Back to Journals » Clinical Ophthalmology » Volume 8
Mutations of the CYP1B1 gene in congenital anterior staphylomas
Authors Judaibi R, Abu-Amero K, Morales J, Al Shahwan S, Edward D
Received 18 August 2013
Accepted for publication 25 September 2013
Published 24 February 2014 Volume 2014:8 Pages 445—448
DOI https://doi.org/10.2147/OPTH.S53200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Ramzi Al Judaibi,1 Khaled K Abu-Amero,2,3 Jose Morales,1 Sami Al Shahwan,1 Deepak P Edward1,4
1King Khaled Eye Specialist Hospital, 2Department of Ophthalmology, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia; 3Department of Ophthalmology, College of Medicine, University of Florida, Jacksonville, FL, USA; 4Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore MD, USA
Purpose: Here, we present two patients with congenital anterior staphyloma, with mutations in the CYP1B1 gene.
Methods: We reviewed the medical records, including the genetic analysis.
Results: Two unrelated patients presented with congenital anterior staphylomas. Both patients showed mutations in the CYP1B1 gene. The first patient, the product of a consanguineous marriage, showed a homozygous misssense mutation g.3987G>A (p.G61E). The second patient had compound heterozygous misssense mutations [g.4160 G>T (p.A119S) and g.8131 C>G (p.L432V)].
Conclusion: CYP1B1 gene mutation may be associated with congenital anterior staphylomas.
Keywords: mutation analysis, congenital glaucoma, consanguinity, congenital aphakia
Introduction
Congenital anterior staphyloma is a rare condition characterized by enlarged, opaque, ectatic corneas that protrude through the interpalpebral fissure. This congenital malformation has been described in isolated case reports, but the etiology remains unclear.1 In this paper, we report two cases of congenital staphyloma that were associated with CYP1B1 gene mutations.
Case reports
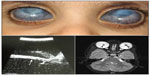
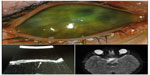
The history, clinical findings at presentation, the clinical course, and findings after genetic testing are summarized in Table 1 and illustrated in Figures 1 and 2.
Discussion
Congenital anterior staphyloma is a rare unilateral or bilateral anomaly and may account for about 11% of congenital corneal opacities.2 The clinical findings include an enlarged, vascularized, ectatic cornea, with thinning and ectasia of the surrounding anterior segment structures. These findings are associated with marked anterior segment anomalies that can include an iris that is adherent to the posterior corneal surface or a partially absent iris. The crystalline lens may be adherent to the posterior corneal surface, similar to Peters anomaly, be subluxed, or show cataractous changes.1,3−5 The degree of staphylomatous changes can be variable. In our patients, the corneal ectasia was not as conspicuous, with the staphylomatous changes being more prominent in the limbal region. Limbal staphylomas can occur due to stretching and thinning of the globe, as a result of high intraocular pressure in severe congenital glaucoma. The clinical course in such patients is progressive, often requiring multiple surgical interventions. In contrast, our patients presented at birth with severe anterior segment changes that remained stable and mild-to-moderate intraocular pressure elevations that appeared controlled with medications. In the differential diagnosis, the pathologies considered included severe primary congenital glaucoma with anterior segment dysgenesis and Peters anomaly.2 There seems to be considerable overlap in the clinical features between these clinical conditions.6 Though the presenting conditions could be considered as a severe form of anterior segment dysgenesis, it was felt that the term was generic since both patients had phenotypic features that were more consistent with those seen in congenital anterior staphylomas. Peters anomaly presents with central corneal opacities with thinning, and with iris adhesions to the posterior surface. Corneal ectasia, staphylomas, and adherence of the crystalline lens have been reported in cases of Peters Plus syndrome with multiple systemic abnormalities.7,8 Both patients in this study showed corneal opacification, ectasia, and vascularization. In addition, our first patient had a dislocated crystalline lenses and the second patient had had congenital aphakia, both of which have been described with Peters anomaly and congenital anterior staphylomas.9,10
Multiple etiological factors have been implicated in the pathogenesis of congenital anterior staphylomas. These include intrauterine infections and specific chromosomal abnormalities with multisystem organ involvement, and an association with amniotic band syndrome has been reported.3,9,11 However, in most cases, the etiology remains undetermined. Both our patients had no family history; however, patient 1 was the product of a consanguineous marriage, alerting us to further look into the association of CYP1B1 mutations with the anterior staphylomas.
We detected a homozygous CYP1B1 mutation g.3987G>A (p.G61E) in patient 1. This mutation is the most common mutation in Saudi Arabian patients with primary congenital glaucoma.12 The mutation was inherited in a homozygous status and was heterozygous in both parents, thus fits well with the fact that both parents were first cousins. A previous study attempting to correlate this mutation with a particular phenotype was unsuccessful.11 This mutation was previously described in Saudi patients with familial juvenile open-angle glaucoma,13 in Peters anomaly,14 and in isolated open-angle glaucoma.15
As for patient 2, he carried compound heterozygous mutations, g.4160 G>T (p.A119S) and g.8131 C>G (p.L432V), which were inherited from his father. Unfortunately, deoxyribonucleic acid (DNA) was not available from the mother, but it is likely that she harbored the other mutation detected in her son. This was consistent with the history that both parents were not related. Both mutations (p.A119S and p.L432V) are less common than the p.G51E, and mutational phenotypes have not been described with the phenotype seen in the two affected patients. Two CYP1B1 mutations detected in our patients (p.G61E and p.A1195) had an established pathogenicity.12 As for the L432V, the literature is inconclusive about its pathogenic role as this mutation has been detected in normal Turkish and Japanese populations, in a heterozygous status with no other mutation in the CYP1B1 gene.16 It is plausible to suggest that this mutation, if inherited in a heterozygous status with no other CYP1B1 mutation, may not cause the development of congenital glaucoma. However, if this mutation is inherited in a homozygous status or in a heterozygous status with another CYP1B1 mutation, then it may be capable of causing the disease.
Homozygous/compound heterozygous mutations in the CYP1B1 gene are typically associated with primary congenital glaucoma.17 Several reports have identified CYP1B1 mutations in patients with other phenotypes, such as isolated Peters or Axenfeld-Rieger anomaly. These phenotypes are typically associated with glaucoma.14,18 Homozygous or compound heterozygous CYP1B1 mutations were identified in eight probands with mild ectropion uvea, partial aniridia, and congenital glaucoma,19 and now this report further expands the ocular phenotype associated with CYP1B1 mutations.
Disclosure
The authors report no conflicts of interest in this work.
References
Lunardelli P, Matayoshi S. Congenital Anterior Staphyloma. J Pediatr Ophthalmol Strabismus. Epub June 25, 2009. | |
Shigeyasu C, Yamada M, Mizuno Y, Yokoi T, Nishina S, Azuma N. Clinical features of anterior segment dysgenesis associated with congenital corneal opacities. Cornea. 2012;31(3):293–298. | |
Stuart JA. Congenital anterior staphyloma. Surv Ophthalmol. 1963;54:393–395. | |
Miller MM, Butrus S, Hidayat A, Wei LL, Pontigo M. Corneoscleral transplantation in congenital corneal staphyloma and Peters’ anomaly. Ophthalmic Genet. 2003;24(1):59–63. | |
Mullaney PB, Risco JM, Heinz GW. Congenital corneal staphyloma. Arch Ophthalmol. 1995;113(9):1206–1207. | |
Verschooten R, Foets B, De Ravel T, Van Ginderdeuren R, Lombaerts R, Casteels I. Clinical spectrum of congenital corneal staphyloma: a case report. Bull Soc Belge Ophtalmol. 2011;(318):7–10. | |
Shimizu R, Saito R, Hoshino K, et al. Severe Peters Plus syndrome-like phenotype with anterior eye staphyloma and hypoplastic left heart syndrome: proposal of a new syndrome. Congenit Anom (Kyoto). 2010;50(3):197–199. | |
Eberwein P, Reinhard T, Agostini H, Poloschek CM, Guthoff R, Auw-Haedrich C. [Intensive intracorneal keloid formation in a case of Peters plus syndrome and in Peters anomaly with maximum manifestation]. Ophthalmologe. 2010;107(2):178–181. German. | |
Schramm C, Rohrbach JM, Reinert S, et al. Amniotic bands as a cause of congenital anterior staphyloma. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):959–965. | |
Trabucchi G, Piantanida A, Bandello F, Freschi M, Nucci P, Brancato R. Congenital aphakia in Peters’ anomaly syndrome. A case report. Acta Ophthalmol Scand. 1997;75(5):595–597. | |
Bessant DA, Anwar K, Khaliq S, et al. Phenotype of autosomal recessive congenital microphthalmia mapping to chromosome 14q32. Br J Ophthalmol. 1999;83(8):919–922. | |
Abu-Amero KK, Osman EA, Mousa A, et al. Screening of CYP1B1 and LTBP2 genes in Saudi families with primary congenital glaucoma: genotype-phenotype correlation. Mol Vis. 2011;17:2911–2919. | |
Khan AO, Al-Abdi L, Mohamed JY, Aldahmesh MA, Alkuraya FS. Familial juvenile glaucoma with underlying homozygous p.G61E CYP1B1 mutations. J AAPOS. 2011;15(2):198–199. | |
Edward D, Al Rajhi A, Lewis RA, Curry S, Wang Z, Bejjani B. Molecular basis of Peters anomaly in Saudi Arabia. Ophthalmic Genet. 2004;25(4):257–270. | |
López-Garrido MP, Sánchez-Sánchez F, López-Martínez F, et al. Heterozygous CYP1B1 gene mutations in Spanish patients with primary open-angle glaucoma. Mol Vis. 2006;12:748–755. | |
Vasiliou V, Gonzalez FJ. Role of CYP1B1 in glaucoma. Annu Rev Pharmacol Toxicol. 2008;48:333–358. | |
Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet. 1997;6(4):641–647. | |
Tanwar M, Dada T, Dada R. Axenfeld-Rieger Syndrome Associated with Congenital Glaucoma and Cytochrome P4501B1 Gene Mutations. Case Rep Med. 2010;2010. | |
Khan AO, Aldahmesh MA, Al-Abdi L, et al. Molecular characterization of newborn glaucoma including a distinct aniridic phenotype. Ophthalmic Genet. 2011;32(3):138–142. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.