance in the internal environment can be regulated by the abovementioned enzymes. The activation of the Nrf2-ARE signal pathway has been demonstrated after TBI and provided a protective effect against TBI.7 Previous studies demonstrated that the Nrf2-ARE pathway played a leading part during the neuroprotection of several drugs after TBI.8–11
N-acetylcysteine (NAC), an FDA-approved drug, is a precursor of glutathione (GSH).12 GSH has antioxidant effects, and TBI is often accompanied by depletion of GSH.13,14 Increasing evidence suggests that NAC can confer neuroprotection after TBI.12,15 However, owing to the very low bioavailability of NAC, the modified compound N-acetylcysteine amide (NACA) has been developed and is more membrane permeable than NAC.16 NACA treatment has been proved to maintain the integrity of mitochondrial glutathione and provide neuroprotection following moderate unilateral controlled cortical impact (CCI) TBI in rats.17 Moreover, NACA prevented brain tissue damage after focal penetrating TBI in rats.18 In a blast-induced TBI rat model, NACA treatment before injury protected against an increase in intracranial pressure.19 These clues demonstrated that NACA improved neurofunctional outcomes and attenuated oxidative stress after TBI in rats.20 However, we still do not understand the function and mechanism of NACA after TBI in mice. Using a mouse model of TBI, our study explored the neuroprotection of NACA and the potential action of the Nrf2-ARE pathway.
Materials and methods
Ethical approval
All procedures were approved by the Animal Care and Use Committee of Nanjing Medical University (SYXK2012-0047) and were conducted in accordance with the Animals Research Reporting of In vivo Experiments guidelines.
Animals
We purchased the Male Imprinting Control Region (ICR) mice (weight 28–32 g) from the Experimental Animal Center of Jinling Hospital in China. Animals ate food and water freely and were given a daily schedule of dark–light cycle. Before tests, they were housed for at least 2 days.
Models of TBI
A previously described weight-drop model was employed.21 At first, a 5 mL/kg dose of 1% chloral hydrate for anesthesia was administered by intraperitoneal (i.p.) injection into mice, and they were moved to a platform under the weight-drop device. Next, we located the hit area on the left anterior frontal lobe which was situated 1.5 mm from the midline. Thereafter, a 200-g weight was vertically released along a stainless steel string onto the skull that was exposed earlier at a height of 2.5 cm. The incision was closed routinely, and the animals were returned to their cages. Sham animals underwent the same procedures but without the blow to the head.
Drugs employed
NACA was purchased from TOCRIS, Bristol, UK and was dissolved in distilled water to form a 100-mM stock solution according to the manufacturer’s instruction. An equal volume of NACA or distilled water was administered to mice by i.p. injection. Information about antibodies and other reagents are listed below.
Groups and experimental design
In total, 96 mice were randomized into four experimental groups: 1) sham (n=24); 2) TBI (n=24); 3) TBI+vehicle (n=24); and 4) TBI+NACA (n=24). Animals in TBI+NACA group were injected with 100 mg/kg NACA (i.p.) 1 hour after TBI, whereas equal volumes of distilled water were administered i.p. to those in the TBI+vehicle group at the same time. This dose of NACA administered was based on a study of significant behavioral recovery following NAC in mice that suffered a weight-drop injury.15 Lastly, animals were returned to their respective cages and given water and food ad libitum. Six mice in each group underwent neurological evaluation, six were subjected to Western blotting analysis, six to histologic staining, and six to real-time quantitative PCR (RT-qPCR) analysis and biochemical measurements.
Behavioral evaluation
The Neurological Severity Score (NSS) was evaluated in the TBI, TBI+vehicle, and TBI+NACA groups at days 1 and 3 for behavioral evaluation.21 Briefly, ten tasks were completed by all the mice in varied groups. Each completed task was scored 0 point, and one point meant the task was not completed (Table 1). Two observers who were unaware of the grouping executed the testing. The order of the missions was randomly assigned.
  | Table 1 Neurologic Severity Score |
Brain tissue processing
Chloral hydrate (1%, 10 mL/kg) for deep anesthesia was administered to animals in different groups 1 day after TBI, and cold heparinized normal saline was infused intracardially for perfusion. For Western blotting, RT-qPCR detection and biochemical measurements, we collected tissue from the ipsilateral cortex which is located 3 mm from the edge of the contusion, immediately froze the specimens with liquid nitrogen, and then transferred them to a −80°C freezer until use. For TUNEL and Fluoro-Jade C (FJC) staining, animals were subsequently immersed in cold 4% paraformaldehyde. Shortly afterwards, we removed the entire brain and soaked it in 4% paraformaldehyde overnight. For immunofluorescence, we initially steeped the specimens in 20% sucrose and, subsequently, in 30% sucrose.
Western blot
As previously described, diverse proteins from the specimens were extracted. The Bradford method was applied for protein concentrations. We used 10% or 12% SDS-PAGE to separate the proteins into different bands. Then the separated proteins were transferred onto polyvinylidene-difluoride films. Films were blocked with 5% milk–Tris-buffered saline/0.05% Tween 20 (TBST) for 2 hours. Next, they were incubated at 4°C overnight using primary antibodies of rabbit anti-Nrf2 (1:1,000; Abcam, Cambridge, MA, USA), rabbit anti-HO-1 (1:1,000; Abcam, Cambridge, MA, USA), rabbit anti-NQO1 (1:1,000; Abcam, Cambridge, MA, USA), rabbit anti-cleaved caspase-3 (1:1,000; Abcam, Cambridge, MA, USA), rabbit anti-GAPDH (1:5,000; Bioworld Technology, St Louis Park, MN, USA), and rabbit anti-Histone 3 (1:1,000, Cell Signaling Technology, Beverly, MA, USA). After being washed in TBST, films were incubated with the appropriate secondary antibodies for 1 hour at room temperature. Finally, proteins were examined using an enhanced chemiluminescence (ECL) kit (Millipore, Billerica, MA, USA). Un-Scan-It 6.1 software (Silk Scientific Inc., Orem, UT, USA) quantified the signal intensities. Data were normalized to appropriate references (GAPDH for cytoplasmic protein and Histone 3 for nuclear protein).
RT-qPCR
Total RNA was extracted from frozen specimens using RNAiso Plus (TaKaRa Bio, Dalian, China). The concentration and purity of total RNA was determined according to the manufacturer’s instructions. The PrimeScript RT reagent kit (TaKaRa Bio, Dalian, China) was then employed as previously described. Primers for the HO-1-, NQO1-, and GAPDH-designed sequences were as follows: HO-1: F, 5′-ATCGTGCTCGCATGAACACT-3′; R, 5′-CCAACACTGCATTTACATGGC-3′; NQO1: F, 5′-CATTCTGAAAGGCTGGTTTGA-3′; R, 5′-CTAGCTTTGATCTGGTTGTCAG-3′; and GAPDH: F, 5′-TATGTCGTGGAGTCTACTGGT-3′; R, 5′-GAGTTGTCATATTTCTCGTGG-3′. RT-qPCR was amplified with the Mx3000P System (Strata gene, San Diego, CA, USA) with the applied SYBR Green PCR Master Mix (Bio-Rad). Relative expression was normalized to GAPDH.
Biochemical measurements of malondialdehyde (MDA), SOD, and GPx
Samples were homogenized and centrifuged. Then, levels of MDA, SOD, and GPx were measured using the appropriate kits (Key gentec Biochemistry Co. Nanjing, China) and spectrophotometry according to previous descriptions in the literature.8,10 The Bradford method was applied to determine the total protein concentrations. The abovementioned three parameters were quantified separately as nmol/mg, U/mg, and U/mg protein.
TUNEL staining
After conventional paraffin embedding, tissues were cut into 4-μm thick slices. According to previous studies, the TUNEL detection kit (Roche, Indianapolis, IN, USA) was used to analyze apoptotic cells.22 As per standard procedure, PBS with Tween 20 was used to wash the slides and they were then counterstained with DAPI. Finally, these slides were subjected to anti-fade treatment before being coverslipped. Two investigators who were blinded to the grouping analyzed the cells. The apoptotic rate, which was an indicator used to evaluate the extent of brain injury, was calculated as the average percentage of TUNEL-positive cells in a high-power field. For each slice, cells in six random high-power fields were counted. In all, we selected eight coronal slices from the identical cortex regions. The average percentage from eight slices was regarded as the data for each sample.
FJC staining
To investigate neuronal degeneration, FJC staining was conducted as previously described.23 Briefly, the 10-μm thick frozen slides were immersed into a basic alcohol solution for 5 minutes, 70% ethanol for 2 minutes, 0.06% potassium permanganate solution for 10 minutes, and then in a working solution of FJC (Bai Ying Bio., Tianjin, China) for 10 minutes in sequential order. In the interval between treatments, slides were soaked in distilled water. Lastly, the slides were dried, coverslipped, and examined under a fluorescence microscope. Microscopic examination was conducted by two pathologists blinded to the grouping. A total of eight coronal sections from each animal were selected. The neuronal degeneration was evaluated by the FJC-positive rate, which was defined as the average percentage of FJC-positive cells in a high-power field. In each section, six random high-power fields were counted. The final average percentage of the eight sections was regarded as the data for each sample.
Immunofluorescence staining
For immunofluorescence, we collected four serial 4-μm thick coronal sections from each sample. According to a previously described method, the slices were incubated in blocking buffer for 2 hours and incubated with rabbit monoclonal anti-Nrf2 (1:100, Abcam, Cambridge, MA, USA) at 4°C overnight. The next day, we incubated the slices with Alexa Fluor 594 (1:200; Invitrogen, Grand Island, NY, USA), counterstained them with DAPI, and then coverslipped them. In the intervals between treatments, the slices was washed with PBS. Images were observed on an Olympus IX71 inverted microscope system and evaluated using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
SPSS 24.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. All experiments were repeated at least three times. Data are presented as the mean ± SEM. Differences between multiple groups were assessed by one-way ANOVA, followed by the Fisher’s LSD post-test or Dunnett-t3 test based on homogeneity of variance. Significance was assigned at P<0.05.
Results
NACA improved the neurologic status of the mice following TBI
As shown in Figure 1, the NSS score in the TBI+NACA group was lower than that in the TBI or TBI+vehicle group at days 1 (P<0.01) and 3 (P<0.05) after TBI. These indicated that NACA attenuated the evolution of early brain injury in mice after TBI. However, no significant difference was found at days 1 or 3 between the TBI group and TBI+vehicle group (P>0.05). The scores of sham group were nearly zero, which showed no difference in the study (data not shown).
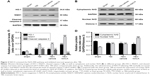
NACA promoted the nuclear import of Nrf2 at 1 day after the TBI
We evaluated the expressions of cytoplasm and nuclear Nrf2 protein (Figure 2B) 1 day post the TBI using Western blotting. Compared with the sham group, the TBI group showed greater expression of nuclear Nrf2 (P<0.001; Figure 2D) and lower expression of cytoplasmic Nrf2 (P<0.001; Figure 2D). Additionally, when compared with the TBI+vehicle group, NACA enhanced the nuclear expression of Nrf2 (P<0.001; Figure 2D) and reduced its cytoplasmic expression (P<0.05; Figure 2D).
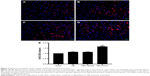
Furthermore, immunofluorescence affirmed the nuclear import of Nrf2. In the sham group, Nrf2 had little immunoreactivity and was located chiefly in the plasma (Figure 3A). However, Nrf2 immunoreactivity was enhanced in the TBI or TBI+vehicle group with partial nuclear import as compared with the sham group (P<0.001; Figure 3B and C). In the NACA-treated group, greater nuclear Nrf2-immunoreactivity was observed 1 day post TBI than in the vehicle-treated group (P<0.001; Figure 3D and E).
NACA enhanced the expression of Nrf2 downstream factors in both protein and mRNA levels 1 day after TBI
As shown in Figure 2, both HO-1 and NQO1 proteins (both P<0.001; Figure 2A and C) were upregulated after TBI as compared with the sham group. Additionally, the TBI+NACA group demonstrated greater protein expression than the TBI+vehicle group (P<0.001 and P<0.001, respectively; Figure 2A and C). However, there were no differences between the TBI and TBI+vehicle groups.
Analogously, the TBI+NACA group showed greater mRNA expressions of HO-1 and NQO1 (both P<0.001; Figure 4) than the TBI+vehicle group. The mRNA expressions of both the TBI (P<0.001; Figure 4) and TBI+vehicle groups (P<0.001; Figure 4) were higher than that in the sham group.
NACA attenuated oxidative stress after TBI
To evaluate whether the oxidative stress after TBI was alleviated by NACA, indicators of lipid peroxidation and levels of antioxidant enzymes such as MDA, SOD, and GPx were measured. Figure 5 showed that both the TBI (P<0.001) and TBI+vehicle (P<0.001) groups had a higher level of MDA than the sham group; however, there was no difference between the TBI and TBI+vehicle groups. Furthermore, NACA treatment significantly suppressed TBI-induced MDA production (P<0.05). The activities of SOD and GPx decreased significantly in the TBI and TBI+vehicle groups as compared with the sham group (all P<0.001). However, NACA significantly promoted SOD and GPx activities as compared with the vehicle-treated group (both P<0.001).
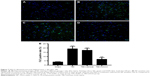
NACA was antiapoptotic in mice after TBI
TUNEL-positive cells were barely noticeable in the sham group (Figure 6A). The rate of apoptosis was elevated in the TBI and TBI+vehicle group than in the sham group (P<0.001, Figure 6B and C), but markedly reduced after NACA administration (P<0.001; Figure 6D and E). Meanwhile, the expression of cleaved caspase-3 also increased in the TBI and TBI+vehicle groups (both P<0.001; Figure 2A and C) and decreased after treatment with NACA (P<0.001; Figure 2A and C). These results indicated that NACA has an antiapoptotic effect after TBI.
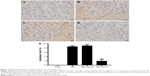
NACA alleviated neuronal degeneration 1 day after TBI
The representative images shown in Figure 7 exhibited a very small number of FJC-positive neurons in the sham group (Figure 7A). In contrast, the FJC-positive rate significantly increased in the TBI and TBI+vehicle groups (both P<0.001; Figure 7B and C). NACA treatment reduced the FJC-positive rate in the cortex as compared to the vehicle-treated group (P<0.001; Figure 7D and E). There was no statistically significant difference between the TBI and vehicle groups.
Discussion
In this study, we investigated the neuroprotection of NACA in a mouse model of TBI and evaluated the potential function of the Nrf2-ARE pathway in the process. Our results showed that, 1 day after TBI: 1) post-injury NACA administration improved neurobehavioral performance; 2) Nrf2 completed the nuclear import and activated downstream HO-1 and NQO1, and NACA promoted these events; and 3) NACA inhibited TBI-induced apoptosis and alleviated neuronal degeneration and oxidative stress. The above results indicate that NACA potentially provides neuroprotection via the activation of the Nrf2-ARE signal pathway after TBI in mice.
The neuroprotective and antioxidative effects of NACA have been demonstrated in some trauma models.19,20 Pandya et al17 reported that NACA after injury resulted in ameliorative behavioral prognosis and incremental tissue sparing in rats following a moderate unilateral CCI injury. It turned out that NACA significantly improved hind-limb function and increased tissue sparing at the injury site in a rat model of contusion spinal cord injury (SCI).24 Similar findings in behavioral performance were noticeable in our study. Meanwhile, we identified an inhibitory effect of NACA on neuronal apoptosis and neuronal degeneration in mice 1 day after TBI. This agrees with a study of focal penetrating TBI in rats where NACA decreased the FJC-positive cells at 1 day after the TBI, whereas NACA reduced the number of TUNEL cells at 2 hours but not at 24 hours.18 However, the underlying molecular mechanisms are still unknown.
Previous studies showed oxidative stress played a vital role in the pathophysiology of TBI and mediated most of the histopathological changes and neurobehavioral defects.23,25,26 With the continuous production of ROS after oxidative stress, lipid peroxidation, protein nitration, and DNA damage may occur subsequently, whereas antioxidant enzymes may be gradually exhausted.3,4,27 In our study, the index of lipid peroxidation (MDA) was found to have accumulated 1 day after TBI. The reductive activity of antioxidant enzymes (SOD and GPx) was detected simultaneously. However, NACA treatment following TBI significantly reduced MDA levels and enhanced the SOD and GPx activities, which indicated that NACA attenuated the oxidative stress caused by TBI. Moreover, other authors found increased levels of manganese superoxide dismutase (MnSOD, an antioxidant enzyme) in a rat model of TBI;18 they reported that NACA had reduced oxidative damage [4-hydroxynonenonal (HNE) levels] 7 days after a CCI injury. Thus, NACA may exert a neuroprotective effect after TBI through its antioxidant mechanism.
Increasing evidence indicates that the antioxidant effect of the Nrf2-ARE pathway after TBI depended on upregulated Phase II detoxifying enzymes, such as NQO1 and HO-1.28–30 To clarify whether the Nrf2-ARE pathway participated in the neuroprotective effects of NACA, the activation of the Nrf2-ARE pathway as well as histological and neurological status were evaluated. Our test displayed the nuclear import of Nrf2 and upregulation of NQO1 and HO-1 levels after TBI. Moreover, NACA enhanced the abovementioned activation of the Nrf2-ARE pathway. NQO1 and HO-1, potent antioxidant and detoxifying enzymes, may contribute to the weakened oxidant damage and brain injury.9 Moreover, because NACA attenuated the oxidative stress and brain injury caused by TBI, the Nrf2-ARE pathway may play a critical role underlying the neuroprotective process.31 Nevertheless, how NACA activates the Nrf2-ARE signal pathway remains undetermined. GSH, a primary intracellular antioxidant, plays a pivotal role in the scavenging of excessive ROS production.15,16 Previous experiments showed that the abatement of mitochondrial GSH levels following injury had been related to augmented injury.17,32 Recent results confirmed that NACA upregulated levels of GSH and improved mitochondrial bioenergetics following TBI.12,24 Li et al8 demonstrated that quercetin improved mitochondrial function in TBI models, possibly by activating the Nrf2 pathway. Therefore, we conclude that the protective effects of NACA after TBI may be closely related to the activation of the Nrf2-ARE pathway.
Conclusion
Our paper documents the neuroprotective effect of NACA and may, for the first time, clarify the role and mechanism of NACA in mice following TBI. NACA post-injury can attenuate secondary brain injury as indicated by improved neurologic status and lessened oxidative stress, neuronal degeneration, and neuronal apoptosis. These effects may correlate with the activation of the Nrf2-ARE signaling pathway. Future preclinical studies are needed to further explore the mechanism of action underlying the effect of NACA. To our knowledge, NACA may be regarded as a novel drug for TBI with good effect.
Acknowledgment
This work was financially supported by the Youth Training Project (grant no 16QNP042), the Key Clinical Subjects of “Strengthening Health by Science Education” Project in Jiangsu (grant no ZDXKB2016023), and the Jinling Hospital of Nanjing (grant no 2016017).
Disclosure
The authors report no conflicts of interest in this work.
References
Logsdon AF, Lucke-Wold BP, Nguyen L, et al. Salubrinal reduces oxidative stress, neuroinflammation and impulsive-like behavior in a rodent model of traumatic brain injury. Brain Res. 2016;1643:140–151. | ||
Khatri N, Thankur M, Pareek V, Kumar S, Sharma S, Datusalia AK. Oxidative stress: major threat in traumatic brain injury. CNS Neurol Disord Drug Targets. 2018;17(9):689–695. | ||
Cornelius C, Crupi R, Calabrese V, et al. Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal. 2013;19(8):836–853. | ||
Wang HC, Lin YJ, Shih FY, et al. The role of serial oxidative stress levels in acute traumatic brain injury and as predictors of outcome. World Neurosurg. 2016;87:463–470. | ||
Wu G, Liu Z. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Mediates Neuroprotection in Traumatic Brain Injury at Least in Part by Inactivating Microglia. Med Sci Monit. 2016;22:2161–2166. | ||
Ding H, Wang X, Wang H, et al. Nrf2-ARE signaling provides neuroprotection in traumatic brain injury via modulation of the ubiquitin proteasome system. Neurochem Int. 2017;111:32–44. | ||
Ding H, Wang H, Zhu L, Wei W. Ursolic acid ameliorates early brain injury after experimental traumatic brain injury in mice by activating the Nrf2 pathway. Neurochem Res. 2017;42(2):337–346. | ||
Li X, Wang H, Gao Y, et al. Protective effects of quercetin on mitochondrial biogenesis in experimental traumatic brain injury via the Nrf2 signaling pathway. PLoS One. 2016;11(10):e0164237. | ||
Dai W, Wang H, Fang J, et al. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res Bull. 2018;140:65–71. | ||
Zhang L, Wang H, Fan Y, et al. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci Rep. 2017;7:46763. | ||
Liu Z, Wang H, Shi X, et al. DL-3-n-Butylphthalide (NBP) provides neuroprotection in the mice models after traumatic brain injury via Nrf2-ARE signaling pathway. Neurochem Res. 2017;42(5):1375–1386. | ||
Semple BD. Early preservation of mitochondrial bioenergetics supports both structural and functional recovery after neurotrauma. Exp Neurol. 2014;261:291–297. | ||
Hagos FT, Daood MJ, Ocque JA, et al. Probenecid, an organic anion transporter 1 and 3 inhibitor, increases plasma and brain exposure of N-acetylcysteine. Xenobiotica. 2017;47(4):346–353. | ||
Clark RSB, Empey PE, Bayır H, et al. Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children. PLoS One. 2017;12(7):e0180280. | ||
Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617. | ||
Penugonda S, Mare S, Goldstein G, Banks WA, Ercal N. Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC12. Brain Res. 2005;1056(2):132–138. | ||
Pandya JD, Readnower RD, Patel SP, et al. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp Neurol. 2014;257:106–113. | ||
Günther M, Davidsson J, Plantman S, Norgren S, Mathiesen T, Risling M. Neuroprotective effects of N-acetylcysteine amide on experimental focal penetrating brain injury in rats. J Clin Neurosci. 2015;22(9):1477–1483. | ||
Kawoos U, McCarron RM, Chavko M. Protective Effect of N-Acetylcysteine Amide on Blast-Induced Increase in Intracranial Pressure in Rats. Front Neurol. 2017;8:219. | ||
Bhatti J, Nascimento B, Akhtar U, et al. Systematic review of human and animal studies examining the efficacy and safety of N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) in traumatic brain injury: impact on neurofunctional outcome and biomarkers of oxidative stress and inflammation. Front Neurol. 2017;8:744. | ||
Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat Protoc. 2009;4(9):1328–1337. | ||
Casili G, Campolo M, Paterniti I, et al. Dimethyl fumarate attenuates neuroinflammation and neurobehavioral deficits induced by experimental traumatic brain injury. J Neurotrauma. 2018;35(13):1437–1451. | ||
Huang YN, Yang LY, Greig NH, Wang YC, Lai CC, Wang JY. Neuroprotective effects of pifithrin-α against traumatic brain injury in the striatum through suppression of neuroinflammation, oxidative stress, autophagy, and apoptosis. Sci Rep. 2018;8(1):2368. | ||
Patel SP, Sullivan PG, Pandya JD, et al. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp Neurol. 2014;257:95–105. | ||
Zhang L, Wang H, Zhou Y, Zhu Y, Fei M. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem Int. 2018;118:304–313. | ||
Halstrom A, MacDonald E, Neil C, Arendts G, Fatovich D, Fitzgerald M. Elevation of oxidative stress indicators in a pilot study of plasma following traumatic brain injury. J Clin Neurosci. 2017;35:104–108. | ||
Cao Y, Gao Y, Xu S, et al. Glutamate carboxypeptidase II gene knockout attenuates oxidative stress and cortical apoptosis after traumatic brain injury. BMC Neurosci. 2016;17(1):15. | ||
Chandran R, Kim T, Mehta SL, et al. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J Cereb Blood Flow Metab. 2018;38(10):1818–1827. | ||
Miller DM, Singh IN, Wang JA, Hall ED. Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp Neurol. 2015;264:103–110. | ||
Xu J, Wang H, Ding K, et al. Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE pathway. Free Radic Biol Med. 2014;71:186–195. | ||
Price TO, Uras F, Banks WA, Ercal N. A novel antioxidant N-acetylcysteine amide prevents gp120- and Tat-induced oxidative stress in brain endothelial cells. Exp Neurol. 2006;201(1):193–202. | ||
Zhang X, Banerjee A, Banks WA, Ercal N. N-Acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res. 2009;1275:87–95. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.