Back to Journals » Clinical Interventions in Aging » Volume 18
Myopenic Obesity Determined by Fat Mass Percentage Predicts Risk of Aspirin-Induced Bleeding in Chinese Older Adults
Authors Wang X , Li L, Cui J, Cheng M, Liu M
Received 8 February 2023
Accepted for publication 7 April 2023
Published 13 April 2023 Volume 2023:18 Pages 585—595
DOI https://doi.org/10.2147/CIA.S405559
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Xiting Wang, Li Li, Jing Cui, Mei Cheng, Meilin Liu
Department of Geriatrics, Peking University First Hospital, Beijing, People’s Republic of China
Correspondence: Meilin Liu, Department of Geriatrics, Peking University First Hospital, Beijing, People’s Republic of China, Email [email protected]
Background: Body mass index (BMI) correlates with aspirin-induced bleeding risk. However, skeletal muscle mass (SMM) loss and fat gain commonly occur with aging, making BMI not a reasonable marker of bleeding risk in older individuals. In the present study, we aimed to investigate the prognostic value of myopenic obesity based on the percent of fat mass (%FM) for aspirin-induced bleeding in Chinese patients over 60 years old.
Methods: We prospectively analyzed 185 patients taking aspirin for primary and secondary prevention of cardiovascular diseases. Body composition parameters were estimated using bioelectrical impedance analysis. We defined myopenic obesity (MO) as a height-adjusted appendicular SMM < 7.0 kg/m2 in males and < 5.7 kg/m2 in females with a %FM > 29% in males and > 41% in females or a BMI ≥ 25 kg/m2. The patients were categorized into four groups by the presence or absence of myopenia and obesity.
Results: Based on the %FM grouping, the bleeding risk was significantly higher in the MO group, followed by the nonmyopenic obesity, myopenic nonobesity, and nonmyopenic nonobesity groups (P = 0.044). No statistically significant differences in the probability of bleeding events were observed among the four BMI-based groups (P = 0.502). Multivariate Cox analysis indicated that MO (hazard ratio [HR] 2.724, 95% confidence interval [CI] 1.073– 6.918, P = 0.035), aspirin dose (100 vs 50 mg/day, HR 2.609, 95% CI 1.291– 5.273, P = 0.008), concomitant use of histamine-2 receptor antagonists and proton pump inhibitors (HR 1.777, 95% CI 1.007– 3.137, P = 0.047), and hemorrhage history (HR 2.576, 95% CI 1.355– 4.897, P = 0.004) were associated with bleeding events independently.
Conclusion: %FM-based MO was an independent predictor of aspirin-induced bleeding in older Chinese individuals. Reducing %FM rather than BMI should be an optimal strategy for the management of myopenic obesity.
Keywords: myopenic obesity, aspirin-induced bleeding, body composition, percent of fat mass, older adults
Introduction
Aspirin significantly reduces the incidence of cardiovascular events and cardiovascular mortality1,2 but also increases the risk of bleeding,3 particularly in older adults.4,5 Owing to a higher prevalence of frailty, associated with increased longevity, and the global epidemic of obesity, extremely low and high body weights are becoming more common in the older population.6–8 Several studies have explored the association between aspirin effects and body weight. Obesity may influence the pharmacodynamics and pharmacokinetics of aspirin and is associated with impaired aspirin responsiveness.9,10 Previous trials11,12 have suggested that the risk of aspirin-induced bleeding is related to the body weight, and participants with a lower body weight seem to be at a high risk of bleeding.
Body mass index (BMI) is the simplest and most universal indicator used to assess the body weight in the clinic. However, BMI is not a reasonable marker of the bleeding risk in older individuals. Aging causes a progressive loss of muscle mass and strength, as well as an increase in fat mass (FM) and visceral fat accumulation, which is called sarcopenic obesity.13 Recently, the relevance of body composition to clinical outcomes has attracted much attention, and mounting evidence has suggested that the skeletal muscle and fat tissue represent different characteristics and functions.14 However, few studies have reported the impact of skeletal muscle and FM on aspirin-induced bleeding. In addition, the available evidence suggests a U-shaped relationship between BMI and spontaneous bleeding.15 Overweight (BMI 25–29.9 kg/m2) and class 1 obese individuals (BMI 30.0–34.9 kg/m2) have better outcomes of bleeding, commonly referred to as the “bleeding–obesity paradox”,16 which may be because BMI does not accurately reflect body fat mass and distribution in older individuals. In addition to BMI, other measures to diagnose obesity include the percent of fat mass (%FM), visceral fat area, waist circumference (WC), and waist-to-hip ratio (WHR). The %FM is a good measure of adiposity, particularly in older individuals and those with sarcopenic obesity. However, whether the coexistence of low skeletal muscle mass and obesity defined on the %FM could better predict the risk of aspirin-induced bleeding in older Chinese patients than BMI remains unknown.
Therefore, the current study aimed to investigate the impact of concomitant abnormal skeletal muscle and/or fat mass on aspirin-induced bleeding and to further explore whether the diagnostic criteria of obesity based on %FM could better predict the bleeding risk in older adults than BMI.
Materials and Methods
Study Design
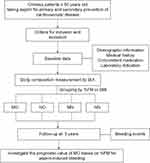
This study was designed as a prospective, observational cohort study, and patients were consecutively recruited from the Department of Geriatrics, Peking University First Hospital, between April 1, 2019 and April 1, 2022. The inclusion criteria were as follows: participants age≥60 years old and regularly taking aspirin for primary and secondary prevention of cardiovascular disease (CVD), who consented to body composition measurements and provided routine laboratory test results in the past 3 months. The exclusion criteria included aspirin-sensitive asthma history or allergies to aspirin. Patients with severe hepatic (Child‒Pugh C) or renal insufficiency (estimated glomerular filtration rate, eGFR≤30 mL/min.1.73 m2) were also excluded from the present study. All patients were followed up via face-to-face or telephone/WeChat visits at intervals of 6 months, and 185 patients were included in the final analysis. A flow chart of the study is shown in Figure 1.
Clinical and Laboratory Parameters
Baseline data of all the participants were collected into a case report form according to standard procedures by independent clinical research coordinators, including demographics, medical history, concomitant medication, and laboratory indication in the past 3 months. Gastrointestinal disease was defined as any self-reported history of reflux esophagitis, erosive gastritis, or gastric or duodenal ulcers. Hemorrhage history was defined as a history of bleeding events at any site and of any severity. The primary outcome was the first occurrence of any bleeding event, which was graded as fatal (Bleeding Academic Research Consortium [BARC]17 type 5), major (BARC type 3–4), and minor bleeding (BARC type 1–2). Time to bleeding event was defined as the number of days from the date of enrollment to the confirmed date of the bleeding event.
Body Composition Measurements
Bioelectrical impedance analysis (BIA) was performed using a body composition analyzer (InBody 720, Korea) to obtain all body composition data, including the skeletal muscle mass (SMM), body FM, %FM, WHR, and appendicular SMM (ASM), of the patients. The Asian Working Group for Sarcopenia (AWGS) has updated the definition of sarcopenia as low muscle strength or physical performance and a low SMM, with cutoffs for height-adjusted ASM of <7.0 kg/m2 in men and <5.7 kg/m2 in women by BIA.18 Owing to the lack of muscle strength and physical performance data, we referred to low SMM as “myopenia” in the present study. In general, obesity is supposed to be defined as BMI ≥30 kg/m2 according to the World Health Organization (WHO) expert report. However, Asian populations are more likely to suffer from obesity-related complications at a lower BMI than Western individuals.19 Thus, the diagnostic criteria for obesity20,21 in the present study include a %FM of >29% in males and >41% in females or a BMI of ≥25 kg/m2 in both sexes. Accordingly, the cohort was divided into four groups based on the height-adjusted ASM and %FM or BMI as follows: nonmyopenic nonobesity (NN), nonmyopenic obesity (NO), myopenic nonobesity (MN), and myopenic obesity (MO).
Statistical Analysis
Continuous variables are presented as the mean ± standard deviation (SD) or median (minimum, maximum) and were compared using an independent Student’s t-test or the Mann‒Whitney U-test. Categorical variables are presented as percentages (%) and were compared using Pearson’s χ²-test or Fisher’s exact test. Multiple comparisons were performed using one-way ANOVA or the Kruskal‒Wallis test with Dunn’s or LSD post hoc test, and Bonferroni correction was performed to correct for multiple comparisons. Kaplan–Meier analysis was used to evaluate the timing of bleeding event occurrences, and a Log rank test was conducted. Hazard ratios (HRs) and 95% confidence intervals (CIs) were obtained from Cox proportional hazards regression models. After adjusting for variables that were known to be strongly associated with the bleeding risk or differed significantly by univariate analysis, multivariate Cox proportional hazards models were developed to identify the predictors of aspirin-induced bleeding. A P value of < 0.05 was considered to be statistically significant for all tests. Statistical analyses were performed using the SPSS software, version 23.0 (IBM, Armonk, NY, USA).
Results
Patient Characteristics and Bleeding Probabilities According to %FM
The baseline characteristics and body composition of all the participants are presented in Table 1. A total of 185 patients (159 males, 85.9%) with a median age of 75.5 years were enrolled in this study. After a median of 738 days (interquartile range: 381, 880 days) of follow-up for all 185 patients, bleeding events occurred in 56 patients. When stratified based on the %FM, all the participants were divided into four groups: NN, n = 85 (46.0%); NO, n = 55 (29.7%); MN, n = 27 (14.6%); and MO, n = 18 (9.7%).
 |
Table 1 Characteristics of the Entire Cohort and Classification by %FM |
Age (P = 0.006), arachidonic acid-induced platelet aggregation rates (P = 0.024), high-density lipoprotein cholesterol (HDL-C) levels (P = 0.034), and body composition were significantly different among the four groups based on the %FM classification. The patients in the MO group had higher age (78.8 vs 73.6 years, P = 0.016), body FM (22.7 vs 18.5 kg, P < 0.001), %FM (34.1 vs 26.2%, P < 0.001), and WHR (0.95 vs 0.91, P = 0.002) and lower SMM (24.1 vs 29.5 kg, P < 0.001) and height-adjusted ASM (6.59 vs 7.64 kg/m2, P < 0.001) than those in the NN group. The patient characteristics for the obesity group (n = 73) and nonobesity group (n = 112), as classified by %FM, are shown in Table S1.
The bleeding event rates in the NN, NO, MN, and MO groups were 21.2% (n = 18), 38.2% (n = 21), 29.6% (n = 8), and 50% (n = 9), respectively. Of these bleeding events, 1 (1.8%) patient in the MN group had a fatal intracranial hemorrhage (BARC type 5), 1 (1.8%) patient in the NO group had severe gastrointestinal bleeding (BARC type 3–4), and 54 (96.4%) patients had minor bleeding events (BARC type 1–2). The probability of bleeding events was significantly higher in the MO group, followed by the NO, MN, and NN groups (P = 0.044) (Figure 2A).
Patient Characteristics and Bleeding Probabilities According to BMI
When stratified based on BMI, all participants were divided into NN, n = 70 (37.8%); NO, n = 70 (37.8%); MN, n = 43 (23.2%); and MO, n = 2 (1.1%). Age (P = 0.007), triglycerides (P = 0.016), low-density lipoprotein cholesterol (P = 0.029), HDL-C levels (P = 0.003), and body composition were significantly different among the four groups based on BMI classification. The patients in the MO group had higher BMI (27.7 vs 23.1 kg/m2, P = 0.001) and %FM (38.5 vs 27.0%, P = 0.006) than those in the NN group (Table 2). The patient characteristics for the BMI ≥25 kg/m2 group (n = 72) and BMI <25 kg/m2 group (n = 113) are shown in Table S2.
 |
Table 2 Characteristics of Entire Cohort and Classified by BMI |
The bleeding event rates in the NN, NO, MN, and MO groups were 27.1% (n = 19), 28.6% (n = 20), 39.5% (n = 17), and 0%, respectively. Of these bleeding events, 1 (1.8%) patient in the MN group had fatal intracranial hemorrhage (BARC type 5), 1 (1.8%) patient in the NN group had severe gastrointestinal bleeding (BARC type 3–4), and 54 (96.4%) patients had minor bleeding events (BARC type 1–2). However, no significant differences in the probability of bleeding events were observed among the four groups (P = 0.502) (Figure 2B).
Risk Factors for Aspirin-Induced Hemorrhage
The predictive value of baseline variables and body composition for aspirin-induced hemorrhage was further investigated using a Cox regression model based on %FM or BMI classification.
The results of univariate analysis showed that the level of hemoglobin, concomitant use of proton pump inhibitors and histamine-2 receptor antagonists (PPI/H2RA), and hemorrhage history were associated with aspirin-induced hemorrhage (Table S3).
Based on the %FM classification, we found that PPI/H2RA use (HR 1.777, 95% CI 1.007–3.137, P = 0.047), hemorrhage history (HR 2.576, 95% CI 1.355–4.897, P = 0.004), aspirin dose (100 vs 50 mg/day, HR 2.609, 95% CI 1.291–5.273, P = 0.008), and presence of MO (HR 2.724, 95% CI 1.073–6.918, P = 0.035) were independent risk factors for aspirin-induced bleeding after adjusting for confounders. Based on BMI classification, hemorrhage history (HR 2.861, 95% CI 1.502–5.451, P = 0.001) and aspirin dose (100 vs 50 mg/day, HR 2.742, 95% CI 1.346–5.587, P = 0.005) were independent risk factors for aspirin-induced bleeding (Table 3).
 |
Table 3 Features Associated with Bleeding Events by Multivariate Cox Analysis of the Entire Cohort |
Discussion
During the aging process, a loss of the SMM and function (sarcopenia) commonly occurs in parallel with relative or absolute body fat gain. From a clinical standpoint, the coexistence of sarcopenia and obesity potentially leads to a cumulative risk derived from these two conditions.19 Aspirin, a platelet aggregation inhibitor that leads to long-lasting suppression of thromboxane (TX) A2 production by acetylated cyclooxygenase-1 (COX-1), has been widely used for the prevention and treatment of CVD for decades. However, aspirin is associated with an increased bleeding risk, especially in older individuals. Therefore, scientific research has recently focused on the association between the coexistence of sarcopenia and obesity and the risk of aspirin-induced bleeding. After being absorbed in the stomach and duodenum, aspirin is rapidly deacetylated by esterases in the plasma, red blood cells, intestinal wall, and liver and then reaches systemic circulation in the form of salicylic acid. Obesity modifies the body composition, including kidney, liver, and heart function and plasma proteins, thus affecting the absorption, volume of distribution, metabolism, and/or elimination of aspirin.22 In obese subjects, high esterase activity has been found in mesenteric fat tissue, which may increase aspirin inactivation before the first passage through the liver and reduce its antithrombotic efficacy.23,24 Obesity has been reported to be associated with impaired aspirin responsiveness,25,26 as indicated by a high residual serum TXB2 level, high platelet function, or elevated urinary levels of TX metabolites. Moreover, inhibition of COX-1 has been shown to be reduced when low-dose aspirin is used in persons with a high body weight and increased BMI,26,27 probably due to increased platelet activation or turnover.23,28 Meanwhile, a decreased lean (muscle) body mass can markedly affect the pharmacokinetic processes by increasing the volume of distribution and half-life of lipophilic aspirin.4,29 When aspirin is administered to patients with a low muscle mass, bleeding events are more likely to occur because of a relatively high level of salicylic acid in systemic circulation and a decrease in prostacyclin production in endothelial cells. In addition to pharmacodynamic studies, observational studies of clinical outcomes have explored the correlation between body weight and aspirin-induced bleeding,12 and it was reported that patients with lower body weight (≤60 kg) were at increased bleeding risk with 100 mg aspirin. However, few studies have been reported on the correlation between body composition and aspirin-induced bleeding. Our previous retrospective study30 indicated that both the SMM and height-adjusted ASM were independently and negatively correlated with aspirin-induced hemorrhage. In the present study, overall bleeding events occurred in 30.3% of all recruited participants, including patients on aspirin monotherapy and those with concomitant use of antiplatelet and anticoagulant medications. We observed that body composition was related to aspirin-induced adverse bleeding events, and patients with MO, as defined by a low height-adjusted ASM and a high %FM, seemed to be at a high bleeding risk during the 738-day follow-up period.
Although the small sample size of MO for either %FM or BMI classification makes it difficult to confirm statistically whether it is better to use %FM rather than BMI for diagnosing MO, our findings may still have some exploratory implications. This may suggest that MO, as determined using %FM rather than BMI, could identify specific subgroups representing a higher bleeding risk. The prevalence of MO considerably varied depending on the use of %FM or BMI. Based on %FM, 18 (9.7%) patients were classified as having MO, compared with 2 (1.1%) patients based on BMI. It seems that BMI cannot recognize nearly 90% of patients with the coexistence of excessive FM and decreased SMM because BMI fails to precisely account for differences in fat and muscle distribution. In the present study, multivariate analysis indicated that MO (based on the %FM) was associated with a higher risk of bleeding. Compared with those in the reference group (individuals without obesity or myopenia), those who had MO had a 172% higher risk of bleeding. Therefore, our findings have implications for practice. When evaluating the risk of aspirin-induced hemorrhage in older patients, body composition measurements and screening for sarcopenic obesity should be carried out to develop an individual therapy strategy, especially for very old patients.
Consistent with the findings of our previous study,31 the aspirin-associated bleeding risk was shown to increase with the aspirin dose. Multivariate Cox analysis indicated that the aspirin dose was independently associated with bleeding events regardless of %FM or BMI stratification. It is believed that lower-dose aspirin can effectively reduce the risk of bleeding and is safer in older individuals, particularly those with a history of bleeding, which is in line with the findings of previous studies.31 A history of ulcer or upper gastrointestinal bleeding, Helicobacter pylori infection and aspirin dose were all risk factors for aspirin-related bleeding and gastrointestinal complications.32 Multivariate analysis revealed that bleeding history and concomitant use of PPI/H2RA were independent risk factors for the occurrence of bleeding events following aspirin administration. Understandably, patients with gastrointestinal disease, Helicobacter pylori infection and upper gastrointestinal bleeding history are more likely to use PPI/H2RAs. However, in this study, we did not observe that the combination of antiplatelet and anticoagulant drugs increased the risk of aspirin-induced bleeding, probably because of the small number of patients who took combined antithrombotic drugs.
The following limitations of this study must be considered. First, it was a single-center study with a relatively short follow-up period, and the majority of patients were males. Owing to the small sample size and short follow-up time, selection bias cannot be excluded. Second, we did not exclude patients with severe peripheral edema when designing the exclusion criteria, which may interfere with the results of BIA measurement. Third, we focused on the association between body composition and bleeding risk with aspirin, while the efficacy of aspirin was not separately analyzed. Fourth, most bleeding events were minor, and only 2 (1.1%) of the 185 patients experienced major and fatal bleeding events during the follow-up, precluding the assessment of associations between severe clinical hemorrhage and body composition. Fifth, we only identified myopenia with SMM loss using BIA. Recently, the AWGS revised the definition of sarcopenia and elevated low muscle strength as a primary indicator of probable sarcopenia. However, the correlation between sarcopenia and aspirin-induced hemorrhage was not analyzed because of the lack of data on muscle strength and physical performance. Additional studies to evaluate the predictive value of handgrip strength, gait speed, frailty, and nutritional status in older participants are still necessary. Sixth, we acknowledge that bias might exist because of the low numbers of cases in the MO groups (18 cases by %FM and 2 cases by BMI). Future studies will be needed to validate our preliminary findings in a large cohort of patients from distinct body composition groups. Seventh, we performed analysis based on %FM and BMI, and further analyses are needed to investigate whether other measures to diagnose obesity, including the visceral fat area, WC, and WHR, could predict the risk of aspirin-induced bleeding in Chinese older individuals. Finally, the ESPEN and EASO consensus statement published in 2022 recommended using weight-adjusted SMM instead of height-adjusted ASM by BIA for assessing sarcopenic obesity.33 The new diagnostic criteria should be used in future studies to evaluate whether they are suitable for the Chinese elderly population.However, our work attempted to explore the underlying mechanism with respect to older population. This special entity has attracted much attention as concomitant abnormal age-dependent muscle wasting and fat accumulation may act synergistically, thus maximizing their health-threatening effects.
Conclusion
In this prospective study, we found that MO, which was determined based on %FM, was an independent predictor of aspirin-induced bleeding in Chinese individuals over 60 years of age. It is plausible to develop preventive strategies by controlling %FM rather than BMI as the optimal target for MO management.
Abbreviations
%FM, percent of fat mass; ASM, appendicular skeletal muscle mass; AWGS, Asian Working Group for Sarcopenia; BARC, Bleeding Academic Research Consortium; BIA, bioelectrical impedance analysis; BMI, body mass index; CI, confidence interval; COX-1, cyclooxygenase-1; CVD, cardiovascular disease; H2RA, histamine-2 receptor antagonist; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; MN, myopenic/nonobesity; MO, myopenic obesity; NN, nonmyopenic/nonobesity; NO, nonmyopenic/obesity; PPI, proton pump inhibitor; SMM, skeletal muscle mass; TX, thromboxane; WC, waist circumference; WHR, waist-to-hip ratio.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The present study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Peking University First Hospital (approval number 2021-432). Each participant signed a written informed consent form.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Youth Clinical Research Project of Peking University First Hospital (2021CR17) and by the PKUBaidu Fund (2019BD019). The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomized trials. Lancet. 2009;373:1849–1860. doi:10.1016/S0140-6736(09)60503-1
2. Casado-Arroyo R, Bayrak F, Sarkozy A, et al. Role of ASA in the primary and secondary prevention of cardiovascular events. Best Pract Res Clin Gastroenterol. 2012;26:113–123.
3. Capodanno D, Angiolillo DJ. Aspirin for primary cardiovascular risk prevention and beyond in diabetes mellitus. Circulation. 2016;134:1579–1594.
4. Andreotti F, Rocca B, Husted S, et al.; Group ESCTW. Antithrombotic therapy in elderly individuals: expert position paper of the European Society of cardiology working group on thrombosis. Eur Heart J. 36;2015:3238–3249. doi:10.1093/eurheartj/ehv304
5. Li L, Geraghty OC, Mehta Z, et al. Age-specific risks, severity, time course, and outcome of bleeding on longterm antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390:490–499. doi:10.1016/S0140-6736(17)30770-5
6. Rao G, Powell-Wiley TM, Ancheta I, et al. American heart association obesity committee of the council on lifestyle and cardiometabolic health. Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: a scientific statement from the American Heart Association. Circulation. 2015;132:457–472. doi:10.1161/CIR.0000000000000223
7. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi:10.1016/S0140-6736(17)32129-3
8. Afshin A, Forouzanfar MH, Reitsma MB, et al.; GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi:10.1056/NEJMoa1614362
9. Furtado RHM, Giugliano RP, Dalcoquio TF, et al. Increased bodyweight and inadequate response to aspirin in individuals with coronary artery disease. J Thromb Thrombolysis. 2019;48(2):217–224. doi:10.1007/s11239-019-01830-z
10. Mourikis P, Zako S, Dannenberg L, et al. Aspirin antiplatelet effects are associated with body weight. Vascul Pharmacol. 2020;125–126:106635. doi:10.1016/j.vph.2019.106635
11. Rothwell PM, Cook NR, Michael Gaziano J, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomized trials. Lancet. 2018;392:387–399. doi:10.1016/S0140-6736(18)31133-4
12. Wang X, Zhu Q, Wu Y, et al. Effect of body weight on bleeding events of aspirin in ischemic stroke or transient ischemic attack patients. J Clin Pharm Ther. 2022;47:1684–1689. PMID: 35748660. doi:10.1111/jcpt.13722
13. Zamboni M, Mazzali G, Fantin F, et al. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18(5):388–395. doi:10.1016/j.numecd.2007.10.002
14. Feng H, Wang X, Zhao T, et al. Myopenic obesity determined by visceral fat area strongly predicts long-term mortality in cirrhosis. Clin Nutr. 2021;40(4):1983–1989. doi:10.1016/j.clnu.2020.09.016
15. Powell BD, Lennon RJ, Lerman A, et al. Association of body mass index with outcome after percutaneous coronary intervention. Am J Cardiol. 2003;91:472–476. doi:10.1016/S0002-9149(02)03252-6
16. Delhaye C, Wakabayashi K, Maluenda G, et al. Body mass index and bleeding complications after percutaneous coronary intervention: does bivalirudin make a difference? Am Heart J. 2010;159:1139–1146. doi:10.1016/j.ahj.2010.03.011
17. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–2747. doi:10.1161/CIRCULATIONAHA.110.009449
18. Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. PMID: 32033882. doi:10.1016/j.jamda.2019.12.012
19. Yuji M. Examination committee of criteria for obesity disease in Japan; Japan Society for the study of obesity. New criteria for “obesity disease”. Jpn Circ J. 2002;66(11):987–992.
20. Batsis JA, Mackenzie TA, Bartels SJ, et al. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes. 2016;40(5):761–767. doi:10.1038/ijo.2015.243
21. Kamo N, Kaido T, Hamaguchi Y, et al. Impact of sarcopenic obesity on outcomes in patients undergoing living donor liver transplantation. Clin Nutr. 2019;38:2202–2209. doi:10.1016/j.clnu.2018.09.019
22. Alpert MA, Omran J, Bostick BP. Effects of obesity on cardiovascular hemodynamics, cardiac morphology, and ventricular function. Curr Obes Rep. 2016;5:424–434. doi:10.1007/s13679-016-0235-6
23. Patrono C, Rocca B. Type 2 diabetes, obesity, and aspirin responsiveness. J Am Coll Cardiol. 2017;69(6):613–615. doi:10.1016/j.jacc.2016.11.049
24. Rocca B, Fox KAA, Ajjan RA, et al. Antithrombotic therapy and body mass: an expert position paper of the ESC Working Group on Thrombosis. Eur Heart J. 2018;39(19):1672–1686f. doi:10.1093/eurheartj/ehy066
25. Petrucci G, Zaccardi F, Giaretta A, et al. Obesity is associated with impaired responsiveness to once‐daily low‐dose aspirin and in vivo platelet activation. J Thromb Hemost. 2019;17:885–895. doi:10.1111/jth.14445
26. Maree AO, Curtin RJ, Dooley M, et al. Platelet response to low-dose enteric-coated aspirin in patients with stable cardiovascular disease. J Am Coll Cardiol. 2005;46:1258–1263. doi:10.1016/j.jacc.2005.06.058
27. Rocca B, Santilli F, Pitocco D, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Hemost. 2012;10:1220–1230. doi:10.1111/j.1538-7836.2012.04723.x
28. Davi G, Guagnano MT, Ciabattoni G, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi:10.1001/jama.288.16.2008
29. Sankaralingam S, Kim RB, Padwal RS. The impact of obesity on the pharmacology of medications used for cardiovascular risk factor control. Can J Cardiol. 2015;31:167–176. doi:10.1016/j.cjca.2014.10.025
30. Xiting W, Meilin L. Correlation between body composition and aspirin-induced bleeding in older patients with coronary heart disease. J Clinical Cardiol. 2021;37(1):32–37. doi:10.13201/j.issn.1001-1439.2021.01.007
31. Wang X, Wang H, Zheng Q, et al. Outcomes Associated with 50 mg/d and 100 mg/d aspirin for the prevention and management of cardiovascular disease in Chinese elderly: single-center interim analysis of a multicenter, prospective, observational study. Int J Gen Med. 2022;15:7089–7100. doi:10.2147/IJGM.S384375
32. Editorial Board of Chinese Journal of Cardiovascular Disease (Online edition). Expert consensus on prevention and treatment of digestive tract injury associated with oral antithrombotic drugs. Chin J Cardiovasc Dis. 2021;4:e1000081.
33. Donini LM, Busetto L, Bischoff SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022;15(3):321–335. doi:10.1159/000521241
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.