Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Multidisciplinary Team (MDT) Discussion Improves Overall Survival Outcomes for Metastatic Renal Cell Carcinoma Patients
Authors Zeng Y, Zhu S, Wang Z, Chen J, Dai J, Liu Z, Sun G, Liang J, Zhang X, Wang Z, Zhao J , Ni Y, Yang J, Wang M, Wei Q, Li X , Chen N, Li Z, Wang X, Shen Y, Yao J, Huang R, Liu J , Cai D, Zeng H , Shen P
Received 21 October 2022
Accepted for publication 17 February 2023
Published 23 February 2023 Volume 2023:16 Pages 503—513
DOI https://doi.org/10.2147/JMDH.S393457
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yuhao Zeng,1,* Sha Zhu,1,* Zilin Wang,1 Junru Chen,1 Jindong Dai,1 Zhenhua Liu,1 Guangxi Sun,1 Jiayu Liang,1 Xingming Zhang,1 Zhipeng Wang,1 Jinge Zhao,1 Yuchao Ni,1 Jiyu Yang,1 Minghao Wang,1 Qiang Wei,1 Xiang Li,1 Ni Chen,2 Zhiping Li,3 Xin Wang,3 Yali Shen,3 Jin Yao,4 Rui Huang,5 Jiyan Liu,6 Diming Cai,7 Hao Zeng,1 Pengfei Shen1
1Department of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Department of Pathology, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 3Department of Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 4Department of Radiology, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 5Department of Nuclear Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 6Department of Biotherapy, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 7Department of Medical Ultrasound, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Pengfei Shen; Hao Zeng, Department of Urology, West China Hospital, Sichuan University, Chengdu, Sichuan, 610041, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Multidisciplinary team (MDT) discussion is a widely used model to manage patients diagnosed with cancer. However, there has been no direct evidence to prove its effect on the prognosis of metastatic renal cell carcinoma (mRCC) patients, so this study explored the impact of MDT discussion on mRCC patient survival.
Methods: The clinical data of 269 mRCC patients were retrospectively collected from 2012 to 2021. The cases were grouped into the MDT and non-MDT groups, then subgroup analysis was performed according to different histology types, as well as exploring the role of MDT in patients who have undergone multiple-line therapy. Overall survival (OS) and progression free survival (PFS) were set as the study endpoint.
Results: Approximately half (48.0%, 129/269) of the patients were in the MDT group, with univariable survival analyses showing these patients had remarkably longer median OS (MDT group: 73.7 months; non-MDT group: 33.2 months, hazard ratio (HR): 0.423 (0.288, 0.622), p< 0.001) and longer median PFS (MDT group: 16.9 months, non-MDT group: 12.7 months, HR: 0.722 (0.542, 0.962), p=0.026). Furthermore, MDT management resulted in longer survival for both ccRCC and non-ccRCC subgroups. Patients in the MDT group were more likely to receive multi-line therapy (MDT group: 79/129, 61.2% vs non-MDT group: 56/140, 40.0%, p< 0.001), and within this patient group, MDT management still resulted in longer OS (MDT group: 94.0 months; non-MDT group: 43.5 months, p=0.009).
Conclusion: MDT is associated with prolonged overall survival in mRCC independent of histology, ensuring that patients receive better management and precise treatment.
Keywords: mRCC, MDT, overall survival, prognosis, ccRCC, non-ccRCC, multi-line treatment
Introduction
Kidney cancer is the third most common cancer of the genitourinary neoplasms,1 and metastatic renal cell carcinoma (mRCC) is usually associated with poor clinical outcomes. Over the last two decades, mammalian targets of rapamycin (mTOR) inhibitors, tyrosine kinase inhibitors (TKIs), and vascular endothelial growth factor receptor (VEGF-R) inhibitors have reshaped the treatment landscape of mRCC2 and prolonged the survival of mRCC patients from less than one to over four years.3–5 Several large clinical trials have shown that immune checkpoint inhibitors (ICIs) have further revolutionized the therapeutics of mRCC.6,7 ICIs plus TKIs, and even nivolumab plus Ipilimumab have been recommended as the first-line treatment for clear cell renal cell carcinoma (ccRCC).8,9 Although the current guidelines recommend first-line drugs, there is still controversy about the choice of a patient’s subsequent treatment when the disease progresses. Therefore, in order to develop a more appropriate therapy plan for the patient, it is necessary to evaluate the patient’s condition by experts from multiple disciplines. Renal cell carcinoma (RCC) is a heterogeneous tumor with diverse subtypes and molecular phenotypes, including clear cell, papillary (types 1 and 2), chromophobe, and other rare RCC subtypes.10 Even though several options are recommended as first-line treatment,9 there is still no standard second or later-line treatments for metastatic ccRCC. Moreover, there is currently no standard treatment for metastatic non-ccRCC. Due to the diversity of RCC treatment for different subtypes, multidisciplinary collaborative approaches are needed to develop appropriate clinical protocols which provide patients with individualized and precise treatment.
A multidisciplinary team (MDT) brings together professionals from various departments who meet regularly and review relevant information on cancer cases to guarantee an individualized and precise treatment plan for each patient.11–13 This disease management model has been implemented in many medical centers worldwide and used in various cancer types.14 In some studies, researchers have reported that MDT could provide more precise treatment and better patient management by flexibly adjusting treatment plans, thus leading to better patient satisfaction.15–17 However, there is still no direct evidence supporting the effect of MDT on mRCC patient clinical outcomes.18 This study summarized the clinical information of mRCC in our center and explored the impact of MDT discussions on their survival.
Materials and Methods
Study Design and Patients
This is a single-centre retrospective cohort study which included 320 patients diagnosed with mRCC at West China Hospital from 2012 to 2021. These patients might be recommended to participate in the MDT discussions, and the MDT team usually consists of urologists, radiologists, oncologists, radiotherapists, pathologists, sonographers, and specialist nurses, sometimes including anesthesiology, general surgery, and endocrinology professionals. All MDT physicians must have complementary skills, qualifications, and experience. MDT roundtable discussions were held weekly in our institute. MDT discussions focus on changes in the patient’s disease status including disease progression, genetic testing and pathological molecular types, and possible changes in treatment modalities including surgical operation, immunotherapy, radiation therapy, chemotherapy and participation in clinical trial. After the discussion, the experts’ opinions would be integrated to develop a detailed, personalized therapy.
All enrolled patients were divided into the MDT group and the non-MDT group. For patients with a specific histology type, imaging feature or type of RCC identified by genetic testing and who progressed to mRCC would be recommended for MDT discussions. If these patients eligible for MDT refused to participate in the discussion, treatment options would be provided based on clinicians’ experience. Additionally, subgroup analysis was performed according to different histology types, and patients were divided into the ccRCC and non-ccRCC subgroups. The role of MDT in patients who have undergone multiple-line therapy was also explored. The 1-, 2-, 3-, 5-, and 8-year survival rates were analyzed among all patients and the two subgroups according to different histology. Patients’ clinical information included age, gender, histology type, clinical T (cT) stage, ISUP (international society of urological pathology) grade, IMDC (International Metastatic renal cell carcinoma Database Consortium) grade, the interval from diagnosis to metastasis, metastatic sites, and treatments.
Follow-Up and Endpoints
Patients were suggested to accept outpatient follow-up at least once every three months and increase the number of visits if required. Participants who could not attend visits were contacted by telephone or email to record their general condition, various vital signs (respiration, blood pressure, pulse, body temperature, etc.), and adverse events. Medical history records, physical examinations, and several laboratory tests (including routine blood tests, liver and kidney function, thyroid function, and adrenal hormone levels) were included in the follow-up protocol. Every three months, patients were required to undergo several imaging examinations (CT, MRI, or bone scan).
All participants were followed until the last study visit or death, and 31 patients did not have completed data and 20 patients did not accept any therapy. Therefore, 269 cases were analyzed in this study. The primary endpoint was overall survival (OS), that is, the time from the diagnosis of mRCC to the last study visit or death. The second endpoint was progression free survival (PFS), which was defined as the time from diagnosis to progression of disease or death.
Statistical Methods
Chi-squared tests were used to analyze the statistical difference between the MDT and non-MDT groups. A Kaplan-Meier survival curve was plotted to compare the OS and PFS, and a Log rank test was performed to analyze the statistical difference. Univariable Cox regression was used to evaluate the predictive value of individual factors in predicting OS. SPSS (version 26.0.) and GraphPad Prism software (version 8.0.2) were used for data analyses and figures. Factors with a p<0.05 were further analyzed in multivariate analyses. Hazard ratios (HR) were complemented with 95% confidence intervals (CI) and supported with significance levels. P<0.05 was considered statistically significant.
Results
Patients and Baseline Characteristics
Of 269 mRCC patients, 129 (48.0%) participated in MDT. The median follow-up time of the entire cohort was 26.3 ± 20.39 months, and a total of 129 patients died. The median OS of the total cohort was 43.5 months. Of all the patients, 235 (87.4%) received Tyrosine kinase inhibitors (TKI) therapy, 27 (10.0%) received Immune checkpoint inhibitors (ICIs) +TKI therapy, and 7 (2.6%) received mammalian target of rapamycin (mTOR) therapy in their first line (Table S1).
There were no significant associations between baseline age (p=0.47), gender (p=0.289), histological type (p=0.273), cT stage (p=0.280), ISUP (p=0.154), nephrectomy rate (p= 0.126), IMDC score (p=0.105), interval from diagnosis to metastasis (p=0.946), number of metastatic organs (p=0.758) and metastasis resection rate (p=0.488) in both groups (Table 1). Patients who participated in MDT discussions tended to be more likely to receive multi-line therapy (MDT group: 79/129, 61.2% vs non-MDT group: 56/140, 40.0%, p<0.001). There were no differences in baselines characteristics between the MDT and non-MDT groups when patients were divided into the ccRCC subgroup (196/269, 72.9%) and non-ccRCC subgroup (73/269, 27.1%) (Table S2).
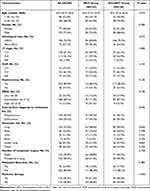 |
Table 1 Patient Baseline Characteristics |
Survival Analyses
Univariable survival analyses showed that the MDT group had a significantly longer median OS (MDT group: 73.7 months, non-MDT group: 33.2 months, HR: 0.423 (0.288, 0.622), p<0.001), and the association remained in multivariable analyses (HR: 0.554 (0.370, 0.827), p=0.004) (Figure 1A and Table 2). The other three independent adverse indexes were: IMDC score >3 (HR: 3.878 (2.091, 7.191), p<0.001), cT>2 (HR: 1.852 (1.285, 2.670), p=0.001), and number of metastatic organs ≥2 (HR: 1.975 (1.381, 2.825), p<0.001) (Table 2). Multi-line therapy was a favorable index (HR: 0.486 (0.342, 0.692), p<0.001) and was more correlated with longer survival than single-line therapy (55.4 vs 31.5 months, p<0.001) (Figure 1B). As expected, patients with low-risk and intermediate-risk IMDC scores had a significantly higher median OS than high-risk patients (48.7 vs 45.3 vs 17.9 months, p<0.001). Similarly, patients with a cT stage less than 3 had a significantly better prognosis (p=0.001); and patients with only single metastases survived longer than those with multiple metastases (p<0.001) (Figure 1C–E).
 |
Table 2 Univariable and Multivariable Analyses for OS |
Furthermore, similar results were obtained for the subgroups of ccRCC and non-ccRCC. Univariable survival analyses showed that MDT dynamic management could also result in longer survival in both ccRCC and non-ccRCC subgroups, and this effect was similar in multivariable analyses (Table S3 and Figure S1). The OS of ccRCC subgroup was just longer than the non-ccRCC subgroup.
Additionally, In the analysis of PFS, the MDT group had a significantly longer median PFS (MDT group: 16.9 months, non-MDT group: 12.7 months, HR: 0.722 (0.542, 0.962), p=0.026) (Figure S2 and Table S4). This result suggests that MDT may also prolong the PFS of these patients.
Subgroup Analyses for the Prognostic Effect of MDT
The ability of MDT to prolong OS was further validated in different subgroups. In 17 out of 23 subgroups, MDT could predict OS of mRCC and showed a trend toward longer OS. In 6 out of 23 subgroups (the age <50 years group, cT stage 1–2 subgroup, ISUP grade 3–4 subgroup, low-risk IMDC grade subgroup, high-risk IMDC grade subgroup, and non-nephrectomy subgroup), the effect of MDT on prognosis was not statistically significant (Figure 2).
 |
Figure 2 Forest plot showing the prognostic significance of MDT in predicting OS for patients of different subgroups. |
Among all cases, the 1-, 2-, 3-, 5- and 8-year survival rates of MDT group were significantly better than the non-MDT group (1-, 2-, 3-, 5- and 8-year: 90.5%, 83.9%, 68.1%, 55.3%, and 23.0% vs 83.4%, 60.2%, 45.1%, 20.5%, and 8.9%), with the 5- and 8-year survival rate of MDT group doubling compared to the non-MDT group (Table 3). In the ccRCC subgroup, the MDT group also achieved better survival rates (1-, 2-, 3-, 5- and 8-year: 90.9%, 81.6%, 73.0%, 57.3%, and 23.9% vs 84.7%, 61.9%, 48.5%, 21.1%, and 7.4%) (Table 3). Similarly, in patients diagnosed with non-ccRCC, the survival rates of MDT group were higher than the non-MDT group (1-, 2- and 3-year: 89.4%, 84.9%, and 56.1% vs 76.4%, 44.4%, and 33.6%) (Table 3).
 |
Table 3 The Overall Survival Rate Between Two Groups (MDT and Non-MDT) Among All Patients, Patients with ccRCC or Non-ccRCC |
The Prognostic Effect of MDT in Patients Receiving Multi-Line Therapy
In the multi-line therapy group (n=135), there was no difference between the MDT group and the non-MDT group in baseline characteristics, except for lung metastasis (Table 4), which was not an independent prognostic factor (Table 5). Notably, even within the multi-line therapy group, MDT dynamic management still resulted in longer survival (MDT group: 94.0 months; non-MDT group: 43.5 months, HR: 0.486 (0.283, 0.834), p=0.009) (Figure 3), and this effect remained in multivariable analyses (Table 5), proving that participating in MDT helped patients obtain more sequential treatments.
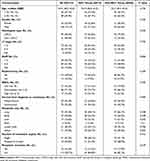 |
Table 4 Baseline Characteristics of Patients with Multi-Line Therapies, According to in MDT Group or Not |
 |
Table 5 Univariable and Multivariable Analysis for OS in Patients with Multi-Line Therapy |
 |
Figure 3 Kaplan-Meier OS estimates in patients with multi-line therapy according to MDT or non-MDT. |
Discussion
This study retrospectively analyzed the clinical data of mRCC patients over ten years to explore the prognostic effect of MDT in mRCC. Patients who participated in MDT showed more prolonged survival, and the survival benefit remained in the ccRCC and non-ccRCC subgroups. Also, patients in MDT group were more likely to receive multi-line therapy than non-MDT group, and the multivariable analyses showed that MDT also resulted in longer survival of these patients. This also indicated that MDT participation enabled patients to receive precise treatment plans and better sequential management. Moreover, patients in MDT group also showed more prolonged PFS in univariable analysis, but the difference was not observed in multivariable analysis. This may imply that the effect of MDT on PFS is interfered by other factors. In general, our finding provides practical and theoretical support for the survival-promoting effect of MDT in mRCC.
Studies in other tumors, including prostate, gastric, lung, breast, and colorectal cancer, indicate that MDT can improve patients’ survival.11,14,19 However, few studies have explored patients more suitable for MDT intervention. Our data suggested that MDT was a favorable factor in patients over 50 years old, with cT stage >2, ISUP grades >2, undergone nephrectomy, and IMDC intermediate-risk group. No significant difference was observed for patients with low cT stage, low ISUP grades, and the IMDC low-risk group, possibly due to their better overall prognosis. Patients with cT stage >2 and ISUP grades >2 may have more complicated conditions. Thus, it is reasonable that they require better management, care, and attention. Similarly, the IMDC high-risk group always had a poorer median OS. Accordingly, our study further supports selecting the patient population who are likely to benefit the most from MDT.
For patients with synchronous metastasis or metachronous metastasis, a tailored treatment plan is challenging for doctors. Although the guidelines provide the current first-line treatment recommendations, clinicians need to be more flexible in formulating treatment plans due to various general patients’ conditions and national conditions.20 Additionally, there is currently no standard treatment regimen for the subsequent treatment of mRCC, and the treatment of non-ccRCC is even more difficult.21 Therefore, clinicians from a single department are unlikely to comprehensively digest all the latest treatment-related information. For some patients with complicated conditions, whether to perform metastasectomy or cytoreductive surgery, or even whether to perform radiotherapy, these can be decided by MDT discussion. It is proved that through MDT, urologists have strengthened communication and cooperation with other specialties, forming groups to update knowledge, thereby providing patients with the latest medical support.17
Through MDT discussions, healthcare professionals can address both diagnostic and therapeutic concerns. Furthermore, with the advancement of imaging, the evaluation of patients’ clinical stage and overall condition is more accurate, enabling clinicians to adjust the treatment method more flexibly according to patients’ conditions. However, urologists or oncologists alone have limited exposure to novel imaging knowledge and may not accurately assess patients’ disease progression, requiring professional imaging physicians to give professional advice. Likewise, urologists have limited exposure to radiation oncology, which requires oncologists to join the team.22 For the life quality of patients, specialist nurses might offer more useful suggestions. All of these can be better resolved with a professional team discussion, and regular MDT meetings may be the best solution.
2The strength of this study is that it provided practical and theoretical support for MDT as a favorable prognostic factor. Also, we illustrated that some specific patient groups are better candidates for MDT participation. Nonetheless, the study has some limitations. Because some patients were initially treated in other hospitals, we could not obtain the precise data on the effectiveness of treatment. Moreover, since MDT mainly plays a role in guiding the treatment plan of patients in the course of their disease, it is of little significance to study the effectiveness of treatment, the specific treatment methods of each person are different from each other, so there is no more description on the effectiveness of treatment in this article. Additionally, there are some other limitations, including being a single-center retrospective study, the lack of cost-effectiveness data and patient quality of life, and the lack of statistics on adverse patient effects due to the diversity of treatment modalities. More prospective clinical trials are warranted to validate our data.
Conclusion
MDT can prolong the overall survival of mRCC patients, as these patients are more likely to gain sequential treatments. It is recommended that more healthcare facilities promote MDT discussions to provide mRCC patients with more comprehensive, personalized treatment plans.
Ethics and Consent Statements
This research was approved by the Ethics Committee of West China Hospital, Sichuan University and complied with the 1964 Helsinki Declaration. Written informed consent was obtained from all patients. No identifiable participant information (such as patients’ images, faces, or names) was disclosed in the study.
Acknowledgments
We would like to acknowledge the specialists of the MDT team: Ni Chen, Yali Shen, Jin Yao, Rui Huang, Jiyan Liu, Diming Cai, Hao Zeng and Pengfei Shen. And we also want to acknowledge Qiang Wei, Xiang Li, Zhiping Li and Xin Wang for their support of the MDT project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by The Science and Technology Support Program of Sichuan Province (2021YFS0119).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Kaelin W. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8(11):865–873. doi:10.1038/nrc2502
3. Tran J, Ornstein MC. Clinical review on the management of metastatic renal cell carcinoma. JCO Oncol Pract. 2022;18(3):187–196. doi:10.1200/OP.21.00419
4. Bedke J, Gauler T, Grunwald V, et al. Systemic therapy in metastatic renal cell carcinoma. World J Urol. 2017;35(2):179–188. doi:10.1007/s00345-016-1868-5
5. Zerdes I, Tolia M, Tsoukalas N, et al. Systemic therapy of metastatic renal cell carcinoma: review of the current literature. Urologia. 2019;86(1):3–8. doi:10.1177/0391560318802166
6. Rini B, Plimack E, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi:10.1056/NEJMoa1816714
7. Motzer R, Tannir N, McDermott D, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi:10.1056/NEJMoa1712126
8. Motzer R, Jonasch E, Boyle S, et al. NCCN guidelines insights: kidney cancer, version 1.2021. J Natl Compr Canc Netw. 2020;18(9):1160–1170. doi:10.6004/jnccn.2020.0043
9. Hahn AW, Klaassen Z, Agarwal N, et al. First-line treatment of metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Oncol. 2019;2(6):708–715. doi:10.1016/j.euo.2019.09.002
10. Williamson S, Gill A, Argani P, et al. Report From the International Society of Urological Pathology (ISUP) consultation conference on molecular pathology of urogenital cancers: III: molecular pathology of kidney cancer. Am J Surg Pathol. 2020;44(7):e47–e65. doi:10.1097/PAS.0000000000001476
11. Zhu S, Chen J, Ni Y, et al. Dynamic multidisciplinary team discussions can improve the prognosis of metastatic castration-resistant prostate cancer patients. Prostate. 2021;81(11):721–727. doi:10.1002/pros.24167
12. Sooriakumaran P, Dick JA, Thompson AC, Morley R. The central urology multidisciplinary team - is it time to change the referral criteria? An audit of practice in a district general hospital in London. Ann R Coll Surg Engl. 2009;91(8):700–702. doi:10.1308/003588409X12486167521190
13. Croke JM, El-Sayed S. Multidisciplinary management of cancer patients: chasing a shadow or real value? An overview of the literature. Curr Oncol. 2012;19(4):e232–238. doi:10.3747/co.19.944
14. Kočo L, Weekenstroo H, Lambregts D, et al. The effects of multidisciplinary team meetings on clinical practice for colorectal, lung, prostate and breast cancer: a systematic review. Cancers. 2021;13(16):4159. doi:10.3390/cancers13164159
15. Betschart P, Babst C, Schmid S, et al. Shared decision-making for patients with advanced urological malignancies: evaluation of a joint urological-oncological clinic model. Oncol Res Treat. 2019;42(7–8):366–374. doi:10.1159/000499721
16. Rao K, Manya K, Azad A, et al. Uro-oncology multidisciplinary meetings at an Australian tertiary referral centre--impact on clinical decision-making and implications for patient inclusion. BJU Int. 2014;114(Suppl 1):50–54. doi:10.1111/bju.12764
17. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes. Health Policy. 2015;119(4):464–474. doi:10.1016/j.healthpol.2014.09.006
18. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev. 2016;42:56–72. doi:10.1016/j.ctrv.2015.11.007
19. Xiang Y-Y, Deng C-C, Liu H-Y, Kuo Z-C, Zhang C-H, He Y-L. The prognostic effect of multidisciplinary team intervention in patients with advanced gastric cancer. Curr Oncol. 2022;29(2):1201–1212. doi:10.3390/curroncol29020102
20. De Ieso PB, Coward JI, Letsa I, et al. A study of the decision outcomes and financial costs of multidisciplinary team meetings (MDMs) in oncology. Br J Cancer. 2013;109(9):2295–2300. doi:10.1038/bjc.2013.586
21. Syn NL, Yong WP, Goh BC, Lee SC. Evolving landscape of tumor molecular profiling for personalized cancer therapy: a comprehensive review. Expert Opin Drug Metab Toxicol. 2016;12(8):911–922. doi:10.1080/17425255.2016.1196187
22. Taggar AS, Martell K, Husain S, Peacock M, Sia M, Gotto G. Exposure to radiation and medical oncology training: a survey of Canadian urology residents and fellows. Can Urol Assoc J. 2018;12(10):321–325. doi:10.5489/cuaj.5147
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

