Back to Journals » International Journal of General Medicine » Volume 7
Myelofibrosis-associated complications: pathogenesis, clinical manifestations, and effects on outcomes
Authors Mughal T, Vaddi K, Sarlis N, Verstovsek S
Received 19 July 2013
Accepted for publication 3 September 2013
Published 29 January 2014 Volume 2014:7 Pages 89—101
DOI https://doi.org/10.2147/IJGM.S51800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Tariq I Mughal,1 Kris Vaddi,2 Nicholas J Sarlis,2 Srdan Verstovsek3
1Tufts University School of Medicine, Boston, MA, 2Incyte Corporation, Wilmington, DE, 3Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX, USA
Abstract: Myelofibrosis (MF) is a rare chronic BCR-ABL1 (breakpoint cluster region-Abelson murine leukemia viral oncogene homologue 1)-negative myeloproliferative neoplasm characterized by progressive bone marrow fibrosis, inefficient hematopoiesis, and shortened survival. The clinical manifestations of MF include splenomegaly, consequent to extramedullary hematopoiesis, cytopenias, and an array of potentially debilitating abdominal and constitutional symptoms. Dysregulated Janus kinase (JAK)-signal transducer and activator of transcription signaling underlies secondary disease-associated effects in MF, such as myeloproliferation, bone marrow fibrosis, constitutional symptoms, and cachexia. Common fatal complications of MF include transformation to acute leukemia, thrombohemorrhagic events, organ failure, and infections. Potential complications from hepatosplenomegaly include portal hypertension and variceal bleeding, whereas extramedullary hematopoiesis outside the spleen and liver – depending on the affected organ – may result in intracranial hypertension, spinal cord compression, pulmonary hypertension, pleural effusions, lymphadenopathy, skin lesions, and/or exacerbation of abdominal symptoms. Although allogeneic stem cell transplantation is the only potentially curative therapy, it is suitable for few patients. The JAK1/JAK2 inhibitor ruxolitinib is effective in improving splenomegaly, MF-related symptoms, and quality-of-life measures. Emerging evidence that ruxolitinib may be associated with a survival benefit in intermediate- or high-risk MF suggests the possibility of a disease-modifying effect. Consequently, ruxolitinib could provide a treatment backbone to which other (conventional and novel) therapies may be added for the prevention and effective management of specific MF-associated complications.
Keywords: extramedullary hematopoiesis, JAK inhibitor, myelofibrosis, myeloproliferative neoplasm, ruxolitinib
Introduction
Myelofibrosis (MF) is a chronic BCR-ABL1 (breakpoint cluster region-Abelson murine leukemia viral oncogene homologue 1)-negative stem cell myeloproliferative neoplasm (MPN) characterized by bone marrow fibrosis, ineffective hematopoiesis, extramedullary hematopoiesis (EMH), splenomegaly, shortened survival and progressive abdominal and constitutional symptoms, as well as other general chronic debilitating complaints.1,2 The MF-associated consequences and medical complications often result in premature death from infection, thrombohemorrhagic events, cardiac or pulmonary failure, and leukemic transformation.3,4 MF is an uncommon malignancy. Recent estimates of MF prevalence in the USA range from 3.6 to 5.7 per 100,000 persons, whereas estimates of MF incidence range from 1.7 to 2.4 per 100,000 persons.5
Although the etiology of MF is unknown, environmental factors may well be relevant since MF has been linked in a small number of patients to radiation and exposure to petrochemicals such as benzene and toluene.6–8 MF can be primary (termed “primary myelofibrosis” [PMF]; formerly termed “idiopathic MF,” “agnogenic myeloid metaplasia,” or “myeloid metaplasia with MF”) or secondary, developing from polycythemia vera (PV; currently termed “post-PV MF” [PPV-MF]) or essential thrombocythemia (ET; currently termed “post-ET MF”).9 The past decade has witnessed considerable progress in the understanding of the cellular and molecular biology of MPNs, and this has recently resulted in the addition of the Janus kinase (JAK) 1 and JAK2 inhibitor ruxolitinib to our therapeutic armamentarium.10 Ruxolitinib is highly effective in the clinical management of patients with intermediate- or high-risk MF, particularly in those with disease-related symptoms and splenomegaly.11–13 Importantly, recent updates from two prospective, randomized, Phase III studies showed that patients with MF treated with ruxolitinib had improved survival over placebo and best available therapy, suggesting an overall survival benefit.14,15 However, the overall prognosis for advanced MF remains guarded, owing to a potentially remaining substantive burden of disease-related morbidities. The basis for these morbidities is the emergence of a remarkably broad array of general medical complications associated with this rare – and, until recently, rather therapeutically neglected – malignancy. Some of these complications are directly linked to excessive clonal myeloproliferation (the end result of which is leukemic transformation); however, most MF-associated complications are of more protean nature and deserve a deeper discourse. Here, we discuss some of the important issues related to the diagnosis and management of these complications.
Definition and pathogenetic features of MF
The current diagnostic criteria for PMF were defined by the World Health Organization in 2008 and are depicted in Table 1.16 Available evidence indicates that PMF is a bona fide clonal stem cell malignancy.17 MPNs comprise clonal hematologic diseases that are thought to arise from a transformation of a hematopoietic stem cell. The notion of “clonality” gained popularity in 1974 due to the astute seminal observations of Prchal and Axelrad,18 and thereafter was confirmed by Fialkow et al,19,20 as well as various other investigators.21 Currently, in contrast to our detailed understanding of chronic myeloid leukemia pathogenesis, which is defined by a single causative molecular lesion, the BCR-ABL1 fusion gene, we only have some essential clues to the molecular pathogenetic mechanisms for PV, ET, and PMF. A major clue was the recognition of increased signaling through the JAK-signal transducer and activator of transcription (STAT) pathway, comprised of JAKs and STATs, as well as through the phosphatidylinositol 3-kinase (PI3K)-AKT (also known as protein kinase B) pathway in erythroid and myeloid cells.22–24 The most significant clue to date came in 2005 with the identification of the somatic mutation JAK2V617F.25–28 This mutation in JAK2 exon 14, which occurs in at least 95% of patients with PV and about 60% of those with PMF and ET, results in a valine (V) to phenylalanine (F) substitution at codon 617.29 This codon is located in the JH2 pseudokinase domain of JAK2, and the mutation is generally considered to negatively affect the JH2-mediated auto-inhibitory functionality of the enzyme, resulting in constitutive activation of the tyrosine kinase function. This in turn results in dysregulation of JAK-dependent signal transduction and activation of multiple downstream effectors, including STAT3 and STAT5.13,30 Dysregulated JAK-STAT signaling is now recognized as the central mechanism of MF pathobiology31 beyond aberrant myeloproliferation (Figures 1 and 2). For example, the efficacy of JAK inhibitors appears to play a role, in large part, in the reversal of secondary disease-related phenomena, such as inflammation and cachexia,11,32 that have no obvious relationship to myeloproliferation but are primary drivers of the MF symptom burden.
  | Table 1 World Health Organization (WHO) diagnostic criteria for primary myelofibrosis (PMF)16 |
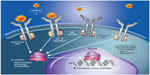  | Figure 1 Pathogenic mechanisms in myelofibrosis involving dysregulated JAK-STAT signaling. Mutations affecting cytokine receptor function (eg, MPL mutations causing ligand-autonomous activation of the thrombopoietin receptor) or JAK2 mutations resulting in constitutive JAK2 activity lead to over-activation of JAK-STAT signaling in hematopoietic stem cells, with consequent myeloproliferation and excess production of proinflammatory cytokines.108 |
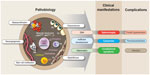  | Figure 2 Pathobiology, main clinical manifestations, and common complications of myelofibrosis. |
Although JAK2V617F is the most prevalent somatic mutation among patients with MF, a large proportion of patients with MF are JAK2V617F negative and, even in those who are JAK2V617F positive, the mutation is unlikely to be the disease-initiating event.33,34 An increasing number of mutations that directly or indirectly affect JAK-STAT signaling are being implicated in the pathobiology of MPNs, including PMF,33–35 revealing an unexpected genetic and epigenetic complexity of these neoplasms.29,36 Notably, findings of multiple mutations in the same patient33 are consistent with the notion that MPNs are multiclonal diseases with as yet poorly understood clonal hierarchies.37–39
In 2006, mutations in exon 10 of the thrombopoietin receptor gene MPL (myeloproliferative leukemia virus oncogene) were noted in some patients with JAK2V617F-negative MPNs,40 and further work confirmed their pathogenicity.33 In 2007, mutations within exon 12 of JAK2 were also described in some patients with JAK2V617F-negative MPNs, but the precise role in the pathogenicity of MPNs remains enigmatic.41,42 To date, mutations in at least 12 genes have been described that can be involved in MPN pathogenesis.43 In addition, recent work has established the important contribution of STAT5 to the molecular pathogenesis of MPNs,44 confirming the central role of the STAT family of phosphorylation-regulatable nuclear transcription factors in the induction and sustenance of excessive proliferation of clonal myeloid progenitor cells in these neoplasms, including MF. Clearly, much has been learned about the molecular biology of MPNs, but the precise causative (“initiator” culprit) lesion remains, for the moment, an enigma.34,45
Natural history of MF
The evolution of bone marrow fibrosis, a cardinal feature of MF, is poorly understood. It is thought to represent a polyclonal reaction to several cytokines, particularly transforming growth factor-β, basic fibroblast growth factor, epidermal growth factor, platelet-derived growth factor, vascular endothelial growth factor, and calmodulin.46 These moieties are mostly locally produced from the malignant clone throughout its various stages of aberrant differentiation (megakaryocytes, monocytes, or both).47 The effects of these growth factors are mainly para- and autocrine; however, in advanced MF, as well as advanced PV and ET, progression of fibrosis appears to involve increasingly altered crosstalk between hematopoietic and stromal cells, resulting in the liberation of fibrogenic cytokines and the escape of malignant stem cells in the circulation with consequent EMH.48,49 Efforts are underway to thoroughly study other candidate factors involved in the development of fibrosis in patients with MPNs. Results of a recent study suggest that the risk of patients with PV developing PPV-MF is increased significantly by high (>50%) JAK2V617F allele burden.50
Results of a recent study suggest that the bone marrow fibrosis grade (determined according to the European consensus on grading bone marrow fibrosis)51 may have prognostic value in patients with PMF,52 and thus may deserve attention during a patient’s risk assessment when allogeneic hematopoietic stem cell transplantation (allo-SCT) is considered. However, although allo-SCT may provide rapid regression of bone marrow fibrosis in some patients53 and at present is the only potentially curative therapy,54 it is also associated with high risks of relapse, morbidity, and mortality.55–57
Transformation into secondary acute leukemia occurs in a small minority of patients with MPNs; it typically involves the myeloid lineages (secondary acute myeloid leukemia [sAML]) but, rarely, lymphoid transformation (secondary acute lymphoid leukemia) also may occur. Patients who develop acute leukemia have a median survival time of less than 3 months.58 Leukemic transformation is rare in patients with non-fibrotic MPNs59 but common in patients with MF.3,58,60 The 10-year risk of leukemic transformation for patients with PMF has been estimated at 12%–31%, depending on the presence of thrombocytopenia and/or unfavorable karyotype.61 A peripheral blood blasts count of ≥2% has been identified as an independent predictor of poor leukemia-free survival.62 The pathogenetic events leading to leukemic transformation are poorly understood, but the presence of IDH (isocitrate dehydrogenase) mutations has been shown to be a significant independent prognostic factor of leukemic transformation.35 In contrast, the presence of JAK2V617F is not essential for nor prognostic of leukemic transformation,63,64 with various case reports indicating that JAK2V617F allele burden may diminish or completely disappear during leukemic transformation of JAK2V617F-positive chronic-phase MF.63,65–67 It has been shown that exposure to select cytoreductive agents may increase the risk of leukemic transformation, possibly by causing additional genetic and/or epigenetic lesions.68,69
Clinical presentation and complications of MF
The median age of patients with MF at the time of diagnosis is approximately 65 years.3,60 In the USA, MF appears to affect slightly more men than women.60 Until recently, it was estimated that up to 21% of all patients with MF are asymptomatic at the time of diagnosis, which is often made by the determination of an abnormal blood count, usually indicating anemia, or an abnormal peripheral blood smear demonstrating leukoerythroblastosis, or after a routine physical examination revealing splenomegaly and sometimes hepatomegaly.70 However, the results of recently published, Internet-based symptom surveys among patients with MPNs showed that the great majority (ie, more than 80%) of patients with MF experienced at least one disease-related symptom at the time of the survey.71,72 The most common presenting symptoms are constitutional symptoms and the consequences of ineffective hematopoiesis.2,73 Fatigue is extremely common; it is often relentless, chronic, and profoundly debilitating, and is also seen in patients who do not have anemia.71
Constitutional symptoms, including fever, night sweats, pruritus, and weight loss, are thought to result from the abnormal production of cytokines.13 Such symptoms not only tend to have a negative impact on a patient’s quality of life (QOL)71 but also have been firmly associated with a poor prognosis for overall survival.3 Both hepatomegaly and splenomegaly are considered fundamentally characteristic of MF and to be largely, although not exclusively, due to EMH. Splenomegaly may result from splenic sequestration of immature myeloid cells or other ill-defined mechanisms. Indeed, palpable splenomegaly (of any degree, from merely palpable to massive) has been noted in more than 80% of non-selected patients with MF3 and can lead to a range of physical complaints, including generalized abdominal discomfort, left upper quadrant/subcostal pain, and early satiety. As splenomegaly worsens, it is not uncommon to witness patients developing severe generalized abdominal pain and sometimes an “acute abdomen”-like clinical picture; some patients may also experience splenic infarcts.74
Progressive bone marrow fibrosis leads to a “myelophthisic” phenotype with worsening cytopenias, particularly thrombocytopenia and anemia.70,75,76 The latter typically results in fatigue, weakness, palpitations, compensatory tachycardia, bone pain, and dyspnea (either exertional or at rest), and may lead to (or exacerbate) symptoms of tissue hypoxia in patients with vasculopathies, such as atherosclerotic cardio-, cerebro-, and renovascular disease, or in those with pre-existing heart failure (combination of hypo-oxygenation and hypoperfusion).77 Anemia also can negatively affect pulmonary function parameters in patients with various pre-existing lung diseases.78 The above considerations are clinically meaningful and important because patients with MF tend to have significant general medical comorbidities owing to their (on average) advanced age. The consequences of thrombocytopenia, which may include bleeding of any grade/severity, can be compounded by acquired platelet dysfunction and sometimes a state of low-grade disseminated intravascular coagulation, resulting occasionally in a complex thrombohemorrhagic picture.79 These latter manifestations may be catastrophic and are potentially life-threatening, particularly in the presence of portal hypertension. Moreover, MF is associated with an increased susceptibility to infections (viral, bacterial, and atypical),70,76 even if there is no evidence of frank leukopenia/neutropenia.80
Hepatomegaly, which in various studies has been observed in 39%–65% of patients with MF at diagnosis,3,4,76 may result in abnormal liver function tests, coagulopathy, and increased abdominal complaints.81 MF is the most common cause of massive splenomegaly82 and, with increased hepatic blood flow (or intrahepatic venous obstruction/stasis), marked splenomegaly may result in portal hypertension.83 This complication is noted in about 7% of patients with MF84 and may present with ascites and esophageal or gastric varices; bleeding in these varices may lead to catastrophic hemorrhage. Splanchnic (portal, mesenteric, or splenic) vein thrombosis and associated complications (eg, Budd–Chiari syndrome) also may occur in MF, although they do so less often in MF than in PV or ET.85 Interestingly, although hyperplasia of Kupffer cells has been detected in MF, neither hepatic stellate cell activation nor hepatic parenchymal fibrosis are features of this malignancy.86 In addition, EMH can result in a wide range of complications other than hepatosplenomegaly, depending on the specific organ involved, with potentially life-threatening consequences. EMH affecting the central nervous system may lead to intracranial hypertension, which in turn may result in chronic headaches, delirium, photophobia, papilledema, gait instability, alterations in the level of consciousness and, rarely, coma, paralysis, and/or death. In cases in which EMH develops in a paraspinal location, spinal cord compression may ensue, occasionally presenting as acute cord or spinal root/nerve compression syndrome.87 Involvement of lymph nodes by this process can lead to generalized lymphadenopathy and, in advanced cases, lymphoceles. Pleural infiltration by EMH may result in pleural effusions and hemothorax.88
EMH in the gastrointestinal tract manifests itself as exacerbation of already existing abdominal pain and/or intestinal lumen obstruction. Rarely, obstructive uropathy may result from EMH infiltration of the kidneys, ureters, and the bladder neck,84 which may lead to renal failure. Other very rare secondary complications of EMH include gastric outlet obstruction, bile duct obstruction, acalculous cholecystitis from gallbladder infiltration, arthritis from synovial involvement, and renal colic from intrarenal or intra- or periureteric obstruction. Skin manifestations of EMH are quite rare and may include erythematous plaques, nodules, ulcers, bullae, myeloid leukemic-like infiltrates, and even neutrophilic dermatosis (Sweet’s syndrome).87,89 The risk of thromboembolic events in patients with MF (particularly PMF) appears to be considerably lower than that in patients with PV or ET. Nonetheless, cardiovascular, thromboembolic, and hemorrhagic complications are common in patients with MF,70,76 and several investigators reported thrombohemorrhagic events to be among the principal causes of death in patients with MF.3,4 The risk of leukemic transformation (mainly to sAML) appears to increase with time, and patients with sAML have a grim prognosis.58,61 Very seldom, leukemic transformation can present with granulocytic sarcomas (also known as “chloromas” in the earlier literature) at any anatomical site.90,91
Prognosis and MF-associated complications
MF is clearly associated with substantial and increasingly burdensome morbidity, which has a very significant negative impact on patients’ QOL and is associated with a substantial long-term risk (which increases over time) of developing potentially life-threatening complications. Because the median age of patients with MF is around 65 years at diagnosis, disease-associated complications are often compounded by concurring medical conditions such as diabetes, hypertension, atherosclerotic or pulmonary disease, and obesity. Current estimates suggest an overall survival of about 5 years for patients with intermediate-risk disease, and less than 2 years for those with high-risk disease.61 Some patients, in particular those with low-risk disease, may survive for longer periods, but as the disease progresses, they often experience relentlessly progressive debilitating symptoms, inexorably worsening QOL, and increasing disability. Therefore, it is prudent to assess individual patients carefully and devise a treatment plan commensurate with each individual patient’s risk stratification and symptoms. The only treatment that can accord long-term remission and possible cure is allo-SCT. This treatment is available to a small minority (around 3%) of eligible patients with MF and carries significant risks and complications.54,74
Over the past two decades, efforts have been made to identify clinical and laboratory parameters that are independently associated with prognosis in MF.3,4,61 The principal candidate prognostic parameters have been age at diagnosis, constitutional symptoms, abnormal karyotype, anemia, thrombocytopenia, low reticulocyte count, peripheral blood CD (cluster of differentiation) 34+ progenitor cells, monocytosis, and peripheral blood blasts. Interestingly, the presence of hepatosplenomegaly has not been included in these prognostication systems. The most widely accepted prognostic scale so far is based on the 2009 International Working Group for Myelofibrosis Research and Treatment initiative and is known as the “International Prognostic Scoring System” (IPSS).3 The IPSS validated the following parameters at diagnosis to be associated with a worse prognosis: age >65 years, presence of constitutional symptoms, hemoglobin <10 g/dL, leukocyte count >25 × 109/L, and ≥1% circulating blasts.3 Compared with the IPSS, the Dynamic International Prognostic Scoring System gives more prognostic weight to the presence of disease-related anemia and is valid not only at diagnosis but at any time during disease progression.4 Recent efforts have established the additional value of abnormal karyotype as an independent negative prognostic factor for overall and leukemia-free survival.61 Conversely, patients with a normal karyotype seem to have a very low risk of transformation to acute leukemia. Nonetheless, even for these patients, substantial residual risk of shortened overall survival remains, suggesting that multiple and diverse clinical events (other than leukemic transformation) contribute to the morbidity and, indeed, the mortality associated with MF. Some of the mechanisms responsible for the emergence of complications in patients with MF (as delineated here) are summarized in Figure 3.
Management of MF-associated complications
Until recently, best available pharmacotherapy for MF consisted of the use of conventional agents that generally had limited and non-lasting efficacy and were often poorly tolerated, including cytoreductive agents such as hydroxyurea (hydroxycarbamide), and immunomodulatory agents.92 Thus, treatment of MF was essentially palliative, without durably effective options for the alleviation of major clinical manifestations such as splenomegaly and MF-associated symptoms. The discovery of the essential role of dysregulated JAK-STAT signaling in the pathobiology of MF led to the development of JAK inhibitors as novel targeted therapies. The recent approval of ruxolitinib in MF in many countries – including the USA, where it is approved for the treatment of intermediate- or high-risk MF – is a validation of JAK inhibitor therapy in MF and a milestone in the development of targeted therapies for this disease. In light of the complexity of MF pathogenetics, experimental therapies targeting additional disease mechanisms are likely to have future roles in MF disease management.92 Table 2 is a summary of current management options for MF-associated complications that highlights the benefits of JAK-targeted therapy.
Ruxolitinib is currently the only approved treatment for patients with MF that has been shown in pivotal randomized clinical trials to be highly effective in alleviating symptom burden and splenomegaly.11,12 In addition, ruxolitinib has been shown to reduce hepatomegaly in patients with prior splenectomy,93 to mitigate cachexia-related weight loss and hypocholesterolemia in MF,32 and to reduce the levels of cytokines driving systemic inflammation in this malignancy.11,13 Preliminary data for experimental therapies suggest that a number of JAK inhibitors currently in development also have the capacity to reduce splenomegaly.94–96 Although hydroxyurea may reduce splenomegaly in some patients with mostly non-massive splenomegaly, its benefit is usually of short duration and tolerability is poor.97 Results of a randomized Phase III study showed that best available therapy, including the use of hydroxyurea in 47% of the patients, was significantly less effective than ruxolitinib in reducing MF-associated splenomegaly or improving several cardinal indices of health-related QOL.12 Splenectomy is an option for patients with refractory symptomatic splenomegaly and/or portal hypertension but should only be considered if the qualifying patient has an adequate life expectancy.98 Palliative splenic irradiation may be indicated for patients with highly symptomatic splenomegaly and adequate platelet count; however, the benefits are usually transient and, in some cases, profound and prolonged cytopenias may develop.98
Currently available therapies have no or limited efficacy in the treatment of MF-related anemia. Thus, management of anemia largely depends on supportive care and the use of androgens or erythropoietin-stimulating agents. Immunomodulators, such as thalidomide and lenalidomide, may be effective in select patients but are generally poorly tolerated in the long term. A recent study of pomalidomide in patients with MF and significant anemia found that dosing was severely limited by tolerability, and treatment at tolerable doses was only moderately effective.99 In addition, the manufacturer of pomalidomide recently announced in a press release that a Phase-3 double-blind, placebo-controlled study of pomalidomide in persons with myeloproliferative-neoplasm-associated myelofibrosis and red blood cell (RBC)-transfusion-dependence myelofibrosis and RBC-transfusion-dependence (RESUME)100 did not meet its primary end point.101 JAK inhibitors, with the possible exception of CYT387,96 appear to have no beneficial effect on MF-related anemia, and, currently, no therapies in development clearly demonstrate efficacy in mitigating MF-related thrombocytopenia. Treatment of additional complications of MF, such as thrombosis, bleeding, portal hypertension, infections, chronic inflammation, and sAML consists mostly of supportive care and/or palliative therapies that are specific for the type of complication.
Because of the essential role of JAK2-STAT signaling in definitive hematopoiesis, decreases in blood cell counts are an expected effect of JAK2 inhibition. Thus, thrombocytopenia, and to a lesser extent anemia, are the most common adverse events associated with ruxolitinib therapy. However, the frequency and severity of these treatment-related cytopenias can be reduced by careful individual patient management. Consequently, the rates of grade 3 and 4 events of thrombocytopenia or anemia tend to be highest within the first 8–12 weeks of therapy,11 and, in the two Phase III studies, cytopenias were rarely a cause for discontinuation of ruxolitinib therapy.11,12 Effective management of ruxolitinib-related thrombocytopenia requires initial dose titration, monitoring of platelet counts, and appropriate dose adjustments based on serially tested platelet counts.10 Treatment-related decreases in hemoglobin level usually occur within the first 8–12 weeks after treatment initiation. However, hemoglobin levels generally recover with appropriate dose modifications and/or the use of RBC transfusions, on average returning to near baseline values by week 24 of therapy.11
Conclusion
Many efforts since the seminal description of the mutations affecting JAK2 and the enhanced understanding of the universal presence of a dysregulated JAK-STAT signaling pathway in MF (even in the absence of a JAK2 mutation) have led to a wide array of therapeutic agents being studied clinically in this malignancy, ranging from adenosine triphosphate-competitive inhibitors of JAKs (eg, ruxolitinib) to immunomodulatory agents (eg, lenalidomide) and other novel approaches.92 Current experience confirms the notion that treating patients with intermediate- and high-risk MF (either primary or secondary) with the JAK1 and JAK2 inhibitor ruxolitinib has an overall favorable benefit–risk profile. Robust clinical trial data11–13 have led to the drug receiving regulatory approval in health authorities in many countries, including the USA,10 Canada,102 and the European Union.103 The drug has a notable clinical impact on the management of patients with intermediate- or high-risk MF, with the majority achieving durable symptomatic responses and reduction of splenomegaly. Moreover, recent updates from two prospective, randomized, Phase III studies showed that patients with MF treated with ruxolitinib had improved survival over placebo (COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment: The COMFORT-I Trial [COMFORT-I])14,104 and best available therapy (COntrolled MyeloFibrosis study with ORal Janus-associated kinase [JAK] inhibitor Treatment-II: The COMFORT-II Trial [COMFORT-II]),15,105 suggesting an overall survival benefit. The median follow-up period for data reported to date remains relatively short for either trial (approximately 2 years), and there are indeed numerous challenges, both clinical and scientific, that need further elucidation, including the precise mode of action of ruxolitinib in this malignancy.
Interestingly, recent data from an exploratory analysis of bone marrow trephine biopsies in patients with MF treated at the MD Anderson Cancer Center who participated in the Phase I/II trial of ruxolitinib10,106 raise the possibility that JAK inhibitor therapy with ruxolitinib for a minimum of 2 years may retard the progression of bone marrow fibrosis in some patients.107 Long-term hydroxyurea therapy had no comparable benefit in a European cohort of patients with MF. Data from randomized controlled studies are needed to substantiate a positive effect of ruxolitinib therapy on bone marrow fibrosis.
In conclusion, given the clinical efficacy and safety profile of ruxolitinib treatment in intermediate- and high-risk MF and with several other candidate agents of the same class currently under development,92 the focus of the treating clinician should now be the prompt identification and effective preventive management of MF-associated complications.
Acknowledgments
Assistance with editing an advanced draft of the manuscript was provided by Roland Tacke, PhD, of Evidence Scientific Solutions, and funded by Incyte Corporation.
Disclosure
TIM consults for Incyte Corporation and is on the speakers’ bureau for Bristol-Myers Squibb. SV has received research support from Incyte, Bristol-Myers Squibb, AstraZeneca, NS Pharma, Roche, Celgene, Gilead, Infinity, Exelixis, YM Bioscience, S*Bio, Geron, and Lilly. NJS and KV are employees of Incyte Corporation.
References
Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112(6):2190–2198. | |
Gregory SA, Mesa RA, Hoffman R, Shammo JM. Clinical and laboratory features of myelofibrosis and limitations of current therapies. Clin Adv Hematol Oncol. 2011;9(9 Suppl 22):1–16. | |
Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. | |
Passamonti F, Cervantes F, Vannucchi AM, et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood. 2010;116(15):2857–2858. | |
Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. Epub July 29, 2013. | |
Aksoy M. Malignancies due to occupational exposure to benzene. Haematologica. 1980;65(3):370–373. | |
Tondel M, Persson B, Carstensen J. Myelofibrosis and benzene exposure. Occup Med (Lond). 1995;45(1):51–52. | |
Bosch X, Campistol JM, Montoliu J, Revert L. Myelofibrosis and focal segmental glomerulosclerosis associated with toluene poisoning. Hum Toxicol. 1988;7(4):357–361. | |
Mesa RA, Green A, Barosi G, Verstovsek S, Vardiman J, Gale RP. MPN-associated myelofibrosis (MPN-MF). Leuk Res. 2011;35(1):12–13. | |
Jakafi® (Ruxolitinib) tablets, for oral use [prescribing information]. Wilmington, DE: Incyte Corporation. Available from: http://www.incyte.com/products/uspi_jakafi.pdf. Accessed September 9, 2013. | |
Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012; 366(9):799–807. | |
Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. | |
Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. | |
Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib treatment in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. Epub 2013 Sept 13. | |
Cervantes F, Kiladjian JJ, Niederwieser D, et al. Long-term safety, efficacy, and survival findings from Comfort-II, a phase 3 study comparing ruxolitinib with best available therapy (BAT) for the treatment of myelofibrosis (MF). Blood (ASH Annual Meeting Abstracts). 2012;120(21):Abstract 801. | |
Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. | |
Lataillade JJ, Pierre-Louis O, Hasselbalch HC, et al; French INSERM and the European EUMNET Networks on Myelofibrosis. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood. 2008;112(8):3026–3035. | |
Prchal JF, Axelrad AA. Bone marrow responses in polycythemia vera. N Engl J Med. 1974;290(24):1382. | |
Adamson JW, Fialkow PJ, Murphy S, Prchal JF, Steinmann L. Polycythemia vera: stem-cell and probable clonal origin of the disease. N Engl J Med. 1976;295(17):913–916. | |
Fialkow PJ, Faguet GB, Jacobson RJ, Vaidya K, Murphy S. Evidence that essential thrombocythemia is a clonal disorder with origin in a multipotent stem cell. Blood. 1981;58(5):916–919. | |
Xu M, Bruno E, Chao J, et al. The constitutive mobilization of bone marrow-repopulating cells into the peripheral blood in idiopathic myelofibrosis. Blood. 2005;105(4):1699–1705. | |
Röder S, Steimle C, Meinhardt G, Pahl HL. STAT3 is constitutively active in some patients with Polycythemia rubra vera. Exp Hematol. 2001;29(6):694–702. | |
Ugo V, Marzac C, Teyssandier I, et al. Multiple signaling pathways are involved in erythropoietin-independent differentiation of erythroid progenitors in polycythemia vera. Exp Hematol. 2004;32(2):179–187. | |
Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118(7):1723–1735. | |
Baxter EJ, Scott LM, Campbell PJ, et al; Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. | |
James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. | |
Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352(17):1779–1790. | |
Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. | |
Cross NC. Genetic and epigenetic complexity in myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2011;2011:208–214. | |
Oku S, Takenaka K, Kuriyama T, et al. JAK2 V617F uses distinct signalling pathways to induce cell proliferation and neutrophil activation. Br J Haematol. 2010;150(3):334–344. | |
Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10(2):127–140. | |
Mesa RA, Verstovsek S, Gupta V, et al. Improvement in weight and total cholesterol and their association with survival in ruxolitinib-treated patients with myelofibrosis from COMFORT-I. Blood (ASH Annual Meeting Abstracts). 2012;120(21):Abstract 1733. | |
Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24(6):1128–1138. | |
Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32(21):2601–2613. | |
Tefferi A, Jimma T, Sulai NH, et al. IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: clinical evidence for leukemogenic collaboration with JAK2V617F. Leukemia. 2012;26(3):475–480. | |
Vannucchi AM, Biamonte F. Epigenetics and mutations in chronic myeloproliferative neoplasms. Haematologica. 2011;96(10):1398–1402. | |
Schaub FX, Looser R, Li S, et al. Clonal analysis of TET2 and JAK2 mutations suggests that TET2 can be a late event in the progression of myeloproliferative neoplasms. Blood. 2010;115(10):2003–2007. | |
Rumi E, Harutyunyan A, Elena C, et al. Identification of genomic aberrations associated with disease transformation by means of high-resolution SNP array analysis in patients with myeloproliferative neoplasm. Am J Hematol. 2011;86(12):974–979. | |
Hummel JM, Kletecka MC, Sanks JK, et al. Concomitant BCR-ABL1 translocation and JAK2(V617F) mutation in three patients with myeloproliferative neoplasms. Diagn Mol Pathol. 2012;21(3):176–183. | |
Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270. | |
Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356(5):459–468. | |
Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood. 2011;117(10):2813–2816. | |
Abdel-Wahab O, Pardanani A, Bernard OA, et al. Unraveling the genetic underpinnings of myeloproliferative neoplasms and understanding their effect on disease course and response to therapy: proceedings from the 6th International Post-ASH Symposium. Am J Hematol. 2012;87(5):562–568. | |
Walz C, Ahmed W, Lazarides K, et al. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and JAK2(V617F) in mice. Blood. 2012;119(15):3550–3560. | |
Constantinescu SN, Vainchenker W. Small-molecule inhibitors in myeloproliferative neoplasms: are we aiming for the right targets? Hematology Am Soc Hematol Educ Program. 2012;2012:553–560. | |
Le Bousse-Kerdilès MC, Martyré MC, Samson M. Cellular and molecular mechanisms underlying bone marrow and liver fibrosis: a review. Eur Cytokine Netw. 2008;19(2):69–80. | |
Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005;23(33):8520–8530. | |
Kreipe H, Büsche G, Bock O, Hussein K. Myelofibrosis: molecular and cell biological aspects. Fibrogenesis Tissue Repair. 2012;5 Suppl 1:S21. | |
Le Bousse-Kerdilès MC. Primary myelofibrosis and the “bad seeds in bad soil” concept. Fibrogenesis Tissue Repair. 2012;5 Suppl 1:S20. | |
Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24(9):1574–1579. | |
Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–1132. | |
Gianelli U, Vener C, Bossi A, et al. The European Consensus on grading of bone marrow fibrosis allows a better prognostication of patients with primary myelofibrosis. Mod Pathol. 2012;25(9):1193–1202. | |
Kröger N, Kvasnicka M, Thiele J. Replacement of hematopoietic system by allogeneic stem cell transplantation in myelofibrosis patients induces rapid regression of bone marrow fibrosis. Fibrogenesis Tissue Repair. 2012;5 Suppl 1:S25. | |
Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood. 2012;120(7):1367–1379. | |
Patriarca F, Bacigalupo A, Sperotto A, et al; GITMO. Allogeneic hematopoietic stem cell transplantation in myelofibrosis: the 20-year experience of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Haematologica. 2008;93(10):1514–1522. | |
Ballen KK, Shrestha S, Sobocinski KA, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2010;16(3):358–367. | |
Zang DY, Deeg HJ. Allogeneic hematopoietic cell transplantation for patients with myelofibrosis. Curr Opin Hematol. 2009;16(2):140–146. | |
Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105(3):973–977. | |
Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep. 2012;7(1):78–86. | |
Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc. 2012;87(1):25–33. | |
Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392–397. | |
Tefferi A, Pardanani A, Gangat N, et al. Leukemia risk models in primary myelofibrosis: an International Working Group study. Leukemia. 2012;26(6):1439–1441. | |
Lopes da Silva R, Ribeiro P, Lourenço A, et al. What is the role of JAK2(V617F) mutation in leukemic transformation of myeloproliferative neoplasms? Lab Hematol. 2011;17(1):12–16. | |
Helbig G, Wieczorkiewicz A, Woźniczka K, Wiśniewska-Piaty K, Rusek A, Kyrcz-Krzemień S. The JAK2V617F tyrosine kinase mutation has no impact on overall survival and the risk of leukemic transformation in myelofibrosis. Med Oncol. 2012;29(4):2379–2384. | |
Kreft A, Springer E, Lipka DB, Kirkpatrick CJ. Wild-type JAK2 secondary acute erythroleukemia developing after JAK2-V617F-mutated primary myelofibrosis. Acta Haematol. 2009;122(1):36–38. | |
Wu YY, Hung HM, Chen TS, Chao TY, Ho CL. Decreased JAK2 V617F allele burden level in a myelofibrosis with myeloid metaplasia patient with leukemic transformation. Leuk Res. 2008;32(11):1783–1786. | |
Theocharides A, Boissinot M, Girodon F, et al. Leukemic blasts in transformed JAK2-V617F-positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood. 2007; 110(1):375–379. | |
Nielsen I, Hasselbalch HC. Acute leukemia and myelodysplasia in patients with a Philadelphia chromosome negative chronic myeloproliferative disorder treated with hydroxyurea alone or with hydroxyurea after busulphan. Am J Hematol. 2003;74(1):26–31. | |
Kiladjian JJ, Chevret S, Dosquet C, Chomienne C, Rain JD. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol. 2011;29(29):3907–3913. | |
Varki A, Lottenberg R, Griffith R, Reinhard E. The syndrome of idiopathic myelofibrosis. A clinicopathologic review with emphasis on the prognostic variables predicting survival. Medicine (Baltimore). 1983;62(6):353–371. | |
Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76. | |
Johansson P, Mesa R, Scherber R, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma. 2012;53(3):441–444. | |
Abdel-Wahab OI, Levine RL. Primary myelofibrosis: update on definition, pathogenesis, and treatment. Annu Rev Med. 2009;60:233–245. | |
Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–770. | |
Michiels JJ. Clinical, pathological and molecular features of the chronic myeloproliferative disorders: MPD 2005 and beyond. Hematology. 2005;10 Suppl 1:215–223. | |
Hasselbalch H. Idiopathic myelofibrosis: a clinical study of 80 patients. Am J Hematol. 1990;34(4):291–300. | |
Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120(6):1185–1196. | |
Ouellette DR. The impact of anemia in patients with respiratory failure. Chest. 2005;128(5 Suppl 2):576S–582S. | |
Prentice CR. Acquired coagulation disorders. Clin Haematol. 1985;14(2):413–442. | |
Wolach B, Gavrieli R, Manor Y, Lishner M. Leukocyte function in chronic myeloproliferative disorders. Blood Cells Mol Dis. 1998;24(4):544–551. | |
Pereira A, Bruguera M, Cervantes F, Rozman C. Liver involvement at diagnosis of primary myelofibrosis: a clinicopathological study of twenty-two cases. Eur J Haematol. 1988;40(4):355–361. | |
O’Reilly RA. Splenomegaly at a United States County Hospital: diagnostic evaluation of 170 patients. Am J Med Sci. 1996;312(4):160–165. | |
Alvarez-Larrán A, Abraldes JG, Cervantes F, et al. Portal hypertension secondary to myelofibrosis: a study of three cases. Am J Gastroenterol. 2005;100(10):2355–2358. | |
Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342(17):1255–1265. | |
Anger BR, Seifried E, Scheppach J, Heimpel H. Budd-Chiari syndrome and thrombosis of other abdominal vessels in the chronic myeloproliferative diseases. Klin Wochenschr. 1989;67(16):818–825. | |
Bioulac-Sage P, Roux D, Quinton A, Lamouliatte H, Balabaud C. Ultrastructure of sinusoids in patients with agnogenic myeloid metaplasia. J Submicrosc Cytol. 1986;18(4):815–821. | |
Koch CA, Li CY, Mesa RA, Tefferi A. Nonhepatosplenic extramedullary hematopoiesis: associated diseases, pathology, clinical course, and treatment. Mayo Clin Proc. 2003;78(10):1223–1233. | |
Nadrous HF, Krowka MJ, McClure RF, Tefferi A, Lim KG. Agnogenic myeloid metaplasia with pleural extramedullary leukemic transformation. Leuk Lymphoma. 2004;45(4):815–818. | |
Altomare G, Capella GL, Frigerio E. Sweet’s syndrome in a patient with idiopathic myelofibrosis and thymoma-myasthenia gravis-immunodeficiency complex: efficacy of treatment with etretinate. Haematologica. 1996;81(1):54–58. | |
Chan AC, Kwong YL, Lam CC. Granulocytic sarcoma of megakaryoblastic differentiation complicating chronic idiopathic myelofibrosis. Hum Pathol. 1996;27(4):417–420. | |
Frohna BJ, Quint DJ. Granulocytic sarcoma (chloroma) causing spinal cord compression. Neuroradiology. 1993;35(7):509–511. | |
Atallah E, Verstovsek S. Emerging drugs for myelofibrosis. Expert Opin Emerg Drugs. 2012;17(4):555–570. | |
Benjamini O, Jain P, Estrov Z, Kantarjian HM, Verstovsek S. Therapeutic effects of ruxolitinib in patients with myelofibrosis without clinically significant splenomegaly. Blood. 2012;120(13):2768–2769. | |
Santos FP, Kantarjian HM, Jain N, et al. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood. 2010;115(6):1131–1136. | |
Pardanani A, Gotlib JR, Jamieson C, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29(7):789–796. | |
Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27(6):1322–1327. | |
Vannucchi AM. Management of myelofibrosis. Hematology Am Soc Hematol Educ Program. 2011;2011:222–230. | |
Mesa RA. How I treat symptomatic splenomegaly in patients with myelofibrosis. Blood. 2009;113(22):5394–5400. | |
Daver N, Shastri A, Kadia T, et al. Modest activity of pomalidomide in patients with myelofibrosis and significant anemia. Leuk Res. Epub July 24, 2013. | |
Celgene Corporation. Phase-3 double-blind, placebo-controlled study of pomalidomide in persons with myeloproliferative-neoplasm-associated myelofibrosis and RBC-transfusion-dependence myelofibrosis and RBC-transfusion-dependence (RESUME). In: ClinicalTrials.gov [website on the Internet]. Bethseda, MD: US National Library of Medicine; 2010 [updated November 13, 2012]. Available from: http://clinicaltrials.gov/show/NCT01178281. NLM identifier: NCT01178281. Accessed September 9, 2013. | |
Celgene Corporation. Celgene reports first quarter 2013 operating and financial results [web page on the Internet]. Summit, NJ: Celgene Corporation; nd. Available from: http://ir.celgene.com/phoenix.zhtml?c=111960&p=irol-newsArticle&ID=1811033&highlight=myelofibrosis. Accessed August 19, 2013. | |
JAKAVI® the first medication to receive health canada approval to treat patients with myelofibrosis [press release]. CNW Group; 2012 [July 5]. Available from: http://www.newswire.ca/en/story/1003655/-pr-jakavi-the-first-medication-to-receive-health-canada-approval-to-treat-patients-with-myelofibrosis. Accessed July 17, 2013. | |
Novartis AG. Novartis drug JAKAVI® first medication to receive European Commission approval to treat patients with myelofibrosis [media release]. Basel: Novartis AG; 2012 [Aug 28]. Available from: http://www.novartis.com/newsroom/media-releases/en/2012/1636508.shtml. Accessed July 17, 2013. | |
Incyte Corporation. COntrolled MyeloFibrosis Study With ORal JAK Inhibitor Treatment: The COMFORT-I Trial. In: ClinicalTrials.gov [website on the Internet]. Bethseda, MD: US National Library of Medicine; 2009 [updated May 13, 2013]. Available from: http://clinicaltrials.gov/show/NCT00952289. NLM identifier: NCT00952289. Accessed September 9, 2013. | |
Novartis Pharmaceuticals. COntrolled MyeloFibrosis study with ORal Janus-associated kinase (JAK) inhibitor Treatment-II: The COMFORT-II Trial. In: ClinicalTrials.gov [website on the Internet]. Bethseda, MD: US National Library of Medicine; 2009 [updated March 12, 2013]. Available from: http://clinicaltrials.gov/show/NCT00934544. NLM identifier: NCT00934544. Accessed September 9, 2013. | |
Incyte Corporation. Open label ruxolitinib (INCB018424) in patients with myelofibrosis and post polycythemia vera/essential thrombocythemia myelofibrosis. In: ClinicalTrials.gov [website on the Internet]. Bethseda, MD: US National Library of Medicine; 2007 [updated October 9, 2012]. Available from: http://clinicaltrials.gov/show/NCT00509899. NLM identifier: NCT00509899. Accessed September 9, 2013. | |
Kvasnicka HM, Thiele J, Bueso-Ramos CE, et al. Exploratory analysis of the effect of ruxolitinib on bone marrow morphology in patients with myelofibrosis. J Clin Oncol. 2013;31(Suppl):Abstract 7030. | |
Vaddi K, Sarlis NJ, Gupta V. Ruxolitinib, an oral JAK1 and JAK2 inhibitor, in myelofibrosis. Expert Opin Pharmacother. 2012;13(16):2397–2407. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.