Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Multifaceted applications of bile salts in pharmacy: an emphasis on nanomedicine
Authors Elnaggar Y
Received 10 February 2015
Accepted for publication 20 March 2015
Published 12 June 2015 Volume 2015:10(1) Pages 3955—3971
DOI https://doi.org/10.2147/IJN.S82558
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Thomas Webster
Yosra SR Elnaggar
Department of Pharmaceutics, Faculty of Pharmacy, Alexandria University, Alexandria, Egypt
Abstract: The human body has long provided pharmaceutical science with biomaterials of interesting applications. Bile salts (BSs) are biomaterials reminiscent of traditional surfactants with peculiar structure and self-assembled topologies. In the pharmaceutical field, BSs were employed on the basis of two different concepts. The first concept exploited BSs’ metabolic and homeostatic functions in disease modulation, whereas the second one utilized BSs’ potential to modify drug-delivery characteristics, which recently involved nanotechnology. This review is the first to gather major pharmaceutical applications of BSs from endogenous organotropism up to integration into nanomedicine, with a greater focus on the latter domain. Endogenous applications highlighted the role of BS in modulating hypercholesterolemia and cancer therapy in view of enterohepatic circulation. In addition, recent BS-integrated nanomedicines have been surveyed, chiefly size-tunable cholate nanoparticles, BS-lecithin mixed micelles, bilosomes, probilosomes, and surface-engineered bilosomes. A greater emphasis has been laid on nanosystems for vaccine and cancer therapy. The comparative advantages of BS-integrated nanomedicines over conventional nanocarriers have been noted. Paradoxical effects, current pitfalls, future perspectives, and opinions have also been outlined.
Keywords: bile salt, nanomedicine, bilosomes, liposomes, size-tunable nanoparticles
Introduction
Human body homeostasis is maintained by numerous endogenous substrates characterized by unique properties that help them to perform specific functions. For years, bile acids were recognized as biosurfactants with crucial roles in endogenous organotropism. They are facial amphiphiles synthesized in the liver and stored in the gall bladder, and exist as ionized bile salts (BSs) under physiological conditions. The unique molecular structure and interfacial properties of these amphiphilic steroidal compounds have made them an intriguing subject for pure academic research. Their extraordinary emulsifying and solubilizing properties have led to their utilization as delivery systems for medicines and cosmetics as well.1,2 Such natural surfactants are synthesized in the liver from cholesterol that is used as a precursor for primary BSs, mainly chenodeoxycholic acid (CDCA) and cholic acid. During fasting, bile is concentrated in the gall bladder, whereas upon food intake, bile is secreted into the small intestine. In the latter, BS are modified by intestinal bacteria into secondary bile acid (lithocholic acid and deoxycholic acid). Before excretion, bile acids are peptide-bound with glycine (75%) or taurine (25%) and self-assembled into micelles. Their peculiar self-assembly characteristics facilitate their beneficial functions in digestion and absorption of water-insoluble nutrients (fats and fat-soluble vitamins) by dispersing them into mixed micelles (MM).2–4 BSs are later reabsorbed either by active transport from the terminal ileum or passively throughout the whole length of the intestine. When the reabsorption process is complete, BSs recirculate to the liver via the portal vein, undergoing this enterohepatic circulation 6–15 times per day. Throughout the enterohepatic circulation, BSs pass via numerous active and passive transport systems in the liver and intestine.5,6 Major differences in the structure of the common bile acids encompass the number, position, and stereochemistry of hydroxyl groups in the steroid nucleus derived from cholesterol. BSs possess unique characteristics differentiating them from conventional surfactants. Instead of a hydrophilic head and a flexible hydrophobic tail, BSs do not exhibit a typical head–tail structure but planar polarity. BSs are rigid, almost flat molecules with weakly separated hydrophobic and hydrophilic faces. The hydrophobic surface lies on the convex side of the rigid steroid ring system, as shown in Figure 1. The concave side of the molecule contains one, two, or three hydroxyl groups and an amino group that can be conjugated with taurine, glycine, or other amino acids.2,7 Owing to their peculiar structure and self-assembly potential, BSs have versatile pharmaceutical applications. Endogenous homeostasis of BSs has been pharmaceutically exploited to enhance the therapeutic effect of many pharmaceuticals, mainly cholesterol-lowering agents in addition to hydrophobic and nonabsorbable actives. Furthermore, transport systems and metabolic pathways for BSs are currently the subject of intense pharmaceutical research efforts.5,6,8–10 By taking advantage of specific transport systems, the combination of bile acids and drugs can lead to liver-specific pharmaceuticals.1,3
  | Figure 1 Illustrative diagram summarizing (A) BS in human digestive system; (B) chemical structure of deoxycholic acid; (C) schematic representation of facial amphiphilic structure of conjugated deoxycholic acid with hydrophobic convex side and hydrophilic concave side; and (D) BS micelle. |
In the pharmaceutical field, BSs have been exploited to improve hydrophilicity of water-insoluble active pharmaceutical ingredients (such as amphotericin B, resveratrol, and oxaprozin) mainly by the wetting effect.11–13 Furthermore, BSs have been employed as permeation enhancers in topical dosage forms including buccal, ocular, nasal, and transdermal routes of administration.14–17 Besides, the serendipitous discovery of BS gelation tendency led to their use as low-molecular-weight cationic hydrogelators.18 A recent era in pharmaceutical research encompassed fabrication of BS-integrated nanocarriers to overcome major obstacles of drug delivery. Poor oral bioavailability and tumor targeting efficiency constitute the two major concerns of recent nanotechnologies. Versatile nanocarriers have captured our interest to improve the delivery characteristics of drugs, including nanoemulsions, solid lipid nanoparticles (NPs), nanostructured lipid carriers, nanosuspensions, nanogels, liposomes, and modified liposomes.14,19–25 Among others, liposomes constitute the classical type of phospholipid (PL) vesicles recognized as delivery systems of choice for hydrophilic drugs, particularly peptide and proteins. Nevertheless, PL vesicles would suffer digestion in the GIT (gastrointestinal tract) by BSs as lipophilic substrates.23 After oral administration, endogenous BSs will be inserted and partitioned into PL bilayers to form MM. Digestion of bilayers is anticipated to lose the hydrophilic drug moiety incorporated in the vesicular core. In consequence, instability, drug leakage, and low entrapment efficiency are the obstacles to oral use of liposomes. Oral digestibility of liposomes triggered the formulation of modified generations of vesicular systems with higher GIT stability.26,27 Next generations of liposomes with stability include phytosomes23 and, more recently, bilosomes.28,29 Bilosomes are BS-integrated liposomes for improving oral delivery of actives. Bilosomes and modified bilosomes (including probilosomes and surface-engineered bilosomes) demonstrate higher oral stability, protective effect, and permeation-enhancing properties compared with conventional liposomes and niosomes.29–32 Such novel nanocarriers could be employed to improve the oral bioavailability of vaccines, hydrophilic drugs,4,29,33–40 and insoluble actives as well.41 In another avenue, injectable size-tunable cholate NPs have been recently developed for cancer targeting with superior properties to their peer nanocarriers. Cholate NPs enjoy tunable physicochemical properties that help them to achieve higher drug loading, tumor targeting, and lower side effects compared with polymeric and lipid NPs.42,43
However, a close search of the literature has found that there is a dearth of articles dealing with the potential roles of BSs in novel nanocarriers. This review is hence the first to provide a comprehensive overview of endogenous and exogenous pharmaceutical applications of BSs, with particular emphasis on intriguing applications in nanomedicine.
Pharmaceutical applications of endogenous functions of BSs
The dissolved components of bile in humans consist of bile acids (67%), PLs (22%, mainly lecithin), cholesterol (4%), gall pigments (0.3%), and various proteins. In most animals and humans, bile is secreted from hepatocytes into the canaliculi, from where it drains into the bile ducts, hepatic ducts, and finally into the common bile duct and gall bladder.44 The hepatic duct shows many individual anatomical variations before it drains into the common bile duct that could explain the intersubject variation in bile amount and digestion process. In humans and most animals, bile is stored in the gall bladder under high concentrations achieved by constant removal of water and electrolytes. The secretory response is initiated as the acid chyme enters the duodenum and is mediated by secretin and cholecystokinin, which stimulates the gallbladder to contract and secrete bile. Rats have no gall bladder, and hence, there is a continuous flow of more diluted bile directly from the liver into the intestine, which peaks after food consumption.1,44 The human bile acid pool contains about 2.5–5 g of bile acids, consisting mainly (~90%) of cholic acid, CDCA, and deoxycholic acid in the ratio of about 2:2:1. Ursodeoxycholic acid and lithocholic acid are found in the remaining 10% of the pool. MM with PL are usually formed in a PL:bile ratio of 1:3 in humans and 1:10 in rats.
Role in digestion and absorption
Endogenous bile plays a crucial beneficial role in the lipid digestion and absorption process. The digestion process of lipid takes place to a limited extent in the stomach (10%–30%) via gastric lipase, and more significantly in the small intestine (70%–90%) via pancreatic lipase. When the digested food reaches the small intestine, it is mixed with bile and pancreatic secretions in the duodenum, forming an emulsion stabilized by endogenous surfactants (Figure 2). In this regard, BSs have been recognized to cover the interface of lipid nutrients, promoting the lipolysis process.2,45
  | Figure 2 Schematic representation of the absorption and digestion mechanisms of fats and fatty carrier (SEDDS) in the GIT.46 |
Bile salts as absorption enhancers
In the pharmaceutical field, a direct relationship between endogenous bile and bioavailability of poorly soluble drugs can be elucidated. PL–BS MM formed after oral administration usually stand behind enhanced solubilization and absorption of poorly soluble drugs.44 In this quest, Figure 2 demonstrates absorption and digestion mechanisms of fats and fatty carrier self-emulsifying drug delivery system (SEDDS) in the GIT. SEDDS are o/w emulsion preconcentrates that entrap lipophilic actives.21,22,25 Formation of MM with BSs was reported as one of three pathways to facilitate digestion and transport of drug-loaded SEDDS to the enterocytes and subsequent absorption process.46
Various in vivo studies with cannulated bile duct of animals showed improvement in oral bioavailability of xenobiotics in the presence of bile juice.47 van Hasselt et al48 have reported that absorption of Vitamin K from polymeric micelles was not achieved in patients lacking bile production (cholestasis). BSs were found important in extracting the vitamin from the dosage form and solubilizing it. Whereas Kim et al49 reported that BSs were additionally capable of inhibiting the precipitation of furosemide drug solutions owing to its pH-dependent solubility. In view of the prominent impact of BSs on absorption of lipophilic API (Active Pharmaceutical Ingredient), Zhang et al50 investigated the effect of BSs on the intestinal absorption of lipid nanocarriers. Their results showed that BSs could improve Candesartan cellular uptake in Caco-2 cell monolayers via the active processes and the lymphatic targeting mechanism.50 The aforementioned examples demonstrate the beneficial effect of BSs on the oral bioavailability of drugs.
Peculiar structure and self-assembled topologies
This peculiar structure results in a complex self-assembly behavior with very distinct aggregate properties,3,7 in particular, the small size of BS micelles formed at low critical micellar concentration, which is a considerable challenge to measurement techniques.7 Another structural feature distinguishing BSs from conventional amphiphiles is their application as low-molecular-weight gelators.18,51 Aggregates formed at low concentration were attributed to hydrophobic interaction between steroidal domains, whereas aggregates formed by further increase in concentration were reported to exhibit hydrogen bonding between hydroxyl and carboxyl groups of various dimeric BS units.52
BSs exhibit intriguing phase behavior in mixtures with other amphiphiles, as mainly exemplified in BS–PL mixtures commonly utilized in solubilizing hydrophobic actives. According to the PL/BS ratio and characteristics of the resultant system, the BS/PL combination may result in either MM (with hydrophobic core) or BS-containing vesicles with a hydrophilic core and PL bilayer. According to the ratios of the three biliary lipids, different topologies may form and vary in particle size ranging from simple micelles (1–2 nm) to MM (4–10 nm), small unilamellar vesicles (40–100 nm) and, finally, large multilamellar vesicles (300–500 nm).11,53 Endogenous self-assembled aggregates of bile lipids were exploited by pharmaceutical researchers to fabricate BS-integrated nanocarriers, as will be discussed in the following section.
BS-integrated nanomedicines
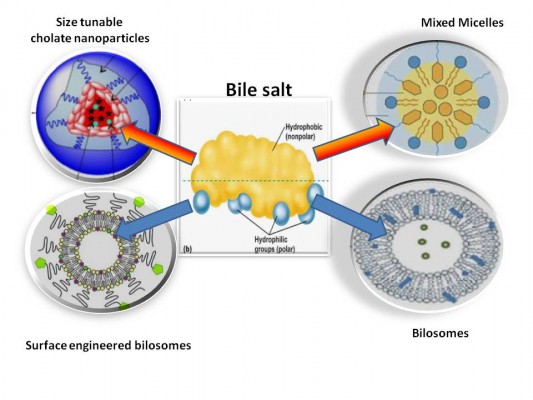
Recently, BSs have been exploited as a core for versatile nanosystems. From a review of the literature, four major categories of BS-integrated nanocarriers can be identified. These include size-tunable cholate NPs, BS–PL MM, bilosomes, and surface-engineered bilosomes (Figure 3).
  | Figure 3 Diagrammatic representation summarizing the four major BS-integrated nanosystems. |
Size-tunable cholate nanocarriers
Recently, a unique class of amphiphilic copolymers was introduced into the field of cancer targeting and gene delivery based on BS core. Such polymers are composed of a hydrophilic linear hydrophilic biodegradable branch (such as PEG [polyethylene glycol], dextran, pullan, chitosan, and PLGA [poly(lactic-co-glycolic acid)]) and a flexible two-arm linear oligomer of cholic acids as a hydrophobic core-forming block. Owing to hydrophilicity of the surface, such amphiphilic structures would spontaneously form self-nanoaggregates in water promoted by intra- and/or intermolecular association between hydrophobic segments to minimize interfacial free energy. Furthermore, the resultant nanocarriers exhibit tunable properties owing to the rigidity of the steroidal polycyclic backbone and the amphiphilic properties of bile acids. Consequently, biodegradable nanocarriers based on bile acids are of captivating potential in the field of drug delivery.54
The biocompatibility and unique amphiphilic structure of cholic acid favored its choice as the building block of copolymers in many studies.42,43,55 Cholic acid is a unique facial amphiphilic molecule displaying two methyl groups and three hydroxyl groups on two opposite faces of a rigid polycyclic steroidal scaffold with one carboxyl group at one end for covalent ligation. These features make cholic acid an excellent candidate for constructing functionalized molecular architectures with well-defined geometrical properties as well as molecular container for host–guest chemistry.56,57 It was reported that physicochemical properties of the amphiphilic polymers and the resulting micelles can be fine-tuned by varying the length of linear PEG chains and the configuration of cholic acid oligomer.These include particle size, critical micelle concentration, and drug-loading capacity. Cholic and deoxycholic acids have been conjugated to natural (such as chitosan, dextran) and synthetic polymers (such as dendrimers) as well.54
Nanocarriers based on BS-polymer conjugates could be successfully loaded with the lipophilic anticancer drug paclitaxel with high drug loading, antitumor activity, and stability compared with marketed products (Taxol® and Abraxane®). Targeted drug delivery to tumor sites with these novel micelles was demonstrated by near-infrared fluorescence imaging in nude mice bearing ovarian cancer xenograft (Figure 4).58
  | Figure 4 Tumor targeting of paclitaxel-loaded PEGylated nanocarrier of cholic acid in ovarian cancer xenograft.58 |
In another study, novel carboxymethylated curdlan (CM-curdlan) conjugate self-assembled NPs were prepared. A lipophilic anticancer drug (epirubicin) was successfully loaded into the hydrophobic deoxycholate core of the self-assembled NPs, and a sustained drug release pattern was demonstrated.43 CM-curdlan has good water solubility, good bioactivity, as well as antitumor activity. Tissue biodistribution study demonstrated that the self-assembled NPs could improve the accumulation of epirubicin in tumor and reduce its uptake in the heart.59
Impact of physicochemical properties on targeting efficiency
In the field of cancer targeting, physicochemical properties of NPs would greatly influence their biodistribution parameters (including opsonization, clearance with macrophage) and hematocompatibility (interaction with blood components). Major physicochemical properties reported to affect NPs behavior are particle size, surface charge, hydrophobicity, and, most recently, surface morphology. The optimum range of nanocarrier particle size for passive tumor targeting has been reported to be around 10–100 nm. In this regard, size-tunable cholate NPs exhibited an advantage over chitosan NPs, whose size range extends from 150 to 500 nm with subsequent significant liver uptake. Cholate NPs exhibited narrow particle size range (20–60 nm) for effective tumor targeting with minimum liver uptake, another advantage over chitosan NPs that generally exhibit larger polydispersity index.60–62
Hydrophilic polymers (such as PEG) have been widely used to coat the surface of NPs. Such PEGylation would counteract the hydrophobic and electrostatic interactions between NPs and plasma proteins (opsonins) or macrophages, resulting in less reticulo-endothelial system uptake and prolonged blood circulation time.63,64 In this context, Xiao et al65 have investigated the impact of surface charge on the cellular uptake and in vivo fate of PEG-oligocholic acid-based micellar NPs. They reported a nonspecific uptake of the NPs by the macrophages when high charge (either positive or negative) was employed, resulting in high liver localization of the drug. In addition, positively charged NPs exhibited dose-dependent hemolytic activities and cytotoxicity against macrophages, whereas negatively charged NPs did not show obvious hemolytic and cytotoxic properties. They attributed hemolysis induced by positively charged NPs to the strong electrostatic interaction between the particles and erythrocyte membrane, which facilitates the insertion of the whole particles into the membrane, resulting in disruption of red blood cells. Slightly negative charge on the particle surface resulted in decreased liver uptake, protecting the NPs from phagocytosis with preferential uptake at the tumor site. As a consequence, the authors recommended a slight negative charge for optimal cancer delivery to minimize the undesirable rapid elimination of NPs from the blood circulation, and facilitate their accumulation at the tumor sites.65
Furthermore, NP morphology has been recently reported to be a crucial parameter affecting NPs’ biodistribution and cellular uptake.66 A review of the literature revealed that nonspherical NPs show great promise as cancer drug-delivery vectors, such as filamentous, wormlike micelles, or needle-like morphologies. This may be explained with reference to facilitated adhesion of NPs on cell membranes and subsequent facilitated endocytosis.67 Traditional spherical micelles remain the dominant shape of nanocarriers described in the literature due to synthesis and testing difficulties. Therefore, efforts are being made to develop facile and versatile NP synthesis methodologies with the flexibility to create different shapes, tunable sizes, and adaptable surface chemistries.68 Therefore, although not so far investigated, nonspherical morphology of size-tunable cholate NPs might stand behind their superiority in cancer targeting and delivery.
Despite the reported advantage of BS–polymeric nanoaggregates as parenteral cancer therapy, it is not the case for oral delivery. Although the oral route is the most convenient one, poor oral bioavailability of drugs is a common handicap usually managed via PL-based nanocarriers. As the oral route is considered the cornerstone of drug delivery, peroral BS–PL MM and BS-vesicles will be covered in the following section.
Bile salt-phospholipid mixed micelles
BS micelles are a clear nanoformulation with a hydrophobic core suitable for incorporation of insoluble actives. BSs in colloidal micelles have been exploited to increase the oral bioavailability of hydrophobic actives, such as phenothiazine and amphotericin B.69 Furthermore, MM demonstrate advantages in parenteral delivery in view of their small size (usually smaller than 60 nm). They show enhanced vascular permeability to leaky vasculature (as in the case of tumors) avoiding opsonization.53 BSs–PL MM demonstrate advantages on BS simple micelles realizing amenability of PL to attenuate membrane damage of BSs.70 According to the packing parameter (PP) concept (p = V/Ao × lc), differential formation of either MM or vesicles when mixing BSs and PL could be explained. V and lc are the total volume and the extended length of the nonpolar chain(s), respectively, and Ao is the optimal head group area of the molecule. It was reported that aggregation of amphiphilic molecules with PP from 0.5 to 1 usually results in bilayer formation (vesicles) due to truncated cone geometry. On the other hand, when PP is lower than 0.5, assembly usually favors micelle formation owing to cone-shape geometry,71 as demonstrated in Figure 5A.53
  | Figure 5 (A) Geometric arrangements of bile salt and phospholipid molecules according to packing parameter (PP) value;53 (B) pseudo-ternary phase diagram for silybin-sodium cholate/phospholipid-mixed micelles.72 |
Figure 5B represents a phase diagram demonstrating how the ratio of BS:PL would determine whether the resultant system either mixed vesicles or mixed micelles.72 The study demonstrated a significant enhancement of silybin bioavailability from oral BS/PL micelles.53 Nevertheless, MM still encompass relatively higher ratio of BS (20%–50%) compared with BS-vesicles (nonclear region in phase diagram) with a relatively higher possibility of membrane damage. Furthermore, MM are not suitable for incorporation of hydrophilic drug candidates, in contrast to BS-vesicles.53,73 Those drawbacks might explain the limited number of mixed micellar drug products being introduced into the commercial pharmaceutical market so far.53
Bilosomes
BSs have been recognized as topical penetration enhancers due to the membrane-destabilizing activities. In a paradoxical effect, BSs were found to subsequently stabilize oral liposomal vesicles against further effects of bile acids when integrated into the vesicular double layer, forming what is called bilosomes.4,29,36 Bilosomes have been recently investigated by many authors under different scientific terminologies, including BS reinforced liposomes, liposomes containing BS,40,74 nanobilosomes,38 and BS stabilized vesicles.37,39 As bilosomes are prepared from naturally occurring lipids and have no apparent toxicity associated with their use, they represent a useful modification of conventional liposomes for the oral delivery of proteins and peptides. Further advantages of bilosomes including higher GIT stability, nanosize, negative surface charge, hydrophobic nature, and technical feasibility to scale up can successfully convert the bilosomes into a clinical reality. According to Mazer’s model, BS molecules are not only positioned around vesicular surfaces but also embedded in the PL bilayer.53 Consequently, bilosomes have been recently utilized to improve the oral bioavailability of many problematic drugs, as will be addressed in the next sections.
Drug candidates
Different APIs have been successful candidates for bilosomes, as detailed in the following sections. Table 1 summarizes variable bilosomal formulations with corresponding loaded drugs and principal research findings.
Oral vaccines and hydrophilic actives
Oral vaccines provide significant advantages as a noninvasive, needle-free administration route with subsequent ease of administration, better patient compliance, and lower susceptibility to cross-contamination. However, pharmaceutical application of this route is marred by the harsh gastrointestinal environment that is detrimental to many vaccine formulations.4,29,33,34,75 Obstacles to oral antigen delivery include degradation of oral antigens by proteolytic enzymes and hydrochloric acid in addition to poor absorption by gut-associated lymphoid tissue. As a consequence, larger and more frequent doses of oral vaccines would be required to give equal immunity to parenteral vaccines, leading to the formation of specific tolerance to such an antigen.4,36–39 It was reported that currently the only orally administered vaccine is the attenuated polio vaccine with the subsequent problem that it can revert to virulence.35
In this quest, a variety of nanocarriers like polymeric micro/NPs, liposomes, and niosomes have been reported with improved immunological performance.29 Bilosomes have been recently addressed, as novel colloidal vehicles have drawn attention in the field of oral immunization, such as cholera toxin vaccine, diphtheria toxoid vaccine, recombinant baculovirus vaccine, influenza vaccines, hepatitis B vaccine, and others.4,29,33–39,75 Vaccines based on nanobilosomes were reported to stimulate comparatively balanced systemic as well as local mucosal immune responses. Oral vaccination using bilosomes was proposed as a needle-free, painless approach to immunization and thereby enhanced patient compliance, consequently increasing vaccination coverage. Furthermore, bilosomes were reported to provide a safer surrogate for oral attenuated vaccines with a lower possibility of reversion to virulence.35 Oral bilosome preparation could induce significant cell-mediated responses against synthetic peptides comparable to those obtained by systemic immunization.39,75,76
Oral hydrophobic drugs
Amenability of bilosomes for enhancing oral availability was not confined to hydrophilic drugs entrapped in hydrophilic core.77 Few hydrophobic actives have been successful candidates for bilosomes as well where the drug would be entrapped in the PL bilayer of the vesicles. In this context, Chen et al41 have investigated the oral bioavailability of fenofibrate-loaded bilosomes and conventional liposomes compared with the micronized form of the drug. Bioavailability from bilosomes was significantly higher than that from liposomes and the micronized form. Contradictory results were demonstrated by in vitro release study, where the fenofibrate micronized form showed the highest percentage of release. The same disparity between in vivo and in vitro release results was observed by Guan et al.78 The authors loaded another lipophilic API (Cyclosporine) in bilosomes in comparison with conventional liposomes and marketed a microemulsion product (Sandimmune® and Neoral® [Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA]). Among others, bilosomes exhibited the lowest in vitro drug release but the highest oral bioavailability. Poor in vitro/in vivo correlation for oral liposomal formulations implied a potential drawback in in vitro release as an assessment tool of bilosomes. In vitro release media are usually deprived of any biosurfactants, enzymes, or bile content and thus may not faithfully mimic in vivo digestion. The in vitro assay failed to take into account the possible disruption of liposomal vesicular structure in physiological conditions. Furthermore, the cellulose artificial membrane does not mimic the PL consistency of the biological GIT barrier.79
Mechanisms of bioavailability enhancement
In view of the complexity of the gastrointestinal physiology, the exact mechanism of enhanced bioavailability of oral bilosomes has not so far been fully elucidated. One can say it is a consequence of the interplay between many factors including the protective effect against GIT harsh conditions, membrane fluidizing ability, and physicochemical properties of incorporated BS.31 Compared with conventional vesicles, bilosomes were reported to possess significantly higher stability and protective properties against gastric gastrointestinal enzymes, pH and bile content. Such an effect could be attributed to the remarkable protective effect against damage by BSs and protease (Figure 6). Maintenance of intact vesicles in GIT for prolonged periods of time would facilitate their uptake by M cells in the Peyer’s patch.29 Furthermore, the ultradeformability of liposomes containing BSs may allow carrier-mediated transmembrane absorption.41
  | Figure 6 Diagrammatic representation of BS stabilizing effect of oral vesicles containing protein (A) ruptured liposomal vesicle prior to reaching M-cell; (B) intact bilosomes retaining antigen offering protection therefore continue to transit toward M-Cell. |
The latter theory was supported by Niu et al40,74 who reported that enhancement of oral bioavailability and the hypoglycemic effect of insulin by bilosomal formulation was dose- and size dependent. Consequently, results proposed absorption of the drug in the form of intact vesicles.
Alternatively, the hypothesis of lipophilic ion-pair formation between BSs and hydrophilic API was proposed.80 Thereby, an increase in the passive diffusion of water-soluble molecules across biological membranes, as well as the membrane fluidizing effect of BSs would also justify bioavailability enhancement.81 Such ion-pair complexes would also be advantageous in terms of increasing the entrapment efficiency of cationic hydrophilic drugs, owing to the formation of more lipophilic compounds and higher affinity to phosphatidylcholine. Yang et al77 have proposed that BSs facilitated the distribution of cationic drug propranolol into cell membranes via electrostatic interactions and formation of ion pairs.
Comparative oral stability of bilosomes
As mentioned in the previous section, the positive effect of bilosomes on bioavailability is mainly ascribed to the gastroprotective effect. Bilosomes were reported to demonstrate higher oral stability in GIT compared with BS-deprived nanovesicles. Conacher et al4 have compared the oral immunization potential of bilosomes with that of nonionic vesicles (niosomes). Both vesicular preparations retained almost all of the entrapped bovine serum albumin when incubated with a 5 mM BS solution. However, this was not the case when the BS concentration was increased to 20 mM, where most of the albumin initially entrapped in niosomes was lost. On the other hand, bilosomes incubated with 20 mM BS solution still retained 85% of albumin relative to the initial load of protein. Results were explained on the basis of higher resistance of bilosomes to gastric degradation by impact of external bile content. Therefore, only bilosomal formulation could significantly generate high titers of virus-specific immunoglobulins compared with niosomes. In another study, Hu et al31 investigated the stability of oral bilosomes versus that of conventional liposomes in simulated gastrointestinal media containing various pH, BS, and enzyme levels. Nanovesicles were loaded with calcein as a small hydrophilic molecule and insulin as a model soluble macromolecule. Both low pH and BS resulted in a significant increase in calcein release from liposomes without significant variation in particle size and distribution. It was proposed that liposomal integrity changed when the liposomes were subjected to BS by forming transient pores but not by a rupture or complete disintegration. Bilosomes retained significantly more encapsulated insulin in simulated gastrointestinal media containing pepsin or pancreatin in comparison with conventional liposome.
Nevertheless, this review does not consider the suggested theory of pore formation to be the optimum explanation. A review of the literature in this quest found that BS integration into PL bilayers would stabilize the PL bilayer by a progressive increase in BS concentration. New mixed micellar and vesicular structures are then formed, while the entrapped material will be released and recaptured accordingly.2,28,29,31,32,40,75,82 The nanostructures formed might explain the insignificant change in the particle size of liposomes, while the release of hydrophilic digestible API into the GIT might explain the decrease in encapsulation efficiency and subsequent possible decrease in oral bioavailability reported by Hu et al.31 The destabilization process of the liposomal bilayers was suggested to occur in two steps. First, BS molecules adsorb at the external membrane surface, followed by their insertion within the vesicle bilayers. The BS destabilizing effect was reported to be of lower potential on multilamellar vesicles compared with small unilamellar vesicles.29,33,82
Rationale choice of BS type
Various BSs have been screened in the literature, and the impact of each slat appears to be case specific. The unique chemical structure of BSs gives them varied physicochemical and biological characteristics. In general, at sublytic concentrations, BS monomers insert into cell membranes with an extent being determined by their lipophilicity. However, other effects, including amphiphilicity and steric hindrance, are involved.83 Regarding physicochemical properties, factors such as solubility, pKa, conjugation, and chemical structure could influence BS selection. Conjugated BSs are generally much more soluble than the unconjugated form. pKa values of different BSs vary according to conjugation and length of the acidic group. In general, BSs are weak acids with pKa of (4.5) for unconjugated forms, (3.5) for glycine conjugates, and (2) for taurine conjugates. Conjugation with amino acids increases the polarity and acidity of side chain leading to lower pKa. Such an effect may explain preferential use of conjugated BSs (particularly taurocholates) as pharmaceutical excipients owing to lower precipitation tendency upon increasing the pH of the formulation.44 Specific BS structure may exhibit higher fitness for ion-pair formation with certain hydrophilic active resulting in better permeation. This was manifested in the superiority of sodium deoxycholate (SDC) to form ion pairs with propranolol with higher membrane permeability compared with other BSs.77
On the other hand, BSs possess variable critical biological properties, affecting their intended outcomes. These include protective potential against GIT drug degradation, safety to epithelial cells, and permeation-enhancing activity measured as impact on intestinal permeability coefficient (Papp). Niu et al40 have investigated the impact of cholate salt type on the hypoglycemic activity and oral bioavailability of insulin. BSs investigated were sodium glycocholate (SGC), sodium taurocholate (STC), or SDC. Among others, SGC exhibited the highest oral bioavailability compared with other cholates and conventional liposomes. Such an effect could be attributed to different protective effects of BSs on the drug encapsulated. It was reported that SGC could provide the best protection of encapsulated insulin against pepsin, trypsin, and a-chymotrypsin, followed by STC. The latter BS demonstrated better protective effects against pepsin and a-chymotrypsin but less protective effects against trypsin. SDC showed the least protection against both trypsin and a-chymotrypsin and similar protection against pepsin as conventional liposomes.40 The role of SGC in intestinal protection was reported by Niu et al84 as well. Conacher et al4 have screened a number of purified BSs (SDC, SGC, STC) to determine which best facilitated an immune response following oral vaccination with influenza antigen. They reported that all types work to varying degrees. Among others, SDC appeared to be most effective in terms of magnitude and reproducibility of the antibody response. The effect of deoxycholate salts as permeation enhancers was confirmed by Song et al81 who compared the potential of ten BSs to enhance the intestinal Papp and to impart damage to epithelial cells. The latter effect was measured by decrease in transepithelial electrical resistance (TEER) across the cell monolayer of the Caco-2 cell line. They reported that sodium taurodeoxycholate (STDC) in the optimal ratio of 0.1% possesses the highest potential to increase GIT permeability with the lowest epithelial damage compared with other surfactants. Such an effect might be ascribed to the ion-pair forming ability of the BSs with calcitonin (CT). In this quest, merit index (MI) was proposed as an important parameter that expresses permeability versus membrane damaging effect of bioenhancers. MI was defined as the ratio of a fold increase in Papp over a fold increase in TEER. The larger the MI, the more merit evaluated for the enhancer. Table 2 demonstrates the superiority of BSs over quaternary ammonium compounds and anionic SDS as inferred from a twofold increase in MI. The results showed superior permeation-enhancing effect with lower membrane damage compared with conventional surfactants.
  | Table 2 Effects of various surfactants on the apical to basolateral permeability of sCT (300 μg/mL) and on TEER values in the Caco-2 cell monolayer (n=3–4) |
Generally, mixing BSs with PL was reported to attenuate their damaging effect on biomembranes.70 Therefore, the proper choice of BS type and concentration in a bilosomal system can combine safety and permeation-enhancing effects. This was contended by Song et al81 who reported biocompatibility of bilosomes with the Caco-2 cells. Bilosomes neither affected the cell viability nor induced apoptosis within a 24 hours investigation with faster uptake as well as more drug transport.81 Such disparities in the superiority of different BSs in the literature implied case-specificity of BS for each drug. A drug with a high liability for hydrolysis would need a differently tailored system than another with poor wettability or a third one suffering from poor permeability. Therefore, the choice of optimum BS type would be the net result of an interplay between different physicochemical and biological factors.4,37,39
Nonoral bilosomes
In a paradoxical effect for oral bilosomes where BS content strengthens the wall against digestion, BS-modified liposomes have been utilized via other routes as permeation enhancers due to elasticity. In this context, transdermal transferosomes have been investigated in numerous research articles for enhancing percutaneous drug absorption.14,19 Transferosomes are ultradeformable liposomes that contain different edge activators including BSs.85 Transferosomes containing BSs demonstrated efficacy in improving transdermal permeation of cosmetics and pharmaceutical molecules when used in concentrations lower than 0.2%.86 Sodium cholate demonstrated efficacy as edge activator as equal as Tween-80 and Span-80 in improving transdermal permeation of estradiol.87
A severe shortage in the research investigating potential of bilosomal delivery via topical routes of administration was observed. For instance, the application of bilosomes in corneal penetration is a novel field that has not so far been fully investigated. Dai et al88 have considered elaboration and corneal permeation of bilosomes as novel ocular delivery systems for tacrolimus. The latter is a new immunosuppressive agent used after corneal transplantation, exhibiting up to 100 times the potency of cyclosporin (A). A previous study has indicated that liposomes loaded with tacrolimus can facilitate penetration of the drug across the cornea into the aqueous humor.88 However, transcorneal permeation from liposomal suspension was still too small to achieve a therapeutic effect. Realizing poor corneal permeation of drugs and delivery systems, the authors investigated ocular tacrolimus bilosomes containing different types of BSs as permeation enhancers. A significant enhancement of corneal permeation was demonstrated by bilosomes compared with liposomes. STC bilosomes exhibited higher corneal permeation compared with SDC and SGC that showed comparative permeation profiles. Such an effect was attributed to amenability of BSs to transiently open tight junction besides higher elasticity compared with cholesterol liposomes. It is worth noting here that SDC was particularly toxic to corneal cells, while STC and SGC are safe for ophthalmic application.
Modified bilosomal formulations
Recent research trends have encompassed elaboration of new modifications of bilosomes with improved characteristics. Being novel platforms, they have not attracted sufficient interest so far. In view of their future potential for drug delivery, the following sections will highlight their fundamentals.
Probilosomes
Recently, nanocarrier preconcentrates (or self-emulsifying nanocarriers) have captured much attention in scientific committees.21,22 Nanocarrier preconcentrates can spontaneously form the corresponding liquid-state nanomedicine upon ingestion. Because they are deprived of water, self-nanocarriers possess higher physical stability compared with liquid nanocarriers. Proliposomes are dry, free-flowing granular products that instantaneously form multilamellar liposomal dispersion upon ingestion. It was reported that oral delivery of liposomes could be improved by enhancing their ability to retain their integrity at the site of absorption, which could be achieved by formulating them into proliposomes.89,90 Despite the higher stability properties on liquid-state liposomes, self-liposomal formulations suffer a low apparent Papp compared with the optimal value favoring intestinal absorption.91
Such a problem entailed incorporation of an absorption enhancer into a liposomal matrix that causes no damage to epithelial cells. In this context, Song et al81 have developed proliposomes using STDC (2.5%, w/w) for the oral delivery of salmon calcitonin. They reported a 7.1-fold increase in the calcitonin bioavailability from probilosomes. They assumed that proliposomes are superior in protecting calcitonin against possible degradation by gastric fluid. Furthermore, entrapment efficiency and permeation of probilosomes were higher compared with the proliposomes that could be attributed to the formation of a lipophilic ion pair between the drug and BS.81
Surface-engineered bilosomes
Surface modification of bilosomes has been recently proposed in a few research articles attempting to increase stability and targeting efficiency of the nanovesicles. In the field of oral immunization, surface-modified vesicles were reported by anchoring a suitable ligand for a variety of receptors (such as mannosyl, galactosyl, folic acid and fibronectin) preferentially and abundantly present on the cell surface of antigen-presenting cells in mucosal linings.92,93 In this context, Jain et al29 developed novel glucomannan-modified (GM) bilosomes for eliciting an immune response following the oral administration of tetanus toxoid. The authors compared three types of vesicular systems, niosomes, bilosomes, and GM-bilosomes. All vesicular systems exhibited comparable in vitro quality attributes (particle size, zeta potential, and entrapment efficiency). Nevertheless, GM-bilosomes showed a higher immunological response parallel to maintained chemical and conformation stability of the tetanus toxoid entrapped. Results revealed significant immunological superiority of GM-bilosomes to conventional bilosomes and superiority of both formulations to niosomes, oral and IM peptides. The authors attributed this improvement to the polymeric nature of GM that increases the surface functionality by increasing mannose molecules density on the surface, which can enhance the uptake of mannosylated bilosomes by the antigen-presenting cells. Furthermore, the polymeric nature of GM can also provide stability against digestive enzymes. The ability of glucomannosylated bilosomes to improve targeting efficiency for oral immunization with bovine serum albumin has also been reported.75
Surface-modified bilosomes for targeting of M cells of Peyer’s patches have been recently investigated. Bilosomal surface was anchored with cholera toxin subunit (CTS) as a ligand targeting Peyer’s patches. The efficient uptake of loaded serum albumin and subsequent elicitation of immune response have been reported.39 In another study, M-cell targeted bilosomes were exploited for oral delivery of hepatitis B surface antigen. Conjugation of bilosomes with CTS increased transmucosal uptake via M cells with elicited immunity comparable to IM injection.36 In these studies, the authors have exploited the reported preferential affinity of CTS to glycolipid receptor on M-cell.94 Consequently, CTS-conjugated bilosomes were proposed to gain access to the gut associated lymphoid tissue to produce an efficient mucosal immune response. From a review of the literature, it is apparent that the enhanced efficacy of surface-modified bilosomes is attributed to the surface ligand rather than the BS.
Paradoxical effects of BS
Earlier in this article, the positive effect of endogenous BSs and BS-integrated dosage forms on bioavailability was discussed.11,44,48 Nevertheless, a contradictory effect was demonstrated when exogenous bile was coadministered with oral solid formulations.91 Administration of exogenous bile was accompanied by decreased oral bioavailability of atenolol95 and nitrendipine.96 It was suggested that coadministration of exogenous unconjugated BSs (such as ursodeoxycholic acid and CDCA) with drug formulation decreases the function of endogenous more soluble BS conjugates.
Another paradoxical effect of BSs was reported by Patra et al52 on curcumin release from BS-containing liposomes. They reported that alteration of membrane fluidity by BSs exhibited an opposing effect in the liquid crystalline phase (55°C) compared with that in the solid gel phase (25°C). Incorporation of monomeric BS molecules within a very low concentration range of 5–50 μM could increase the wetting and membrane fluidity in the solid gel phase. However, membrane fluidity decreased in the liquid crystalline phase that was reported to possess lyophobic nature.97 Paradoxical effect of BS in liquid crystalline phase could be ascribed to an interaction between hydrophobic tail of sodium cholate and the membrane (Figure 7). In the liquid crystalline phase, the liposomal bilayer is relatively less dense compared with the solid gel phase, with subsequent easier blurring of BSs into the bilayer. Less hydrophilic salt (SDC) was reported to be more densely integrated into the hydrophobic bilayer compared with sodium cholate. Higher BS concentration would lead to higher incorporation into the bilayer with higher expulsion of the lipophilic drug incorporated. Researchers should therefore pay close attention to PL, BS ratio, and type that may dramatically alter the composition and characteristics of delivery systems.
  | Figure 7 Schematic images of curcumin embedded in DPPC liposomes without bile salt (left image); in the presence of Na cholate (center image); and in the presence of Na deoxycholate (right image).52 |
Current restrains, future perspectives, and opinions
A close examination of the literature accentuates a major restraint during bilosomal development. Poor in vitro/in vivo correlation is a common drawback with a lack of in vitro method that mimics the actual conditions. Regular in vitro release methods lack the biological constituents significantly affecting vesicular digestion (BSs and enzymes). Using ex vivo permeation models and regular cell lines to assess vesicular permeability would lack this factor as well. A proposed solution would involve employment of the in vitro digestion-Caco-2 cell model.79 This would encompass digestive enzymes and BS content in addition to amenability for assessment of depot liposomal formulations.
The promising characteristics of bilosomes propose them as imperative cores for surface modifications. A proposed nanoformulation for future investigation is polymer-coated bilosomes. Polymeric NPs with bilosomal core would combine the advantages of PL content and permeation enhancer with targeting and entrapment properties of the shell. Furthermore, surface modification of bilosomes using ligands with preferential tumor accumulation would pave the way for cancer targeting and elaboration of stealth bilosomes. Another novel BS-integrated nanocarrier proposed in this review is BS-integrated chylomicrons. Such a novel nanocarrier is anticipated to provoke the preferential affinity of chylomicron for oral lymphatic targeting in addition to stabilizing and permeation-enhancing effects of BS. Paliwal et al98 estimated chylomicron mimicking carrier emulsome for lymph targeting of methotrexate after oral delivery. After taking samples from both blood and lymph, higher uptake and longer residence time of methotrexate molecules in lymphatics was reported. Certain lipid constituents of chylomicron trigger lymphatic targeting even after digestion. Finally, self-dispersing bilosomes to be filled in capsules are proposed as a portable solid dosage form with enhanced permeability of liquid bilosomes and superior in vitro stability. Some of those systems are currently under investigation by our work groups.
Although bilosomes proved to be a successful carrier for cationic water-soluble actives, sufficient loading of anionic active is still an obstacle. Taking into consideration negative charge and hydrophilicity of BSs, incorporation of cationic active would hold the BS in the bilayer to exert its membrane-stabilizing effect. Nevertheless, incorporation of anionic hydrophilic drugs would be accompanied by low entrapment efficiency, migration of both hydrophilic BSs and active to external phase, simulating migration of surfactant in type IV of the lipid formulation classification system.21 The challenge of incorporating anionic hydrophilic drugs (like risedronate) into bilosomes is currently being addressed by our work group.
Conclusion
In this article, the pharmaceutical importance of BSs as endogenous modulators, signaling agents, and nanocarrier excipients has been emphasized. Endogenous BS-based strategies to treat hypercholesterolemia and liver cancer have been highlighted. BS-integrated nanocarriers have been surveyed with crucial applications based on the physicochemical properties of BSs. The criteria for a rationale choice of BS type in nanomedicine have been explained. Important considerations of researchers in the selection and assessment of proper BS-integrated nanocarriers appropriate for certain drugs were highlighted. The advantages and paradoxical effects of BSs have been discussed. Future perspectives have been suggested in view of current limitations and obstacles.
Disclosure
The author reports no conflicts of interest in this work.
References
Enhsen A, Kramer W, Wess G. Bile acids in drug discovery. Drug Discov Today.1998;3(9):409–418. | ||
Maldonado-Valderrama J, Wilde P, Macierzanka A, Mackie A. The role of bile salts in digestion. Adv Colloid Interface Sci. 2011;165(1):36–46. | ||
Li, G. Intestinal probiotics: interactions with bile salts and reduction of cholesterol. Procedia Environ Sci. 2012;12(Part B):1180–1186. | ||
Conacher M, Alexander J, Brewer JM. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes). Vaccine. 2001;19(20–22):2965–2974. | ||
Fu ZD, Klaassen CD. Increased bile acids in enterohepatic circulation by short-term calorie restriction in male mice. Toxicol Appl Pharmacol. 2013;273(3):680–690. | ||
Pols TWH, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54(6):1263–1272. | ||
Madenci, D, Egelhaaf SU. Self-assembly in aqueous bile salt solutions. Curr Opin Colloid Interface Sci. 2010;15(1–2):109–115. | ||
Baptissart M, Vega A, Maqdasy S, et al. Bile acids: from digestion to cancers. Biochimie. 2013;95(3):504–517. | ||
Degirolamo C, Modica S, Palasciano G, Moschetta A. Bile acids and colon cancer: solving the puzzle with nuclear receptors. Trends Mol Med. 2011;17(10):564–572. | ||
Morimoto K, Uehara Y, Iwanaga K, et al. Influence of absorption enhancers (bile salts) and the preservative (benzalkonium chloride) on mucociliary function and permeation barrier function in rabbit tracheas. Eur J Pharm Sci. 1998;6(3):225–230. | ||
Atanackovic M, Posa M, Heinle H, Gojković-Bukarica L, Cvejić J. Solubilization of resveratrol in micellar solutions of different bile acids. Colloids Surf B Biointerfaces. 2009;72(1):148–154. | ||
Maestrelli F, Cirri M, Mennini N, Zerrouk N, Mura P. Improvement of oxaprozin solubility and permeability by the combined use of cyclodextrin, chitosan, and bile components. Eur J Pharm Biopharm. 2011; 78(3):385–393. | ||
Selvam, S, Andrews ME, Mishra AK. A photophysical study on the role of bile salt hydrophobicity in solubilizing amphotericin B aggregates. J Pharm Sci. 2009;98(11):4153–4160. | ||
Elnaggar YS, El-Refaie WM, El-Massik MA, Abdallah OY. Lecithin-based nanostructured gels for skin delivery: an update on state of art and recent applications. J Control Release. 2014;180:10–24. | ||
Romeo VD, deMeireles JC, Gries WJ, et al. Optimization of systemic nasal drug delivery with pharmaceutical excipients. Adv Drug Deliv Rev.1998;29(1–2):117–133. | ||
Shin S-C, Kim J-Y. Enhanced permeation of triamcinolone acetonide through the buccal mucosa. Eur J Pharm Biopharm. 2000;50(2):217–220. | ||
Shin S-C, Cho C-W, Yang K-H. Development of lidocaine gels for enhanced local anesthetic action. Int J Pharm. 2004;287(1–2):73–78. | ||
Bhat S, Maitra U. Low molecular mass cationic gelators derived from deoxycholic acid: remarkable gelation of aqueous solvents. Tetrahedron. 2007;63(31):7309–7320. | ||
El-Refaieb WM, Elnagger YS, El-Massikb MA, Abdallaha OY. Novel self-assembled, gel-core hyaluosomes for non-invasive management of osteoarthritis: in-vitro optimization, ex-vivo and in-vivo permeation. Pharm Res. Epub March 17, 2015. | ||
Elnaggar, YS, El-Massik MA, Abdallah OY. Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int J Nanomedicine. 2011;6:3195–3205. | ||
Elnaggar YS, El-Massik MA, Abdallah OY. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: design and optimization. Int J Pharm. 2009;380(1–2):133–141. | ||
Elsheikh MA, Elnaggar YS, Gohar EY, Abdallah OY. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: optimization and in vivo appraisal. Int J Nanomedicine. 2012;7:3787–3802. | ||
Freag MS, Elnaggar YS, Abdallah OY. Lyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: optimization and ex vivo permeation. Int J Nanomedicine. 2013;8:2385–2397. | ||
Freag MS, Elnaggar YS, Abdallah OY. Development of novel polymer-stabilized diosmin nanosuspensions: in vitro appraisal and ex vivo permeation. Int J Pharm. 2013;454(1):462–471. | ||
Elnaggar YS, El-Massik MA, Abdallah OY. Sildenafil citrate nanoemulsion vs self-nanoemulsifying delivery systems: rational development and transdermal permeation. Int J Nanotechnol. 2011;8(8):749–763. | ||
Harde H, Siddhapura K, Agrawal AK, Jain S. Development of dual toxoid-loaded layersomes for complete immunostimulatory response following peroral administration. Nanomedicine (Lond). 2015;10(7):1077–1091. | ||
Yingsukwattana K, Puttipipatkhachorn S, Ruktanonchai U, Sarisuta N. Enhanced permeability across Caco-2 cell monolayers by specific mannosylating ligand of buserelin acetate proliposomes. J Liposome Res. Epub 2015;6:1–11. | ||
Parmentier J, Becker MM, Heintz U, Fricker G. Stability of liposomes containing bio-enhancers and tetraether lipids in simulated gastro-intestinal fluids. Int J Pharm. 2011;405(1–2):210–217. | ||
Jain S, Harde H, Indulkar A, Agrawal AK. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine. 2014; 10(2):431–440. | ||
Gadras C, Santaella C, Vierling P. Improved stability of highly fluorinated phospholipid-based vesicles in the presence of bile salts. J Control Release. 1999;57(1):29–34. | ||
Hu S, Niu M, Hu F, et al. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int J Pharm. 2013;441(1–2):693–700. | ||
Andrieux K, Forte L, Lesieur S, Paternostre M, Ollivon M, Grabielle-Madelmont C. Solubilisation of dipalmitoylphosphatidylcholine bilayers by sodium taurocholate: a model to study the stability of liposomes in the gastrointestinal tract and their mechanism of interaction with a model bile salt. Eur J Pharm Biopharm. 2009;71(2):346–355. | ||
Martin C, Thongborisute J, Takeuchi H, Yamamoto H, Kawashima Y, Alpar HO. Cholesterol–bile salt vesicles as potential delivery vehicles for drug and vaccine delivery. Int J Pharm. 2005;298(2):339–343. | ||
Premanand B, Prabakaran M, Kiener TK, Kwang J. Recombinant baculovirus associated with bilosomes as an oral vaccine candidate against HEV71 infection in mice. PLoS One. 2013;8(2):e55536. | ||
Senior K. Bilosomes: the answer to oral vaccine delivery? Drug Discov Today. 2001;6(20):1031–1032. | ||
Shukla A, Katare OP, Singh B, Vyas SP. M-cell targeted delivery of recombinant hepatitis B surface antigen using cholera toxin B subunit conjugated bilosomes. Int J Pharm. 2010;385(1–2):47–52. | ||
Shukla A, Khatri K, Gupta PN, Goyal AK, Mehta A, Vyas SP. Oral immunization against hepatitis B using bile salt stabilized vesicles (bilosomes). J Pharm Pharm Sci. 2008;11(1):59–66. | ||
Shukla A, Singh B, Katare OP. Significant systemic and mucosal immune response induced on oral delivery of diphtheria toxoid using nano-bilosomes. Br J Pharmacol. 2011;164(2b):820–827. | ||
Singh P, Prabakaran D, Jain S, Mishra V, Jaganathan KS, Vyas SP. Cholera toxin B subunit conjugated bile salt stabilized vesicles (bilosomes) for oral immunization. Int J Pharm. 2004;278(2):379–390. | ||
Niu M, Tan Y, Guan P, et al. Enhanced oral absorption of insulin-loaded liposomes containing bile salts: a mechanistic study. Int J Pharm. 2014;460(1–2):119–130. | ||
Chen Y, Lu Y, Chen J, et al. Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. Int J Pharm. 2009;376(1–2):153–160. | ||
Khatun Z, Nurunnabi M, Cho KJ, Lee YK. Imaging of the GI tract by QDs loaded heparin-deoxycholic acid (DOCA) nanoparticles. Carbohydr Polym. 2012;90(4):1461–1468. | ||
Gao F, Li L, Zhang H, et al. Deoxycholic acid modified-carboxymethyl curdlan conjugate as a novel carrier of epirubicin: in vitro and in vivo studies. Int J Pharm. 2010;392(1–2):254–260. | ||
Holm R, Mullertz A, Mu H. Bile salts and their importance for drug absorption. Int J Pharm. 2013;453(1):44–55. | ||
Torcello-Gómez A, Maldonado-Valderrama J, De Vicente J, Cabrerizo-Vílchez MA, Gálvez-Ruiz MJ, Martín-Rodríguez A. Investigating the effect of surfactants on lipase interfacial behaviour in the presence of bile salts. Food Hydrocolloids. 2011;25(4):809–816. | ||
Araya H, Nagao S, Tomita M, Hayashi M. The novel formulation design of self-emulsifying drug delivery systems (SEDDS) type O/W microemulsion I: enhancing effects on oral bioavailability of poorly water soluble compounds in rats and beagle dogs. Drug Metab Pharmacokinet. 2005;20(4):244–256. | ||
Miyake K, Arima H, Irie T, Hirayama F, Uekama K. Enhanced absorption of cyclosporin A by complexation with dimethyl-beta-cyclodextrin in bile duct-cannulated and -noncannulated rats. Biol Pharm Bull. 1999;22(1):66–72. | ||
van Hasselt PM, Janssens GE, Slot TK, et al. The influence of bile acids on the oral bioavailability of vitamin K encapsulated in polymeric micelles. J Control Release. 2009;133(2):161–168. | ||
Kim EJ, Han KS, Lee MG. Enhanced absorption of oral furosemide by bile juice in rats. Res Commun Mol Pathol Pharmacol. 1999;104(1):107–110. | ||
Zhang Z, Gao F, Jiang S, et al. Bile salts enhance the intestinal absorption of lipophilic drug loaded lipid nanocarriers: mechanism and effect in rats. Int J Pharm. 2013;452(1–2):374–381. | ||
Lofman M, Koivukorpi J, Noponen V, Salo H, Sievänen E. Bile acid alkylamide derivatives as low molecular weight organogelators: systematic gelation studies and qualitative structural analysis of the systems. J Colloid Interface Sci. 2011;360(2):633–644. | ||
Patra D, Ahmadieh D, Aridi R. Study on interaction of bile salts with curcumin and curcumin embedded in dipalmitoyl-sn-glycero-3- phosphocholine liposome. Colloids Surf B Biointerfaces. 2013;110:296–304. | ||
Rupp C, Steckel H, Muller BW. Mixed micelle formation with phosphatidylcholines: the influence of surfactants with different molecule structures. Int J Pharm. 2010;387(1–2):120–128. | ||
Gautrot JE, Zhu XX. Biodegradable polymers based on bile acids and potential biomedical applications. J Biomater Sci. 2006;17(10): 1123–1139. | ||
Yang J, Gao C, Lü S, Zhang X, Yu C, Liu M. Physicochemical characterization of amphiphilic nanoparticles based on the novel starch-deoxycholic acid conjugates and self-aggregates. Carbohydr Polym. 2014;102:838–845. | ||
Tang X, Cai S, Zhang R, et al. Paclitaxel-loaded nanoparticles of star-shaped cholic acid-core PLA-TPGS copolymer for breast cancer treatment. Nanoscale Res Lett. 2013;8(1):420. | ||
Zeng X, Tao W, Mei L, Huang L, Tan C, Feng SS. Cholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancer. Biomaterials. 2013;34(25):6058–6067. | ||
Li Y, Xiao K, Luo J, Lee J, Pan S, Lam KS. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. J Control Release. 2010;144(3):314–323. | ||
Gao F-P, Zhang, HZ, Liu, LR, et al. Preparation and physicochemical characteristics of self-assembled nanoparticles of deoxycholic acid modified-carboxymethyl curdlan conjugates. Carbohydr Polym. 2008;71(4):606–613. | ||
He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–3666. | ||
Xiao K, Luo J, Fowler WL, et al. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30(30):6006–6016. | ||
Luo J, Xiao K, Li Y, et al. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjug Chem. 2010;21(7):1216–1224. | ||
Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. | ||
Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol). Langmuir. 2006;22(19):8178–8185. | ||
Xiao K, Li Y, Luo J, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32(13):3435–3446. | ||
Ma N, Ma C, Li C, et al. Influence of nanoparticle shape, size, and surface functionalization on cellular uptake. J Nanosci Nanotechnol. 2013;13(10):6485–6498. | ||
Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Åberg C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc. 2013;135(4):1438–1444. | ||
Truong NP, Whittaker MR, Mak CW, Davis TP. The importance of nanoparticle shape in cancer drug delivery. Expert Opin Drug Deliv. 2015;12(1):129–142. | ||
Mahajan S, Mahajan RK. Interactions of phenothiazine drugs with bile salts: micellization and binding studies. J Colloid Interface Sci. 2012;387(1):194–204. | ||
Tan Y, Qi J, Lu Y, Hu F, Yin Z, Wu W. Lecithin in mixed micelles attenuates the cytotoxicity of bile salts in Caco-2 cells. Toxicol In Vitro. 2013;27(2):714–720. | ||
Israelachvili JN, Mitchell DJ, Ninham BW. Theory of self-assembly of lipid bilayers and vesicles. Biochim Biophys Acta. 1977;470(2):185–201. | ||
Yu JN, Zhu Y, Wang L, et al. Enhancement of oral bioavailability of the poorly water-soluble drug silybin by sodium cholate/phospholipid-mixed micelles. Acta Pharmacol Sin. 2010;31(6):759–764. | ||
Wacker M, Schubert R. From mixed micelles to liposomes: critical steps during detergent removal by membrane dialysis. Int J Pharm. 1998;162(1–2):171–175. | ||
Niu M, Lu Y, Hovgaard L, et al. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur J Pharm Biopharm. 2012;81(2):265–272. | ||
Jain S, Indulkar A, Harde H, Agrawal AK. Oral mucosal immunization using glucomannosylated bilosomes. J Biomed Nanotechnol. 2014;10(6):932–947. | ||
Aburahma MH. Bile salts-containing vesicles: promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptide/protein-based therapeutics or vaccines. Drug Deliv. 2014:1–21. | ||
Yang L, Tucker IG, Ostergaard J. Effects of bile salts on propranolol distribution into liposomes studied by capillary electrophoresis. J Pharm Biomed Anal. 2011;56(3):553–559. | ||
Guan P, Lu Y, Qi J, et al. Enhanced oral bioavailability of cyclosporine A by liposomes containing a bile salt. Int J Nanomedicine. 2011;6:965–974. | ||
Elsheikh MA, Elnaggar YS, Abdallah OY. Rationale employment of cell culture versus conventional techniques in pharmaceutical appraisal of nanocarriers. J Control Release. 2014;194:92–102. | ||
Heinen CA, Reuss S, Amidon GL, Langguth P. Ion pairing with bile salts modulates intestinal permeability and contributes to food-drug interaction of BCS class III compound trospium chloride. Mol Pharm. 2013;10(11):3989–3996. | ||
Song K-H, Chung S-J, Shim C-K. Enhanced intestinal absorption of salmon calcitonin (sCT) from proliposomes containing bile salts. J Control Release. 2005;106(3):298–308. | ||
Kokkona M, Kallinteri P, Fatouros D, Antimisiaris SG. Stability of SUV liposomes in the presence of cholate salts and pancreatic lipases: effect of lipid composition. Eur J Pharm Sci. 2000;9(3):245–252. | ||
Kiselev MA, Janich M, Hildebrand A, Strunz P, Neubert RHH, Lombardo D. Structural transition in aqueous lipid/bile salt [DPPC/NaDC] supramolecular aggregates: SANS and DLS study. Chem Phys. 2013;424:93–99. | ||
Niu M, Lu Y, Hovgaard L, Wu W. Liposomes containing glycocholate as potential oral insulin delivery systems: preparation, in vitro characterization, and improved protection against enzymatic degradation. Int J Nanomedicine. 2011;6:1155–1166. | ||
Montanari J, Vera M, Mensi E, Morilla M, Romero E. Nanoberries for topical delivery of antioxidants. J Cosmet Sci. 2013;64(6):469–481. | ||
Paolino D, Cosco D, Cilurzo F, et al. Improved in vitro and in vivo collagen biosynthesis by asiaticoside-loaded ultradeformable vesicles. J Control Release. 2012;162(1):143–151. | ||
El Maghraby GM, Williams AC, Barry BW. Oestradiol skin delivery from ultradeformable liposomes: refinement of surfactant concentration. Int J Pharm. 2000;196(1):63–74. | ||
Dai Y, Zhou R, Liu L, Lu Y, Qi J, Wu W. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int J Nanomedicine. 2013;8:1921–1933. | ||
Fu Q, Fu HL, Huan L, et al. Preparation of cefquinome sulfate proliposome and its pharmacokinetics in rabbit. Iran J Pharm Res. 2013; 12(4):611–621. | ||
Velpula A, Jukanti R, Janga KY, et al. Proliposome powders for enhanced intestinal absorption and bioavailability of raloxifene hydrochloride: effect of surface charge. Drug Dev Ind Pharm. 2013;39(12):1895–1906. | ||
Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175(3):880–885. | ||
Cuna M, Alonso-Sandel M, Remuñán-López C, Pivel JP, Alonso-Lebrero JL, Alonso MJ. Development of phosphorylated glucomannan-coated chitosan nanoparticles as nanocarriers for protein delivery. J Nanosci Nanotechnol. 2006;6(9–10):2887–2895. | ||
Jain S, Vyas SP. Mannosylated niosomes as adjuvant-carrier system for oral mucosal immunization. J Liposome Res. 2006;16(4):331–345. | ||
Owen RL, Pierce NF, Apple RT, Cray WC Jr. M cell transport of Vibrio cholerae from the intestinal lumen into Peyer’s patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986;153(6):1108–1118. | ||
Barnwell SG, Laudanski T, Dwyer M, et al. Reduced bioavailability of atenolol in man: the role of bile acids. Int J Pharm. 1993;89(3):245–250. | ||
Sasaki M, Maeda A, Sakamoto K, Fujimura A. Effect of bile acids on absorption of nitrendipine in healthy subjects. Br J Clin Pharmacol. 2001;52(6):699–701. | ||
Amenitsch H, Edlund H, Khan A, Marques EF, La Mesa C. Bile salts form lyotropic liquid crystals. Colloids Surfaces A Physicochem Eng Aspects. 2003;213(1):79–92. | ||
Paliwal R, Paliwal SR, Mishra N, Mehta A, Vyas SP. Engineered chylomicron mimicking carrier emulsome for lymph targeted oral delivery of methotrexate. Int J Pharm. 2009;380(1–2):181–188. | ||
Wilkhu JS, McNeil SE, Anderson DE, Perrie Y. Characterization and optimization of bilosomes for oral vaccine delivery. J Drug Target. 2013;21(3):291–299. | ||
Basha M, Abd El-Alim SH, Shamma RN, Awad GE. Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J Liposome Res. 2013;23(3):203–210. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

