Back to Journals » Infection and Drug Resistance » Volume 12
Multidrug-Resistant Tuberculosis In A Referral Center In Rome: 2011– 2016
Authors Cannas A, Butera O, Gualano G , Parracino MP, Venditti C, Mazzarelli A, Palmieri F, Girardi E , Di Caro A
Received 8 June 2019
Accepted for publication 12 August 2019
Published 18 October 2019 Volume 2019:12 Pages 3275—3281
DOI https://doi.org/10.2147/IDR.S218744
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
A Cannas,1 O Butera,1 G Gualano,2 MP Parracino,3 C Venditti,1 A Mazzarelli,1 F Palmieri,2 E Girardi,3,* A Di Caro1,*
1Microbiology and Bio-Repository Unit, National Institute for Infectious Diseases “L. Spallanzani” IRCCS, Rome, Italy; 2Respiratory Infectious Diseases Unit, National Institute for Infectious Diseases “L. Spallanzani” IRCCS, Rome, Italy; 3Clinical Epidemiology Unit, National Institute for Infectious Diseases “L. Spallanzani” IRCCS, Rome, Italy
*These authors contributed equally to this work
Correspondence: A Cannas
National Institute for Infectious Diseases “L. Spallanzani” IRCCS, Via Portuense 292, Rome 00149, Italy
Tel +39 06 5517 0905
Email [email protected]
Purpose: Multidrug-resistant tuberculosis (MDR-TB) is a major burden to public health in low incidence countries in Europe. The aim of this study was to attempt to have a better insight into the trends of MDR-TB in the metropolitan area of Rome, within the Italian and the foreign-born population, based on microbiological and demographic data.
Patients and methods: We performed a prospective study, collecting microbiological data based on phenotypic drug-resistant testing (DST) of TB strains consecutively isolated in a referral hospital in Rome, the capital city of a low TB incidence country, over a 6-year period, and correlated them to the geographical origin of patients. This study was carried out in a referral hospital for patients with drug-resistant TB from the whole region.
Results: Drug-resistance data from 926 patients with a microbiological diagnosis of TB from 2011 to 2016 show a 5.5% rate of MDR-TB, mostly occurring in patients born in a single East European country, that has a high incidence of MDR-TB. The strains isolated from these patients frequently carry additional resistances, leading to an increased risk of developing extensively drug-resistant (XDR) TB.
Conclusion: In the great metropolitan area of Rome, MDR-TB more frequently occurs in patients who were born in a single country from Eastern Europe known to have high rates of MDR-TB and long-time residents in Italy. Recent immigrants from non-European countries do not appear to contribute to the rates of MDR-TB reported in this article. This knowledge of local TB trends could help improve the measures of surveillance and prevention of disease.
Keywords: tuberculosis, drug-susceptibility testing, MDR-TB, XDR-TB
Introduction
Tuberculosis (TB) is still a public health priority in both low- and high-resource countries. Although TB control has been effective in some regions of the world, the gains are threatened by the increasing burden of multidrug-resistant (MDR, resistant to at least isoniazid and rifampicin) and extensively drug-resistant (XDR, resistant to isoniazid and rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs) disease. Indeed, MDR and XDR tuberculosis are associated with high morbidity and substantial mortality, are a threat to health-care workers, and much more expensive to treat than drug-susceptible TB.1
In 2017, 2.7% (275,000) of the total 10.0 million TB cases occurred in the WHO European Region,2 and 77,000/330,000 of the estimated multidrug-resistant and rifampicin-resistant cases (MDR/RR) also occurred in this region, which includes eastern European and central Asian countries, posing a serious threat to TB control and, eventually, TB elimination.2–6
The distribution of sensitive and drug-resistant tuberculosis in different countries in Europe has changed during recent years, mainly due to the intense population movements. In the WHO European region, 27.9% of pulmonary TB cases tested for drug susceptibility had MDR-TB, while multidrug resistance was reported in 3.8% of all TB cases tested in the European Union and European Economic Area (EU/EEA), with values up to 10–20% in few countries, i.e. in the Baltic region. In some of these same countries, XDR TB was reported in 20% of MDR cases undergoing second-line drug susceptibility testing.3
These evidence emphasize the need for a better understanding of the trends of resistant TB also in western European countries that have a low burden of the disease,7 since migration from geographical regions with a high incidence of resistant strains could increase the challenges posed to prevention and care and require modifications of the control measures.8
Italy is a low incidence country for tuberculosis and, following a general trend, has reduced the number of new cases during the last decade, with 6.6 cases/100,000 inhabitants reported in 2016.3 Nevertheless, Italy has become in recent years one of the main refugee entry points to Europe, and the conditions in the shelter centers and the complexity of management of migrants in highly populated metropolitan areas, where most of the asylum-seekers converge, can increase the probability of tuberculosis transmission.9 As other metropolitan cities in western Europe, Rome and the surrounding area host an ethnically heterogeneous population, well represented by individuals migrating from countries with a high incidence of the disease, leading to TB rates of 10.5/100,000 cases, much higher than those found in Italy as a whole.10 As stated by WHO in its latest report on TB in the European region, migrants to countries with a low incidence of TB have been shown to be at increased risk of MDR-TB compared with the host population, suggesting that testing and treatment for drug-resistant TB in all individuals diagnosed with TB should be part of a basic package of care.11,12 This could be the basis for a surveillance activity especially focused on city districts where large communities of immigrants from countries with a high prevalence of tuberculosis tend to gather.
In the studied area, a relevant number of TB patients were born in countries of East Europe that present also a high rate of MDR-TB; thus, in our opinion, an evaluation of the distribution of resistant TB within our population could help us to improve the public health measures in order to limit the spread of resistant strains or the acquisition of new resistances in TB patients originating from certain regions.
In this study, we characterized the trends of drug-resistant TB in the Rome metropolitan area, based on microbiology and epidemiology data.
Materials And Methods
Setting And Study Population
The study was conducted at the National Institute for Infectious Diseases L. Spallanzani (INMI), located in Rome, Italy. INMI is both a hospital and a research institute dedicated to infectious diseases, with a capacity of 152 beds, of which 80 are located in negative pressure rooms adequate for patient respiratory isolation. Respiratory (95% of the total samples) and other specimens (5%) collected from patients admitted to the hospital during a six-year period from 2011 to 2016 with a suspect of tuberculosis were tested by routine microbiology, according to national and local guidelines.13 Briefly, two samples (until the beginning of 2016 three samples according to the guidelines present at that time) were collected, at least 8 hrs apart, from each patient, liquefied and concentrated by centrifugation, and analyzed by smear microscopy (Ziehl-Nielsen, hot staining method) and by molecular tests that specifically detect rRNA [E-MTD (Gen-Probe), substituted in 2014 by TRCReady-80 (Tosoh Bioscience)]. Samples were decontaminated and inoculated into liquid media for rapid automated cultivation (MGIT 960; Becton Dickinson), and subsequent automated drug-sensitivity testing (DST) was performed with the same system or manually using the proportion dilution method (solid medium 7H11, MICROBIOL Diagnostici) for first-line drugs.
A portion of the isolates, based on medical request or upon identification of a MDR status, underwent DST for second-line drugs, using non-automated culture or a semi-automated system (BD EpiCenter™ TB-eXiST). Molecular DST tests, such as Xpert MTB/RIF (Cepheid), GenoType MTBDR plus/sl (Hain Lifescience GmbH) or AnyplexTM II MTB/MDR or MTB/XDR Detection (Seegene), were additionally performed on selected biological samples in order to confirm and support the phenotypic testing.
The quality system operated by the laboratory of Microbiology is in compliance with the standard ISO9001:2008 and participates in the drug susceptibility proficiency testing of the Istituto Superiore di Sanità (ISS, Rome, Italy) with an achievement of 95 ≥X% agreement for FLD and SLD since 2008.
This analysis was conducted in the context of an observational study on tuberculosis (DB/TB) approved by the Ethics Committee at INMI (decision n. 12/2015).
An informed consent was obtained from these patients at hospital admission, allowing for the retrospective use of clinical and demographic data related to their case for research studies.
Demographic data include age, sex, patient’s country of origin, and information on previous episodes of TB.
For analysis of the collected data, the patients were classified as italian born (IB) or foreign-born (FB).
Results from microbiological and molecular tests were collected from the records of the laboratory of Microbiology and Bio-repository of INMI and linked to demographic data for analysis. The associations between strains drug resistances and geographical area were analyzed, and differences in proportion were assessed using Chi-squared test or Fisher Exact test.
Results
Study Population
Nine hundred and twenty-six (926) TB strains were isolated from 926 patients hospitalized at INMI between January 2011 and December 2016 and were subsequently assayed by DST in order to modulate an adequate therapy.
Demographic data related to the studied population are shown in Table 1. Two-hundred twenty (23.7%) patients were born in Italy, 706 (76.3%) outside of Italy. Information on previous TB episodes was available for 855 patients (92% of the study population). Of these, 759 (89%) were new cases (181 IB, 578 FB), while 96 (11%) were classified as previously treated (17 IB, 79 FB).
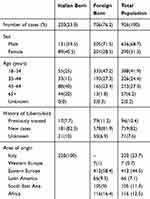 |
Table 1 Demographic And Clinical Characteristics Of The Studied Population |
Mean age of patients was 39.8 years (IB 50.0, FB 36.6) and 289 (31.3%) cases occurred in females (89 IB-30.8%, 200–69.2% FB) and 634 (68.7%) in males (131–20.6% IB; 505–79.4% FB).
All continents except Oceania were represented: 420 patients were born in Europe outside of Italy (420–59.5% of FB TB patients); 116 in Africa (16.5%); 104 in Asia (14.7%), and 66 in The Americas (9.3%).
Figure 1 lists the 10 countries with the highest number of TB patients within our FB population, showing Romania as the most represented with 370 TB patients, accounting for 52.4% of all FB cases. The Philippines follows with 32 cases (4.5%), then Brazil, Bangladesh, Peru and India covering 3.3% of FB cases. The remaining patients were distributed within 52 countries, with ≤17 patients/country.
 |
Figure 1 Number of cases per country of origin (only FB cases, first ten countries). |
Drug-Resistant Tuberculosis
Phenotypic susceptibility to first-line drugs was tested on all isolated strains (874/926 obtained from respiratory samples), while testing for second-line drugs was performed on 75 of these. Additionally, 234/926 patients received a commercial molecular DST besides the phenotypic assay (Xpert MTB/RIF and/or GenoType MTBDR plus or AnyplexTM II), in order to confirm or to support the culture-based results (data not shown).
Based on phenotypic testing, 120 strains showed drug resistances. Of these, 51 were MDR (5.5% of the total study population), being resistant to at least rifampicin (RMP) and isoniazide (INH). Within the MDR cases, 17 were additionally resistant to second-line fluoroquinolones (FQ) or injectable drugs and defined as pre-XDR, and 2 were resistant to both, thus defined as XDR. Eleven additional patients were infected with strains resistant to RMP only and were thus eligible for MDR treatment. INH resistance was found in 52 of non-MDR cases. Six patients harbored strains with resistance to EMB only.
Further analysis of MDR, pre-XDR and XDR cases shows that 22 (43%) of them were previously treated cases. Table 2 lists the most represented resistances to first- and second-line drugs in the studied population and their relation to new or previously treated cases, in the IB and FB population.
 |
Table 2 Anti-Tuberculosis Drug Resistance In The Studied Population |
Further analysis shows that 42/51 MDR-TB cases were isolated from foreign patients (82%), who were born in 15 different countries. The rate of MDR-TB in the Italian (4.1%) and in the foreign patient population (5.9%) did not differ significantly. Figure 2 shows the geographical distribution of MDR cases. The most represented country was Romania (22 MDR patients) and included 8 pre-XDR and 1 XDR cases. Ukraine and Republic of Moldavia were well represented with 4 MDR cases each. MDR foreign-born patients were aged 25–34 years, while italian MDR were 45–54 years. Male–female ratio was 3.5 in the IB and 1.47 in the FB (data not shown).
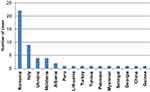 |
Figure 2 Number of MDR, pre-XDR and XDR cases per country of origin. |
Discussion
Recent population flows around the globe have dramatically changed the dynamics of tuberculosis, posing new challenges to national TB programs. Thus, improving the understanding of tuberculosis transmission dynamics and trends of drug-resistant TB will be of foremost importance in order to develop a locally targeted response for tuberculosis.14–16
Our study, based on microbiological data collected in one of the referral hospitals in the metropolitan area of Rome, indicates that more than 70% of the TB patients hospitalized between 2011 and 2016 were foreign, most of them were males and aged between 25 and 34 years. More than half of these patients were born in one single country in East Europe, Romania (Table 1).
The same trend is found when analyzing the results in respect to drug resistances: more than 80% of the MDR patients studied here were of foreign origin and half of these were from Romania, a country in which high incidence of MDR-TB is reported.2–4 We do not find a large difference when we calculate separately the rate of MDR-TB in the Italian (4.1%) and in the foreign patient population (5.9%). Overall, our MDR rates account for 5.5% of the studied TB population, regardless of origin.
Additionally, we observe that pre-XDR and XDR status is found almost exclusively in the FB population migrating from the already described geographical region and accounts for 40% of the FB MDR population, although this difference is not statistically significant. The opposite trend is found for mono-resistance to INH or RMP that are more prevalent within the Italian patients.
Surprisingly, we did not find a strong correlation between the MDR, pre-XDR or XDR status and a previous treatment for tuberculosis, at least based on the available information, contrasting with the findings of Faustini et al,12 who state, although based on earlier data, that previous treatment is the strongest determinant of MDR-TB. Memory bias or language problems during clinical visits could attempt to explain these results. Furthermore, we did not observe a significant variation of MDR-TB rates during the study period (data not shown). The TB population studied here is a fair representation of the situation reported for the entire region of Lazio. Indeed, according to the 2005–2017 report on tuberculosis in Lazio,17 our patients account for about a third of all notifications, with a similar demographic and geographical origin distribution. In our study, we find a higher proportion of foreign-born individuals among the TB population, compared to those found in the regional report (76% versus 63%). No regional data are available on resistant TB; thus, we compared our findings with the national rates, as provided by the 2018 surveillance tuberculosis report by ECDC, related to 2016 data, where 2.6% of MDR TB was reported for Italy.4
In a study recently published by Mustazzoli et al18 based, as our study, on culture-confirmed TB and phenotypic DST results collected during approximately the same period (2009–2016) in the whole country, 54.7% of TB cases in Italy were of foreign origin and 4.4% of these were MDR (3% in the overall TB population). Similarly to our results, the MDR cases presented in the national study were mostly from East European countries, although they found the highest rates of patients born in Ukraine.
Another study, carried out at a large hospital in Naples, and representative of southern Italy, reports that 53% of the TB culture confirmed cases were of foreign origin. They find a rate of MDR-TB of 4.5% and 77% of MDR were FB.19
The higher rates of foreign TB patients found in our study population compared to regional, national and Naples data, as well as the higher rates of MDR-TB in both, IB and FB, are in our opinion due to two main factors. The higher immigration rates observed in the metropolitan area of Rome, compared to Lazio region and to Italy as a whole and the higher rate of migrants that arrived here from east European countries, especially from Romania, where high incidences of MDR-TB are present, could explain our data. Another factor that we should take into account when observing these results is the role that our setting has achieved during the last decade, as a referral hospital for tuberculosis in the adult population in the extended area of the capital Rome, and largely attended by foreign patients. This referral role is probably even more pronounced in the context of drug-resistant TB, the hospital receiving a large number of treatment failures and known resistant tuberculosis cases from other centers in the area. The evidence that a selected population of TB patients attends our hospital could also explain the higher rates of MDR-TB cases among the native population compared to the national situation.
Finally, our results support the view that, to address the issue of MDR tuberculosis among migrants, a comprehensive policy of cross-border collaboration is needed.20
The aims of this policy should be to ensure continuation of care for persons moving between different countries and to provide a correct management of MDR-TB treatment along all its course, through communication among health professionals; to offer screening for active tuberculosis for migrants at higher risk and to provide free access to care to all those diagnosed with tuberculosis regardless of their documents status; to implement contact investigation crossing borders and to share results of molecular epidemiology investigations in order to identify outbreaks of drug-resistant mycobacteria.21
Conclusions
In conclusion, our results suggest that, in the great metropolitan area of Rome, some communities could be at higher risk to develop MDR tuberculosis and maybe to progress to pre-XDR and XDR.
Importantly, the high-risk communities here identified are documented residents and generally well-informed users of the public health services; thus, the information provided here could support an increased attention on these groups in order to address a more rapid diagnosis of tuberculosis and an adequate drug-resistance testing, leading to a better control of the disease.
These groups are mostly represented by documented immigrants from areas with high incidences of TB and MDR-TB, such as those born in Romania.
The extra-European TB patients in our population, represented mostly by recent migrants from sub-saharan Africa and Asia, do not appear to majorly contribute to the MDR-TB burden in the studied area.
Acknowledgments
The authors would like to acknowledge all of the personnel of the Diagnostic Laboratory of Microbiology for their daily work in contributing to a rapid and efficient management of TB cases and for making available the microbiological data used in this study.
Disclosure
Dr E Girardi reports grants from Mylan, grants from Gilead, personal fees from ViiV, personal fees from Gilead, personal fees from Angelini, personal fees from Mylan, personal fees from Otsuka, and non-financial support from Gilead, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Dheda K, Gumbo T, Maartens G, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;5(4):291–360. doi:10.1016/S2213-2600(17)30079-6
2. World Health Organization. Global tuberculosis report 2018. Available from: http://www.who.int/tb/publications/global_report/en/.
3. Tuberculosis surveillance and monitoring report in Europe 2019. WHO and ECDC report. Available from: http://www.euro.who.int/en/health-topics/communicable-diseases/tuberculosis/publications/2019/tuberculosis-surveillance-and-monitoring-report-in-europe-2019.
4. Tuberculosis surveillance and monitoring in Europe 2018. European Centre for Disease Prevention and Control. Available from: https://ecdc.europa.eu/sites/portal/files/documents/ecdc-tuberculosis-surveillance-monitoring-Europe-2018-19mar2018.pdf.
5. Falzon D, Mirzayev F, Wares F, et al. Multidrug-resistant tuberculosis around the world: what progress has been made? Eur Respir J. 2015;45(1):150–160. doi:10.1183/09031936.00101814
6. Jenkins HE, Crudu V, Soltan V, Ciobanu A, Domente L, Cohen T. High risk and rapid appearance of multidrug resistance during tuberculosis treatment in Moldova. Eur Respir J. 2014;43(4):1132–1141. doi:10.1183/09031936.00203613
7. Pareek M, Greenaway C, Noori T, Munoz J, Zenner D. The impact of migration on tuberculosis epidemiology and control in high-income countries: a review. BMC Med. 2016;14:48. doi:10.1186/s12916-016-0595-5
8. D’Ambrosio L, Centis R, Dara M, et al. European policies in the management of tuberculosis among migrants. Int J Infect Dis. 2017;56:85–89. doi:10.1016/j.ijid.2016.11.002
9. Hargreaves S, Lönnroth K, Nellums LB, et al. Multidrug-resistant tuberculosis and migration to Europe. Clin Microbiol Infect. 2017;23(3):141–146. doi:10.1016/j.cmi.2016.09.009
10. Sañé Schepisi M, Scognamiglio P, D’Amato M, Girardi E, Puro V. Letter to the editor: trends in tuberculosis notification rates by country of origin in the metropolitan area of Rome, 2010 to 2015. Euro Surveill. 2017;22(27). doi:10.2807/1560-7917.ES.2017.22.27.30570
11. WHO report on TB in the European region, 2018. What constitutes an effective and efficient package of services for the prevention, diagnosis, treatment and care of tuberculosis among refugees and migrants in the WHO European region? Available from: http://www.euro.who.int/__data/assets/pdf_file/0003/371145/who-hen-report-56.pdf?ua=1.
12. Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61(2):158–163. Epub 2005 Oct 27. doi:10.1136/thx.2005.045963
13. INMI-guidelines for management of tuberculosis. Available from: http://www.inmi.it/linee_guida/Protocollo%20Tubercolosi%20Rev.%207_2017.pdf.
14. Sotgiu G, Dara M, Centis R, et al. Breaking the barriers: migrants and tuberculosis. Presse Med. 2017;46(2 Pt 2):e5–e11. doi:10.1016/j.lpm.2017.01.013
15. Hollo V, Beauté J, Ködmön C, van der Werf MJ. Tuberculosis notification rate decreases faster in residents of native origin than in residents of foreign origin in the EU/EEA, 2010 to 2015. Euro Surveill. 2017;22(12). doi:10.2807/1560-7917.ES.2017.22.12.30486
16. Theron G, Jenkins HE, Cobelens F, et al. Data for action: collection and use of local data to end tuberculosis. Lancet. 2015;386(10010):2324–2333. doi:10.1016/S0140-6736(15)00321-9
17. Bollettino tubercolosi regione Lazio anni 2005–2017. Servizio Regionale per l’Epidemiologia Sorveglianza e Controllo delle Malattie Infettive (SERESMI). Available from: http://www.inmi.it/file/seresmi/Tubercolosi.%20Lazio%202005-2017.pdf.
18. Mustazzolu A, Borroni E, Cirillo DM, Giannoni F, Iacobino A, Fattorini L. Trend in rifampicin-, multidrug- and extensively drug-resistant tuberculosis in Italy, 2009–2016. Eur Respir J. 2018;52:1800070. doi:10.1183/13993003.00070-2018
19. Del Giudice A, Mustazzolu A, Iacobino A, et al. Drug-resistant tuberculosis in Naples, 2008–2013. Ann Ist Super Sanita. 2016;52(4):603–607. doi:10.4415/ANN_16_04_23
20. Matteelli A, Centis R, Sulis G, Tadolini M. Crossborder travel and multidrugresistant tuberculosis (MDRTB) in Europe. Travel Med Infect Dis. 2016;14(6):588–590. doi:10.1016/j.tmaid.2016.11.017
21. Dara M, Sulis G, Centis R, et al. Cross-border collaboration for improved tuberculosis prevention and care: policies, tools and experiences. Int J Tuberc Lung Dis. 2017;21(7):727–736. doi:10.5588/ijtld.16.0940
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
